Abstract
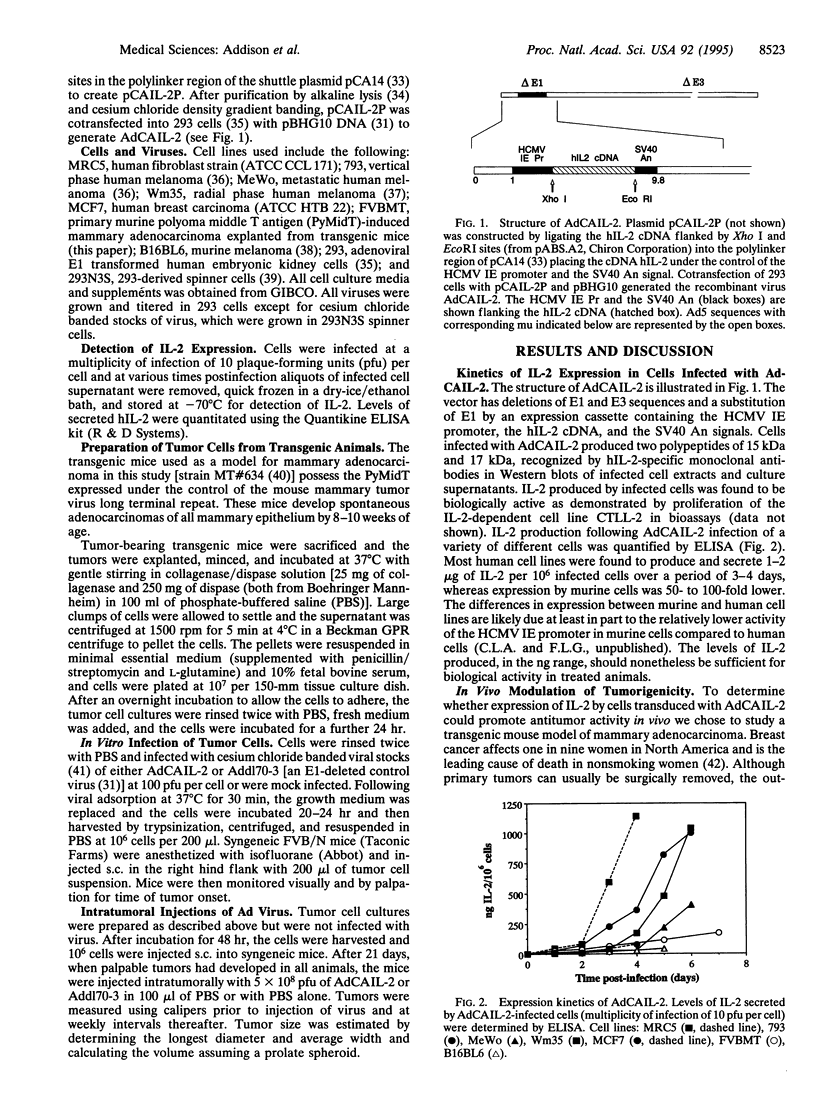
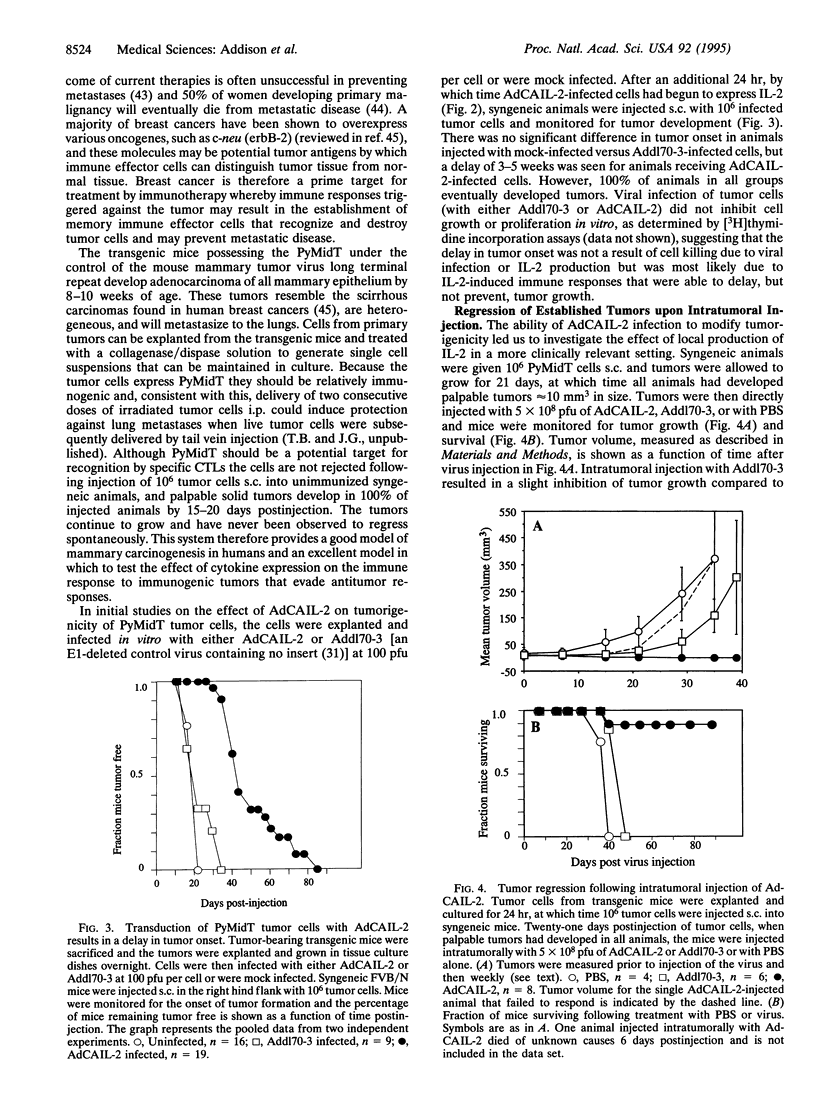
Rodent tumor cells engineered to secrete cytokines such as interleukin 2 (IL-2) or IL-4 are rejected by syngeneic recipients due to an enhanced antitumor host immune response. An adenovirus vector (AdCAIL-2) containing the human IL-2 gene has been constructed and shown to direct secretion of high levels of human IL-2 in infected tumor cells. AdCAIL-2 induces regression of tumors in a transgenic mouse model of mammary adenocarcinoma following intratumoral injection. Elimination of existing tumors in this way results in immunity against a second challenge with tumor cells. These findings suggest that adenovirus vectors expressing cytokines may form the basis for highly effective immunotherapies of human cancers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannerji R., Arroyo C. D., Cordon-Cardo C., Gilboa E. The role of IL-2 secreted from genetically modified tumor cells in the establishment of antitumor immunity. J Immunol. 1994 Mar 1;152(5):2324–2332. [PubMed] [Google Scholar]
- Bean M. A., Bloom B. R., Herberman R. B., Old L. J., Oettgen H. F., Klein G., Terry W. D. Cell-mediated cytotoxicity for bladder carcinoma: evaluation of a workshop. Cancer Res. 1975 Oct;35(10):2902–2913. [PubMed] [Google Scholar]
- Bett A. J., Haddara W., Prevec L., Graham F. L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R. B., Spiess P. J., Rosenberg S. A. Synergistic antitumor activity of tumor-infiltrating lymphocytes, interleukin 2, and local tumor irradiation. Studies on the mechanism of action. J Exp Med. 1990 Jan 1;171(1):249–263. doi: 10.1084/jem.171.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff R. D., Muller W. J. Transgenic mouse models of mammary tumorigenesis. Cancer Surv. 1993;16:97–113. [PubMed] [Google Scholar]
- Chen S. H., Shine H. D., Goodman J. C., Grossman R. G., Woo S. L. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J., Bannerji R., Saito S., Heston W., Fair W., Gilboa E. Regression of bladder tumors in mice treated with interleukin 2 gene-modified tumor cells. J Exp Med. 1993 Apr 1;177(4):1127–1134. doi: 10.1084/jem.177.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier L., Duffour M. T., Sabourin J. C., Lee M. G., Cabannes J., Ragot T., Perricaudet M., Haddada H. Complete recovery of mice from a pre-established tumor by direct intratumoral delivery of an adenovirus vector harboring the murine IL-2 gene. Gene Ther. 1995 Jan;2(1):16–21. [PubMed] [Google Scholar]
- Coulie P. G., Weynants P., Lehmann F., Herman J., Brichard V., Wölfel T., Van Pel A., De Plaen E., Brasseur F., Boon T. Genes coding for tumor antigens recognized by human cytolytic T lymphocytes. J Immunother Emphasis Tumor Immunol. 1993 Aug;14(2):104–109. doi: 10.1097/00002371-199308000-00004. [DOI] [PubMed] [Google Scholar]
- Cox A. L., Skipper J., Chen Y., Henderson R. A., Darrow T. L., Shabanowitz J., Engelhard V. H., Hunt D. F., Slingluff C. L., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994 Apr 29;264(5159):716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Eberlein T. J. Current management of carcinoma of the breast. Ann Surg. 1994 Aug;220(2):121–136. doi: 10.1097/00000658-199408000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberlein T. J., Schoof D. D. The role of interleukin-2 in cancer immunotherapy. Compr Ther. 1991 Jan;17(1):49–56. [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975 Jan;35(1):218–224. [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimmi C. D., Freeman G. J., Gribben J. G., Sugita K., Freedman A. S., Morimoto C., Nadler L. M. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6575–6579. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L. Growth of 293 cells in suspension culture. J Gen Virol. 1987 Mar;68(Pt 3):937–940. doi: 10.1099/0022-1317-68-3-937. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C. T., Cardiff R. D., Muller W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992 Mar;12(3):954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddada H., Ragot T., Cordier L., Duffour M. T., Perricaudet M. Adenoviral interleukin-2 gene transfer into P815 tumor cells abrogates tumorigenicity and induces antitumoral immunity in mice. Hum Gene Ther. 1993 Dec;4(6):703–711. doi: 10.1089/hum.1993.4.6-703. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Thurin J., Balaban G., Bennicelli J. L., Herlyn D., Elder D. E., Bondi E., Guerry D., Nowell P., Clark W. H. Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res. 1985 Nov;45(11 Pt 2):5670–5676. [PubMed] [Google Scholar]
- Karp S. E., Farber A., Salo J. C., Hwu P., Jaffe G., Asher A. L., Shiloni E., Restifo N. P., Mulé J. J., Rosenberg S. A. Cytokine secretion by genetically modified nonimmunogenic murine fibrosarcoma. Tumor inhibition by IL-2 but not tumor necrosis factor. J Immunol. 1993 Feb 1;150(3):896–908. [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C. H., Robbins P. F., Rivoltini L., Topalian S. L., Miki T., Rosenberg S. A. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Nishimura M. I., Restifo N. P., Topalian S. L., O'Neil B. H., Shilyansky J., Yannelli J. R., Rosenberg S. A. T-cell recognition of human melanoma antigens. J Immunother Emphasis Tumor Immunol. 1993 Aug;14(2):88–93. doi: 10.1097/00002371-199308000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey J. L., Berkowitz G. S. Breast cancer epidemiology. Cancer Res. 1988 Oct 15;48(20):5615–5623. [PubMed] [Google Scholar]
- Klijn J. G., Berns P. M., Bontenbal M., Alexieva-Figusch J., Foekens J. A. Clinical breast cancer, new developments in selection and endocrine treatment of patients. J Steroid Biochem Mol Biol. 1992 Sep;43(1-3):211–221. doi: 10.1016/0960-0760(92)90210-a. [DOI] [PubMed] [Google Scholar]
- Lindgren C. G., Thompson J. A., Higuchi C. M., Fefer A. Growth and autologous tumor lysis by tumor-infiltrating lymphocytes from metastatic melanoma expanded in interleukin-2 or interleukin-2 plus interleukin-4. J Immunother Emphasis Tumor Immunol. 1993 Nov;14(4):322–328. doi: 10.1097/00002371-199311000-00012. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M. T., Grimm E. A., Mazumder A., Strausser J. L., Rosenberg S. A. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981 Nov;41(11 Pt 1):4420–4425. [PubMed] [Google Scholar]
- Mandelboim O., Berke G., Fridkin M., Feldman M., Eisenstein M., Eisenbach L. CTL induction by a tumour-associated antigen octapeptide derived from a murine lung carcinoma. Nature. 1994 May 5;369(6475):67–71. doi: 10.1038/369067a0. [DOI] [PubMed] [Google Scholar]
- Oldfield E. H., Ram Z., Culver K. W., Blaese R. M., DeVroom H. L., Anderson W. F. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993 Feb;4(1):39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M. Cancer vaccines. Trends Pharmacol Sci. 1993 May;14(5):202–208. doi: 10.1016/0165-6147(93)90209-3. [DOI] [PubMed] [Google Scholar]
- Ragot T., Vincent N., Chafey P., Vigne E., Gilgenkrantz H., Couton D., Cartaud J., Briand P., Kaplan J. C., Perricaudet M. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993 Feb 18;361(6413):647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L. E., Päkkö P. K., Gilardi P., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Siegel J. P., Puri R. K. Interleukin-2 toxicity. J Clin Oncol. 1991 Apr;9(4):694–704. doi: 10.1200/JCO.1991.9.4.694. [DOI] [PubMed] [Google Scholar]
- Tohmatsu A., Okino T., Stabach P., Padula S. J., Ergin M. T., Mukherji B. Analysis of cytolytic effector cell response in vitro against autologous human tumor cells genetically altered to synthesize interleukin-2. Immunol Lett. 1993 Jan;35(1):51–57. doi: 10.1016/0165-2478(93)90147-t. [DOI] [PubMed] [Google Scholar]
- Topalian S. L., Kasid A., Rosenberg S. A. Immunoselection of a human melanoma resistant to specific lysis by autologous tumor-infiltrating lymphocytes. Possible mechanisms for immunotherapeutic failures. J Immunol. 1990 Jun 1;144(11):4487–4495. [PubMed] [Google Scholar]
- Topalian S. L., Solomon D., Rosenberg S. A. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989 May 15;142(10):3714–3725. [PubMed] [Google Scholar]
- Townsend S. E., Allison J. P. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993 Jan 15;259(5093):368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Tsomides T. J., Eisen H. N. T-cell antigens in cancer. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3487–3489. doi: 10.1073/pnas.91.9.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills K. N., Maneval D. C., Menzel P., Harris M. P., Sutjipto S., Vaillancourt M. T., Huang W. M., Johnson D. E., Anderson S. C., Wen S. F. Development and characterization of recombinant adenoviruses encoding human p53 for gene therapy of cancer. Hum Gene Ther. 1994 Sep;5(9):1079–1088. doi: 10.1089/hum.1994.5.9-1079. [DOI] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung R. S., Vollmer C., Taylor D. D., Palazzo J., Weese J. L. Intratumoral rIL2-based immunotherapy in B16 melanoma. J Surg Res. 1992 Aug;53(2):203–210. doi: 10.1016/0022-4804(92)90036-y. [DOI] [PubMed] [Google Scholar]



