Abstract
Shank is a specialized scaffold protein present in high abundance at the postsynaptic density (PSD). Using pre-embedding immunogold electron microscopy on cultured hippocampal neurons, we had previously demonstrated further accumulation of Shank at the PSD under excitatory conditions. Here, using the same experimental protocol, we demonstrate that a cell permeable CaMKII inhibitor, tatCN21, blocks NMDA-induced accumulation of Shank at the PSD. Furthermore we show that NMDA application changes the distribution pattern of Shank at the PSD, promoting a 7–10 nm shift in the median distance of Shank labels away from the postsynaptic membrane. Inhibition of CaMKII with tatCN21 also blocks this shift in the distribution of Shank. Altogether these results imply that upon activation of NMDA receptors, CaMKII mediates accumulation of Shank, preferentially at the distal regions of the PSD complex extending toward the cytoplasm.
Keywords: CaMKII, tatCN21, ProSAP, Shank, Homer, PSD
INTRODUCTION
Shanks, also known as ProSAP, Synamon, SSTRIP, CortBP and Spank, are a family of scaffold proteins at the postsynaptic density (PSD, reviews: [1], [2]). Shank proteins are thought to promote enlargement and maturation of spines [3], [4] and have been implicated in a number of neuronal diseases including autism (review: [5]).
Shanks have multiple protein-interaction domains and can bind to other PSD scaffolds, GKAPs and Homers (review: [1]). Homer and Shank are localized within the same region of the PSD complex, an area ~30–100 nm from the postsynaptic membrane, right below the electron dense and readily visible PSD core [6], [7]. Using immuno-electron microscopy, we have previously demonstrated that under acute excitatory conditions more Shank accumulates at the PSD [6] while the levels of Homer remain unchanged [7].
In the present study we tested a possible role of CaMKII in the activity-induced recruitment of Shank to the PSD. CaMKII, a Ca2+-regulated protein kinase, is a major component of the PSD and accumulates at the PSD under excitatory conditions [8]. CaMKII activation, autophosphorylation and/or accumulation at the PSD is essential for NMDA receptor-dependent long-term modification of synaptic efficacy (review: [9]). Previous work indicated that activation and or accumulation of CaMKII at the PSD is necessary for NMDA-induced redistribution of two other PSD components, SynGAP [10], a small G protein regulator, and CYLD [11], a deubiquitinase.
We used a cell-permeable CaMKII inhibitor, tatCN21, to examine the role of CaMKII in NMDA-induced accumulation of Shank at the PSD. The inhibitor tatCN21 suppresses Ca2+-dependent as well as autonomous activities of CaMKII towards exogenous substrates [12] and also inhibits the accumulation of CaMKII at the PSD [13]. Our results show that tatCN21 inhibits NMDA-induced changes in the levels and distribution of Shank at the PSD.
MATERIAL AND METHODS
Materials
Mouse monoclonal antibody against pan Shank (clone N23B/49) was from NeuroMab (Davis, CA). Mouse monoclonal antibody against Homer 1 (pan Homer 1, clone 2G8), rabbit polyclonal antibodies against Homer 1b/c (raised against aa 152–354 of human Homer 1b) were from Synaptic Systems (Göttingen, Germany).
The membrane-permeable peptide CaMKII inhibitor used in this study, tatCN21 [12], contains a cell-penetrating “tat” sequence and a 21-amino acid peptide (CN21, amino acid sequence KRPPKLGQIGRSKRVVIEDDR) derived from CaMKIIN [14]. The control peptide contains the tat sequence fused to a scrambled sequence (VKEPRIDGKPVRLRGQKSDRI) of CN21 [15]. N-methyl-D-asparic acid (NMDA) was from Tocris (Ellisville, MO).
Dissociated hippocampal neuronal cultures and treatments
The animal protocol was approved by the NIH Animal Use and Care Committee and conforms to NIH guidelines. Hippocampal cells from 21-day embryonic Sprague-Dawley rats were dissociated and grown on a feeder layer of glial cells for 3–4 weeks. During experiments, culture dishes were placed on a floating platform in a water bath maintained at 37°C. Control incubation medium was: 124 mM NaCl, 2mM KCl, 1.24 mM KH2PO4, 1.3 mM MgCl2, 2.5 mM CaCl2, 30 mM glucose in 25 mM HEPES at pH 7.4. Whenever indicated, control medium was modified to include 50 μM NMDA, 20 μM tatCN21 or tat-control peptide. Cell cultures were washed with control medium and preincubated for 20 min in control medium, or in media containing tatCN21 or control peptide, as indicated. Samples were then treated for 2 min with control medium or NMDA-containing media with the same additions (none, tatCN21, tat-control peptide) used in preincubation. Cells were fixed with 4% paraformaldehyde (EMS, Fort Washington, PA) in PBS for 30–45 min, and thoroughly washed before immunolabeling.
Pre-embedding immunogold labeling and electron microscopy
Fixed cells were washed, and then blocked and made permeable with 5% normal goat serum and 0.1% saponin for 40–60 min. Samples were incubated with primary and secondary antibodies (Nanogold, Nanoprobes, Yaphand, NY) for 1–2 hr, fixed with 2% glutaraldehyde in PBS for 30 min- overnight, silver enhanced (HQ kit, Nanoprobes), treated with 0.2% osmium tetroxide in 0.1M phosphate buffer at pH 7.4 for 30 min, en block stained with 0.25–0.5% uranyl acetate in acetate buffer at pH 5.0 for 1 hr, dehydrated in graded ethanols, and embedded in epoxy resin.
Sampling of synapses and morphometry
Synapses were identified by their typical structural characteristics: clustered synaptic vesicles in the presynaptic terminals, rigidly apposed synaptic cleft, and prominent postsynaptic density. At least five randomly chosen grid openings from each thin-sectioned sample were sampled. Every cross-sectioned synaptic profile encountered was photographed with a digital CCD camera (AMT XR-100, Danvers, MA, USA).
Intensity of label at the PSD was measured by counting all labels located within the PSD complex and divided by the length of the PSD. The measurement area of the PSD complex was bordered by the postsynaptic membrane, two parallel lines perpendicular to the postsynaptic membrane, and a parallel line to the postsynaptic membrane at a 120 nm distance (Fig. 1A). The mean values for intensity of label at the PSD under different conditions were compared via ANOVA.
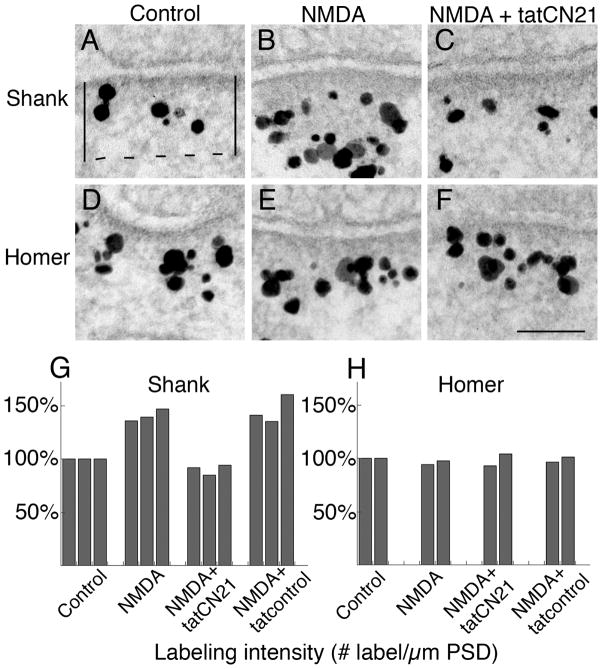
Figure 1. NMDA-induced Shank increase at the PSD is dependent on CaMKII activity.
Hippocampal neurons in culture were pre-incubated for 20 min with or without tat-peptides, as indicated, and then were treated with NMDA-containing media with the same additions or control medium for another 2 min. The cells were then immediately fixed and prepared for antibody labeling.
Above Electron micrographs of synapses labeled for Shank (A–C) or Homer (D–F). Gold labels appear as black particles of heterogeneous size. Label for Shank increased at the PSD complex after NMDA treatment (B vs. A), and this increase was blocked by the CaMKII inhibitor, tatCN21 (C). In contrast, distribution of label for Homer remained unchanged under these same conditions.
Below bar graphs. PSD measurement area was marked as shown in (A) and labeling intensity was calculated as number of labels per μm PSD length. Labeling intensity at the PSD complexes was normalized to control values for Shank (G, three experiments with a pan Shank antibody) and Homer (H, one experiment with a pan Homer1 antibody and a second experiment with a Homer 1b/c antibody). Statistical analyses were carried out via ANOVA with Tukey’s post test within each experiment:
For Shank, between control and NMDA, P< 0.005 for exp 1, P<0.001 for exp 2, P<0.01 for exp 3; between NMDA and NMDA+tatCN21, P<0.0001 for all 3 exp; between NMDA+tatCN21 and NMDA+tatcontrol, P<0.0001 for all 3 exp. For Homer, there were no statistically significant differences among any two treatment conditions.
Distance of label from the postsynaptic membrane was measured from the center of the silver-enhanced label to the outer edge of the postsynaptic membrane for every label within the PSD complex. Because the distribution of distance measurements for Shank is skewed, statistical analysis was carried out with a non-parametric comparison of the median values, Wilcoxin rank sum test (KaleidaGraph, Synergy Software, Reading, PA).
RESULTS
To study the involvement of CaMKII in the NMDA-induced accumulation of Shank at the PSD, cultured hippocampal neurons were treated with NMDA in the presence of a CaMKII inhibitor (tatCN21) or an inactive control peptide (tatcontrol) followed by pre-embedding immuno-electron microscopy. The redistribution of Shank under these conditions was also compared to that of Homer, another scaffold protein that occupies a similar location at the PSD. The results are shown in Figure 1 with typical electron micrographs from one experiment presented in the top panels (Figure 1A–F) and quantified results for label intensities at the PSD from 2–3 experiments presented in bottom panels (Figure 1G, H).
As previously reported [6], immunogold label for Shank under basal conditions is typically located in a broad band within the PSD complex, ~ 30–100 nm from the postsynaptic membrane (Fig. 1A), and shows a marked increase upon application of NMDA (Fig. 1B). In order to test an involvement of CaMKII in the observed recruitment of Shank, a cell permeable inhibitor tatCN21 was used. We have previously demonstrated that unlike other CaMKII inhibitors such as KN93, tatCN21 effectively blocks the translocation of CaMKII to the PSD [13]. NMDA-induced increase in Shank label at the PSD is blocked when cells were pretreated with tatCN21 (Figure 1C).
Data from three experiments show that labeling intensity for Shank (Fig. 1G) in NMDA-treated samples is 141 ± 3% of controls (significant in all three experiments), whereas in samples treated with NMDA in the presence of tatCN21, the labeling intensity for Shank is 90 ± 3 % of controls (not significant in all three experiments). The effect of tatCN21 is not due to the ‘tat’ sequence used to confer cell permeability, because a control peptide with the tat sequence did not have any inhibitory effect (not significantly different from NMDA alone in all three experiments). Altogether these results indicate that, NMDA-induced Shank accumulation at the PSD is mediated by CaMKII. On the other hand, tatCN21 does not have any effect on the labeling intensity of Homer (Fig. 1H) implying that, unlike Shank, CaMKII does not regulate the distribution of Homer.
Compared to the narrow laminar distribution seen for PSD-95 [16], Shank exhibits a broader distribution of label within the PSD complex (Figure 1), extending deeper into the cytoplasm. In order to assess whether CaMKII-mediated recruitment confers a shift in the distribution of Shank molecules at the PSD, median distances of labels in the presence and absence of tatCN21 were compared. Distance measurement of label for Shank from the postsynaptic membrane indicates that application of NMDA promoted a 7–10 nm increase in the median distance. Inclusion of CaMKII inhibitor, tatCN21, but not the tat-control peptide, blocked this NMDA-induced shift in median distance (Table 1). These results indicate that the NMDA-induced, CaMKII-dependent addition of Shank occurs preferentially to the distal part of the PSD that is further away from the postsynaptic membrane.
Table 1.
Median distance (nm) of label for Shank from the postsynaptic membrane under different conditions
| 1. control | 2. NMDA | 3. NMDA+ tatCN21 | 4. NMDA+ tatcontrol | |
|---|---|---|---|---|
| Exp 1 | 53.3 (n=268) | 60.0 (n=419) | 54.6 (n=220) | 64.0 (n=242) |
| Exp 2 | 56.7 (n=276) | 66.7 (n=359) | 53.3 (n=326) | 60.0 (n=300) |
| Exp 3 | 53.3 (n=131) | 63.3 (n=263) | 53.3 (n=354) | 60.0 (n=150) |
(n) number of labels measured.
Distributions were compared by Wilcoxin rank sum test within each experiment with significance level at P<0.05: significant in all three experiments between 1 vs. 2, 2 vs. 3, 3 vs. 4, 1 vs. 4.
DISCUSSION
Acute excitatory conditions promote short-term redistribution of select PSD components. We have so far established that certain enzymes, a protein kinase CaMKII, a small G-protein regulator SynGAP and a deubiquitinase CYLD, redistribute in response to depolarization or NMDA application [8], [17], [16], [18], and that the movements of all three molecules are blocked upon inhibition of CaMKII [13], [10], [11].
Among the scaffold proteins, Shank accumulates at the PSD in response to acute excitatory conditions, whereas the levels and distributions of at least two other scaffolds, PSD-95 and Homer, remain unchanged [16], [7]. In the present study we demonstrate that CaMKII mediates NMDA-induced recruitment of Shank at the PSD. The present result, together with the previous studies on SynGAP and CYLD reveal that activation and accumulation of CaMKII at the PSD are the primary events that trigger short-term redistribution of several other PSD components.
At the present the target protein(s) or phosphorylation site(s) responsible for the CaMKII-mediated recruitment of Shank at the PSD are not clear. CaMKII is known to phosphorylate numerous PSD components including Shank [19]. However, in in vitro experiments investigating the association of Shank and Homer into a complex, addition of CaMKII under phosphorylating conditions did not increase the amount of polymerized Shank [20]. While this result suggests that CaMKII-mediated phosphorylation does not promote the association of Shank with Homer or with other Shank molecules in vitro, additional mechanisms may come into play in the intact cell, maybe ones that alter Shank’s affinity to other components.
Shanks bind to actin regulating factors, cortactin, Abp1 and IRSp53 (BAIP2) [21], [22], [23], [24]. Thus, accumulation of Shank at the distal parts of the PSD bordering the actin cytoskeleton could be followed by the recruitment of these actin-regulating proteins at a critical location to regulate the actin cytoskeleton. The role of CaMKII in the regulation of AMPA receptor recruitment and activity is well established [25], [26]. The demonstration of CaMKII-mediated recruitment of Shank at the distal area of the PSD offers a mechanism for the integration of regulation of AMPA receptors and actin cytoskeleton through a common upstream event: accumulation and activation of CaMKII at the PSD.
NMDA-induces accumulation of Shank at the postsynaptic density.
Shank accumulation is preferential to the distal region of the postsynaptic density.
Shank accumulation is mediated by CaMKII.
Acknowledgments
We thank Christine A. Winters for hippocampal cultures, Rita Azzam and Virginia Crocker for EM technical assistance. Supported by the Intramural Research Program of the NIH, NINDS, and by R01NS052644 (to K.U.B.).
Abbreviations
- NMDA
N-methyl-D-asparic acid
- PSD
postsynaptic density
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sheng M, Kim E. The Shank family of scaffold proteins. J Cell Sci. 2000;113(Pt 11):1851–1856. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- 2.Boeckers TM, Bockmann J, Kreutz MR, Gundelfinger ED. ProSAP/Shank proteins - a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J Neurochem. 2002;81:903–910. doi: 10.1046/j.1471-4159.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- 3.Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- 4.Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF, Weinberg RJ, Sheng M. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grabrucker AM, Schmeisser MJ, Schoen M, Boeckers TM. Postsynaptic ProSAP/Shank scaffolds in the cross-hair of synaptopathies. Trends Cell Biol. 2011;21:594–603. doi: 10.1016/j.tcb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Tao-Cheng JH, Dosemeci A, Gallant PE, Smith C, Reese T. Activity induced changes in the distribution of Shanks at hippocampal synapses. Neuroscience. 2010;168:11–17. doi: 10.1016/j.neuroscience.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao-Cheng JH, Thein S, Yang Y, Reese TS, Gallant PE. Homer is concentrated at the postsynaptic density and does not redistribute after acute synaptic stimulation. Neuroscience. 2014;266:80–90. doi: 10.1016/j.neuroscience.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dosemeci A, Tao-Cheng JH, Vinade L, Winters CA, Pozzo-Miller L, Reese TS. Glutamate-induced transient modification of the postsynaptic density. Proc Natl Acad Sci U S A. 2001;98:10428–10432. doi: 10.1073/pnas.181336998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hell JW. CaMKII: claiming center stage in postsynaptic function and organization. Neuron. 2014;81:249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Tao-Cheng JH, Bayer KU, Reese TS, Dosemeci A. Camkii-Mediated Phosphorylation Regulates Distributions of Syngap-alpha1 and -alpha2 at the Postsynaptic Density. PLoS One. 2013;8:e71795. doi: 10.1371/journal.pone.0071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thein S, Tao-Cheng JH, Li Y, Bayer KU, Reese TS, Dosemeci A. CaMKII Mediates Recruitment and Activation of the Deubiquitinase CYLD at the Postsynaptic Density. PLoS One. 2014;9:e91312. doi: 10.1371/journal.pone.0091312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vest RS, Davies KD, O’Leary H, Port JD, Bayer KU. Dual mechanism of a natural CaMKII inhibitor. Mol Biol Cell. 2007;18:5024–5033. doi: 10.1091/mbc.E07-02-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao-Cheng JH, Yang Y, Bayer KU, Reese TS, Dosemeci A. Effects of CaMKII inhibitor tatCN21 on activity-dependent redistribution of CaMKII in hippocampal neurons. Neuroscience. 2013;244:188–196. doi: 10.1016/j.neuroscience.2013.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci U S A. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vest RS, O’Leary H, Coultrap SJ, Kindy MS, Bayer KU. Effective post- insult neuroprotection by a novel Ca(2+)/ calmodulin-dependent protein kinase II (CaMKII) inhibitor. J Biol Chem. 2010;285:20675–20682. doi: 10.1074/jbc.M109.088617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Tao-Cheng JH, Reese TS, Dosemeci A. SynGAP moves out of the core of the postsynaptic density upon depolarization. Neuroscience. 2011;192:132–139. doi: 10.1016/j.neuroscience.2011.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosemeci A, Vinade L, Winters CA, Reese TS, Tao-Cheng JH. Inhibition of phosphatase activity prolongs NMDA-induced modification of the postsynaptic density. Journal of Neurocytology. 2002;31:605–612. doi: 10.1023/a:1025735410738. [DOI] [PubMed] [Google Scholar]
- 18.Dosemeci A, Thein S, Yang Y, Reese TS, Tao-Cheng JH. CYLD, a deubiquitinase specific for lysine63-linked polyubiquitins, accumulates at the postsynaptic density in an activity-dependent manner. Biochem Biophys Res Commun. 2013;430:245–249. doi: 10.1016/j.bbrc.2012.10.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dosemeci A, Jaffe H. Regulation of phosphorylation at the postsynaptic density during different activity states of Ca2+/calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 2010;391:78–84. doi: 10.1016/j.bbrc.2009.10.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM, Li H, Sala C, Hayashi Y. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137:159–171. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Y, Weed SA, Xiong WC, Marshall TD, Parsons JT. Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol Cell Biol. 1998;18:5838–5851. doi: 10.1128/mcb.18.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 23.Qualmann B, Boeckers TM, Jeromin M, Gundelfinger ED, Kessels MM. Linkage of the actin cytoskeleton to the postsynaptic density via direct interactions of Abp1 with the ProSAP/Shank family. J Neurosci. 2004;24:2481–2495. doi: 10.1523/JNEUROSCI.5479-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bockmann J, Kreutz MR, Gundelfinger ED, Bockers TM. ProSAP/Shank postsynaptic density proteins interact with insulin receptor tyrosine kinase substrate IRSp53. J Neurochem. 2002;83:1013–1017. doi: 10.1046/j.1471-4159.2002.01204.x. [DOI] [PubMed] [Google Scholar]
- 25.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation [see comments] Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]