Abstract
The positive transcription elongation factor b (P-TEFb), composed of cyclin-dependent kinase 9 and cyclin T1, stimulates the elongation of transcription by hyperphosphorylating the C-terminal region of RNA polymerase II. Aberrant activation of P-TEFb results in manifestations of cardiac hypertrophy in mice, suggesting that P-TEFb is an essential factor for cardiac myocyte function and development. Here, we present evidence that P-TEFb selectively activates transcription mediated by the myocyte enhancer factor 2 (MEF2) family of transcription factors, key regulatory factors for myocyte development. Knockdown of endogenous cyclin T1 in murine C2C12 cells abolishes MEF2-dependent reporter gene expression as well as transcription of endogenous MEF2 target genes, whereas overexpression of P-TEFb enhances MEF2-dependent transcription. P-TEFb interacts with MEF2 both in vitro and in vivo. Activation of MEF2-dependent transcription induced by serum starvation is mediated by a rapid dissociation of P-TEFb from its inhibitory subunit, HEXIM1, and a subsequent recruitment of P-TEFb to MEF2 binding sites in the promoter region of MEF2 target genes. These results indicate that recruitment of P-TEFb is a critical step for stimulation of MEF2-dependent transcription, therefore providing a fundamentally important regulatory mechanism underlying the transcriptional program in muscle cells.
Keywords: P-TEFb, CycT1, MEF2, differentiation, transcriptional elongation
Introduction
The elongation step of eukaryotic transcription is a highly regulated process that is tightly controlled by several positive and negative transcription elongation factors (N-TEFs).1,2 Shortly after initiation, transcription machinery containing RNA polymerase II (RNAPII) is arrested by N-TEFs.3 This results in the accumulation of prematurely terminated short transcripts (abortive transcription).2 Positive transcription elongation factors (P-TEFs) are required for RNAPII to overcome this barrier and complete transcription. A well-studied positive factor is P-TEFb, which mediates the transition from premature short transcripts to mature long transcripts.3 P-TEFb is composed of the cyclin-dependent kinase 9 (Cdk9) and one of four C-type cyclin subunits [cyclin T1 (CycT1), alternatively spliced cyclin T2a and T2b (CycT2a and CycT2b, respectively), and cyclin K (CycK)].2,4 The Cdk9 subunit of P-TEFb preferentially phosphorylates the second serine (2S) of the carboxyterminal domain heptapeptide repeats (YSTPSPS) of RNAPII and N-TEFs such as the DRB (5,6-dichloro-1-β-d-ribofuranosylbenzimidazole) sensitivity inducing factor and the negative elongation factor.5–7 This phosphorylation is required for the high processivity of the transcriptional elongation complex and for the removal of the negative effectors.7–9
Recently, increasing numbers of studies have uncovered mechanisms of cellular transcription regulated by P-TEFb.2,10 Studies using flavopiridol, a potent Cdk9 kinase inhibitor, have suggested that P-TEFb regulates transcription of many cellular genes.11 In HeLa cells, approximately 50% of P-TEFb is associated with 7SK small nuclear RNA and the HEXIM1 protein. This complex maintains P-TEFb in an inactive state.12,13 Furthermore, when not bound to HEXIM1 and 7SK RNA, P-TEFb can interact with a chromatin-associated protein, the bromodomain protein 4 (Brd4). This interaction results in recruitment of P-TEFb to transcriptionally active promoters.14,15 Recent studies from the Zhou laboratory indicate that the equilibrium between P-TEFb-HEXIM1-7SK and P-TEFb-Brd4 is tightly regulated in order to control P-TEFb-mediated transcriptional elongation.16
One of the unanswered questions about transcriptional elongation regulated by P-TEFb is how P-TEFb is recruited to its target genes. Several cellular transcription factors and growth factors have been reported to bind directly to P-TEFb.17 Indeed, direct tethering of P-TEFb subunits to DNA is sufficient to activate transcription.18–20 Taken together, there are at least two distinct mechanisms for the recruitment of P-TEFb to its target genes: (1) gene-specific recruitment through an interaction between P-TEFb and transactivators and (2) indirect recruitment via the interaction between P-TEFb and Brd4.
P-TEFb initially attracted attention when it was identified as the cellular cofactor for the transactivator (Tat) protein of the human immunodeficiency virus type 1 (HIV-1).21,22 However, further investigation into P-TEFb functions suggests that P-TEFb also plays an important role in muscle function and development.23–25 First, in cardiac myocytes, hypertrophic reaction induced by endothelin 1 was blocked by dominant-negative Cdk9 or a Cdk9 kinase inhibitor, DRB. Hypertrophic stimulation led to the dissociation of P-TEFb from HEXIM1/7SK. Targeted disruption of the complex between P-TEFb and 7SK by overexpression of antisense DNA against 7SK resulted in cardiac myocyte growth.26 Additionally, transgenic mice overexpressing the CycT1 subunit and knockout mice lacking CLP-1 (cardiac lineage protein 1), a murine equivalent of HEXIM1, showed hypertrophic manifestations.27 Finally, it was demonstrated that CycT2 interacts with MyoD, a basic helix–loop–helix transcription factor, and recruits CycT2-containing P-TEFb to MyoD-dependent promoters during myocyte differentiation.28,29 These observations suggest that P-TEFb is an essential regulator for the complex transcriptional program in muscle cell function and development.
In the present study, we demonstrate that P-TEFb is an essential cofactor for the activation of transcription by the myocyte enhancer factor 2 (MEF2) family of transcription factors in murine skeletal-musclederived C2C12 cells. MEF2-dependent transcription is abolished when the endogenous P-TEFb expression is reduced by small interfering RNA (siRNA) against CycT1. Conversely, overexpression of P-TEFb enhances MEF2-dependent reporter gene expression. P-TEFb interacts with MEF2 both in vitro and in vivo. Serum starvation of C2C12 cells leads to a dissociation of P-TEFb from its inhibitory subunit, HEXIM1, resulting in the activation of MEF2-dependent transcription. Moreover, the expression of endogenous MEF2 target genes is diminished in the presence of siRNA against CycT1 in C2C12 cells. Finally, P-TEFb is detected at the promoter region that contains MEF2 binding elements in muscle creatine kinase (MCK), glucose transporter protein 4 (GLUT4), Nur77, and c-Jun genes. Promoter recruitment of P-TEFb occurs within 30 min after starvation, which coincides with the dissociation of P-TEFb from HEXIM1. These results indicate that P-TEFb selectively enhances MEF2-dependent transcription and thereby helps to orchestrate the transcriptional program during muscle growth and differentiation.
Results
P-TEFb stimulates MEF2-dependent transcription
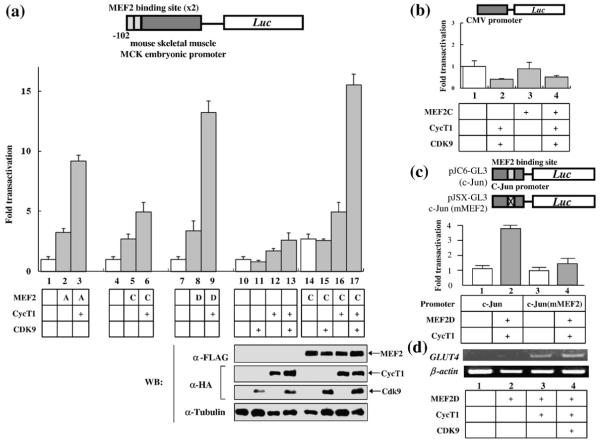
It was demonstrated that P-TEFb is upregulated upon hypertrophic signal. Conversely, mice over-expressing P-TEFb show manifestations of cardiac hypertrophy.26 Since P-TEFb can activate its target genes by interacting with promoter-associated transcription transactivators,29–37 we examined whether P-TEFb can stimulate transcription mediated by myocyte-specific transcription factors. MEF2 is a MADS (MCM1, agamus, deficiens, serum response factor) superfamily transcription factor known to be upregulated during cardiac hypertrophy.38–41 Therefore, we investigated whether there is a functional relationship between P-TEFb and MEF2 using a reporter gene system. P-TEFb subunits (CycT1 and Cdk9) were overexpressed in HeLa cells with a rat MCK promoter-driven luciferase (MCK-Luc) reporter gene that contains two MEF2 binding sites, in the presence or absence of three members of MEF2 (MEF2A, C, and D). As shown in Fig. 1a, MEF2A, C, and D alone activated MCK-Luc by 2- to 3-fold (lanes 2, 5, and 8). Coexpression of CycT1 greatly stimulated MEF2-mediated luciferase expression by 5- to 15-fold in the presence of MEF2A, C, or D (lanes 3, 6, and 9). Overexpression of CycT1 in the absence of MEF2 slightly activated reporter activity (~2-fold, lane 12), presumably due to endogenous MEF2 activity. Addition of the Cdk9 subunit further activated MEF2C-dependent transcription (lane 15), although Cdk9 alone did not activate MCK-Luc expression in the presence or absence of MEF2 (lanes 11 and 15). Western blot analysis indicates that the elevated MEF2 activity was not due to increased expression of MEF2 by CycT1 and/or Cdk9 (Fig. 1a, lower panel). Neither CycT1 nor Cdk9 activated the cytomegalovirus immediate early gene promoter-driven luciferase (CMV-Luc) reporter gene in the presence or absence of MEF2, suggesting that the effect of P-TEFb overexpression is specific to MEF2 (Fig. 1b). Activation of MEF2-dependent transcription by P-TEFb was further confirmed by using a c-Jun promoter-driven luciferase reporter gene that contains an intact (pJC6-GL3) or mutated (pJSX-GL3) MEF2 binding site.42 CycT1 together with MEF2D activated the reporter gene expression from pJC6-GL3 (4-fold) but not from pJSX-GL3 (Fig. 1c), which indicates that the stimulation of transcription by P-TEFb depends on a functional MEF2 binding site in the promoter. In order to analyze the effect of P-TEFb overexperssion on an endogenous MEF2 promoter, we quantified the mRNA levels of GLUT4, a known MEF2 target gene, by reverse transcription (RT)-PCR analysis.43 As shown in Fig. 1d, GLUT4 mRNA expression was stimulated in the presence of CycT1 (Fig. 1d, lane 3). Addition of Cdk9 further enhanced GLUT4 mRNA expression (Fig. 1d, lane 4). Thus, we conclude that P-TEFb and MEF2 synergistically activate MEF2-dependent transcription.
Fig. 1.
Overexpression of P-TEFb enhances MEF2-dependent transcription. (a) Upper panel: HeLa cells were transfected with rat skeletal MCK promoter-driven luciferase reporter gene (MCK-Luc), containing two MEF2 binding sites, with MEF2A (lanes 2 and 3), 2C (lanes 5, 6, 14–17), or 2D (lanes 8 and 9) (0.2 μg) with or without human CycT1 (lanes 3, 6, 9, 12, 13, 16, and 17) (0.3 μg) and Cdk9 (lanes 11, 13, 15, and 17) (0.3 μg). Forty-eight hours after transfection, luciferase activity was measured as described in Materials and Methods. Lower panel: The expression of Flag-epitope-tagged MEF2C, HA-CycT1, and HA-Cdk9 was detected by Western blot analysis. The expression of α-tubulin was also detected by Western blot analysis and used as a loading control. Luciferase activity is shown as fold transactivation relative to the value obtained without MEF2s, CycT1, and Cdk9 (lanes 1, 4, 7, and 10). Data are representative of three independent assays. (b) HeLa cells were transfected with CMV-Luc reporter plasmid, in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of MEF2C, with or without human CycT1 and Cdk9 (lanes 2 and 4). Twenty-four hours after transfection, luciferase activity was measured. (c) HeLa cells were transfected with a reporter construct containing a luciferase gene under the control of the c-Jun promoter with an intact (pJC6-GL3, lanes 1 and 2) or a mutated MEF2 binding site (pJSX-GL3, lanes 3 and 4) in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of MEF2D and human CycT1 expression plasmids. Twenty-four hours after transfection, luciferase activity was measured. (d) P-TEFb activates the transcription of an endogenous MEF2 target gene (GLUT4). MEF2D (lanes 2 to 4), CycT1 (lanes 3 and 4), and Cdk9 (lane 4) were overexpressed in HeLa cells. Twenty-four hours after transfection, the mRNA for GLUT4 and β-actin was amplified from the total cellular RNA by RT-PCR.
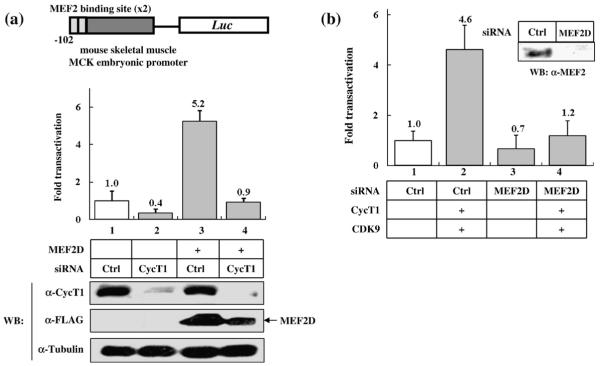
Next, in order to test whether CycT1 is essential for MEF2-dependent transcription, we knocked down the expression of endogenous CycT1 by siRNA and examined its effect on MEF2-dependent transcription. MCK-Luc expression was diminished by the addition of siRNA against CycT1 but not control siRNA, in the presence of MEF2D (Fig. 2a, lanes 3 and 4). The reduction of CycT1 expression by specific siRNA was verified by Western blotting (Fig. 2a, lower panel) and HIV-Luc reporter gene assays in which Luc gene expression is dependent on the interaction between CycT1 and viral Tat protein (data not shown).44–46 The siRNA against CycT1 also slightly decreased basal MCK-Luc expression regulated by endogenous MEF2 activity (from 1.0-fold to 0.4-fold, Fig. 2a, lanes 1 and 2), which is consistent with the observation that overexpression of CycT1 slightly (~2-fold) increased MCK-Luc expression in the absence of ectopically expressed MEF2 proteins (Fig. 1a, lane 12). A slight reduction of ectopically expressed MEF2 protein by siRNA against CycT1 was observed (Fig. 2a, lower panel, lanes 3 and 4), which might partially contribute to the complete abolishment of MCK-Luc expression (Fig. 2a). The siRNA against MEF2D reduced basal MCK-Luc expression to a level similar to that obtained with siRNA against CycT1 in HeLa cells (Fig. 2b, lane 3). Importantly, overexpression of CycT1/Cdk9 failed to activate MEF2-Luc when endogenous MEF2 expression was reduced by siRNA (Fig. 2b, lane 4), which further supports the hypothesis that P-TEFb activates MCK-Luc expression in a MEF2-dependent manner. Therefore, we conclude that P-TEFb is required for MEF2-dependent transcription.
Fig. 2.
Knockdown of endogenous CycT1 or MEF2 by siRNA abolishes MEF2-dependent transcription. (a) Upper panel: HeLa cells were transfected with MCK-Luc with (lanes 3 and 4) or without (lanes 1 and 2) MEF2D in the presence of 20 nM of a control oligoribonucleotide (lanes 1 and 3) or siRNA against CycT1 (lanes 2 and 4). Twenty-four hours after transfection, luciferase activity was measured. Luciferase activity is shown as fold transactivation relative to the value obtained without MEF2D (lane 1). Lower panel: The effect of siRNA was verified by Western blot analysis. Ectopically expressed MEF2D proteins were detected with anti-Flag antibody. (b) HeLa cells were transfected with MCK-Luc and a control oligoribonucleotide (lanes 1 and 2) or siRNA against MEF2D (lanes 3 and 4) in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of Cdk9/CycT1. Twenty-four hours after transfection, luciferase activities were measured. Luciferase activity is shown as fold transactivation relative to the value obtained without MEF2C (lane 1). Data are representative of three independent assays. The effect of siRNA against MEF2 was confirmed by Western blot analysis.
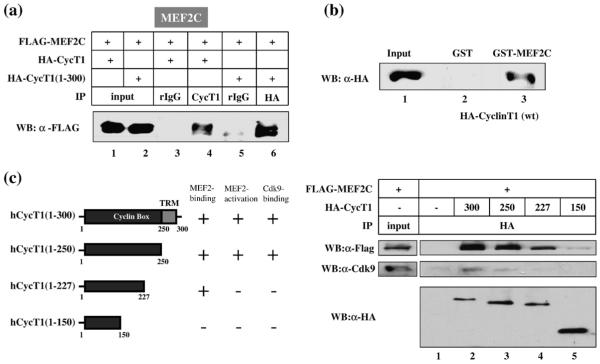
The 250 N-terminal amino acids of CycT1 form cyclin box domains that are critical for interaction with its kinase partner, Cdk9.4,47 In addition, the 300 N-terminal amino acids of CycT1 are sufficient to support HIV-1 transcription.45,46,48,49 Also, this region interacts with several cellular transcription activators.29–37,50,51 Therefore, we next examined whether this region is sufficient to stimulate MEF2-dependent transcription. MCK-Luc reporter assays were performed in HeLa cells by overexpressing increasing amounts of full-length (hCycT1) or C-terminally truncated [hCycT1(1–300)] CycT1 proteins in the presence or absence of MEF2C. As shown in Fig. 3, both hCycT1 (Fig. 3a) and hCycT1 (1–300) (Fig. 3b) stimulated MCK promoter-driven luciferase expression in the presence of MEF2C. Similar results were obtained in the presence of MEF2D (data not shown). In contrast, a more C-terminally truncated CycT1(1–227) failed to stimulate MCK-Luc gene expression in the presence of MEF2C (data not shown) or MEF2D (Fig. 3c, lanes 5 to 8). These results indicate that the 300 N-terminal amino acids of CycT1 are sufficient for stimulating MEF2-dependent transcription.
Fig. 3.
The N-terminal cyclin box region of CycT1 is sufficient for mediating MEF2-dependent transcription. Schematic representations of the full-length (hCycT1) and truncated [hCycT1(1–300) and hCycT1(1–227)] forms of CycT1 used in this study are shown on the top of each panel. Amino acid motifs of cyclin boxes (amino acids 1–250), Tat-TAR recognition motif (TRM, amino acids 250–272), 7SK RNA-interaction domain (7SK ID, amino acids 255–333), coiled-coil region (amino acids 379 to 530), and PEST (amino acids 706–726) sequence are depicted. (a and b) HeLa cells were transfected with MCK-Luc reporter gene in the absence (lanes 1 to 4) or presence of MEF2C (0.1 μg; lanes 5 to 8) with increasing amounts of hCycT1 [(a): 0, 0.1, 0.2, and 0.3 μg] or hCycT1(1–300) [(b): 0, 0.05, 0.1, and 0.15 μg]. Twenty-four hours after transfection, luciferase activity was measured. Luciferase activity is shown as fold transactivation relative to the value obtained without MEF2s, CycT1, and Cdk9 (lanes 1). (c) HeLa cells were transfected with MCK-Luc reporter gene in the presence of MEF2D (0.1 μg) with increasing amounts of hCycT1(1–300) (0, 0.05, 0.1, and 0.15 μg: lanes 1 to 4) or hCycT1(1–227) (0, 0.05, 0.1, and 0.15 μg: lanes 5 to 8). Twenty-four hours after transfection, luciferase activity was measured.
P-TEFb interacts with MEF2 in vitro and in vivo
A possible mechanism by which P-TEFb stimulates MCK-Luc expression is through its association with MEF2. To test this hypothesis, we next examined whether P-TEFb interacts with MEF2 by coimmunoprecipitation analyses. Both full-length and C-terminally truncated CycT1 interacted with MEF2C (Fig. 4a, lanes 4 and 6), MEF2D (Fig. S1a, lanes 4 and 6), and MEF2A (Fig. S1b, lane 3 and data not shown). The interaction between CycT1 and MEF2C was further confirmed by glutathione S-transferase (GST) pull-down assays (Fig. 4b, lanes 2 and 3). The region in CycT1 that interacts with MEF2C was determined by coimmunoprecipitatin using various truncation mutants of CycT1 (Fig. 4c). The truncation mutant hCycT1(1–227) proteins that failed to activate MCK-Luc reporter gene activation (Fig. 3c) interacted with MEF2C, whereas a further C-terminally truncated hCycT1(1–150) lost the ability to bind MEF2C (Fig. 4c, right top panel, lanes 4 and 5). On the other hand, neither hCycT1(1–227) nor hCycT1(1–150) interacted with the endogenous Cdk9 (Fig. 4c, right middle panel, lanes 4 and 5), which is consistent with previous observation.47 Since Cdk9 is required for activation of P-TEFb-mediated transcription, these results provide an explanation for the inability of hCycT1(1–227) to activate MCK-Luc. The results of coimmunoprecipitation between the CycT1 mutants and MEF2C or the endogenous Cdk9, and reporter assays with these mutants, are summarized in Fig. 4c. Based on these data, we conclude that CycT1 interacts with MEF2 through its N-terminal cyclin box region (between amino acids 150 and 227).
Fig. 4.
CycT1 interacts with MEF2 in vitro and in vivo. (a) HA-tagged hCycT1 (lanes 1, 3, and 4) and hCycT1(1–300) (lanes 2, 5, and 6) proteins were coexpressed with Flag-MEF2C and immunoprecipitated from the cell lysates using anti-CycT1 (lane 4) or anti-HA antibody (lane 6). Normal rIgG was used as a negative control (lanes 3 and 5). MEF2 proteins associated with HA-CycT1 were detected by anti-Flag antibody. (b) Lysates from 293T cells overexpressing HA-hCycT1 were incubated with GST or GST-MEF2C in the presence of glutathione–Sepharose beads. After washing extensively, HA-CycT1 proteins associated with GST-MEF2C were detected by Western blot analysis using anti-HA antibody. (c) Mapping of the MEF2-interaction domain in CycT1. Left panel: A schematic representation of the CycT1 truncation mutants used in this study. The cyclin box region and the TRM are indicated by dark and light gray boxes, respectively. The results of coimmunoprecipitation between CycT1 and MEF2 or Cdk9 and MCK-Luc reporter gene assays using the mutant CycT1 proteins were also indicated. Right panel: Coimmunoprecipitation assays were carried out as described above. Flag-tagged MEF2 (top panel) and endogenous Cdk9 (middle panel) coimmunoprecipitated with HA-tagged truncated CycT1 proteins were detected by anti-Flag and anti-Cdk9 antibody, respectively. The expression of the CycT1 mutants was also confirmed by Western blot analysis with anti-HA antibody (bottom panel).
P-TEFb stimulates MEF2-dependent transcription in skeletal muscle cells
Next, in order to study the functional relationship between P-TEFb and MEF2 in a more physiologically relevant system, we examined whether P-TEFb is required for MEF2-dependent transcription in myocyte-derived cells. Mouse skeletal myocyte C2C12 cells were used as models. First, similarly to what we observed in HeLa cells, overexpression of CycT1 stimulated MCK-Luc gene expression up to 2.4-fold in C2C12 cells (Fig. 5a). Conversely, overexpression of a dominant-negative form of Cdk9 (D167N)52 decreased MCK-Luc reporter gene expression (Fig. 5b). The expression of endogenous MEF2 proteins was unaffected by overexpression of CycT1 or Cdk9 (D167N) (Fig. 5a and b). In addition, MEF2-dependent transcription was inhibited by flavopiridol, a potent inhibitor for Cdk9 kinase activity with an IC50 of approximately 10 nM, which is within a similar concentration range as had been determined for inhibiting HIV transcription53 (Fig. 5c). Previously, He et al. demonstrate that P-TEFb activity can be stimulated by N′,N′-hexamethylene bis-acetamide (HMBA), through a mechanism in which P-TEFb is released from its negative regulator, HEXIM1.16 In C2C12 cells, HMBA enhanced MCK-Luc reporter activity by 2.9-fold (Fig. 5d). Furthermore, coimmunoprecipitation assays indicated that dissociation of P-TEFb from HEXIM1 occurred within 30 min after addition of HBMA (Fig. 5d, lower panel). Similar results were also obtained with rat cardiac-myocyte-derived h9c2 cells (data not shown).
Fig. 5.
CycT1 is required for MEF2-dependent transcription in cultured myocytes. (a) Overexpression of CycT1 stimulates MCK-reporter gene expression in C2C12 cells. Increasing amounts (0, 0.3, and 0.5 μg) of HA-CycT1 proteins were overexpressed in C2C12 cells in the presence of MCK-Luc reporter plasmid. Forty-eight hours after transfection, luciferase activities were measured. The results are shown as fold transactivation relative to the value obtained with the cells expressing no CycT1. The expression of the endogenous MEF2 proteins and overexpressed CycT1 proteins was confirmed by Western blot analysis of the cell lysates using anti-MEF2 and anti-HA antibodies, respectively. (b) Increasing amounts (0, 0.3, and 0.5 μg) of HA dominant-negative (dn) Cdk9 (D167N) proteins were overexpressed in C2C12 cells in the presence of MCK-Luc reporter construct. The results were shown as a percentage relative to the value obtained with cells expressing no HA-dnCdk9. The expression of endogenous MEF2 proteins and overexpressed HA-dnCdk9 proteins was confirmed by Western blot analysis of the cell lysate using anti-MEF2 and anti-HA antibodies, respectively. (c) C2C12 cells were transfected with the MCK-Luc reporter gene. Twenty-four hours after transfection, the cells were incubated with the indicated concentration of flavopiridol for 12 h before luciferase assays. The presented data are representative of three independent experiments. (d) Upper panel: C2C12 cells were transfected with the MCK-Luc reporter gene. Twenty-four hours after transfection, the cells were incubated with 5 mM HMBA for 12 h prior to luciferase assays. Lower panel: Dissociation of P-TEFb from HEXIM1 occurred 30 min after HMBA, assessed by coimmunoprecipitation assays. C2C12 cells were incubated with HMBA for 0 and 30 min prior to NE preparation. HEXIM1 proteins coimmunoprecipitated with CycT1 were detected by anti-HEXIM1 antibody.
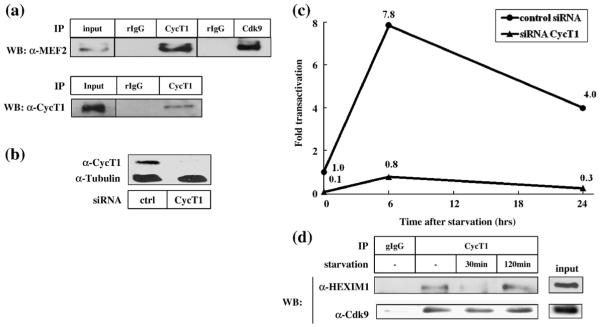
It has been demonstrated that MEF2-dependent transcription can be upregulated when C2C12 cells are induced to differentiate by serum starvation.54,55 Therefore, we measured MEF2-dependent transcription in C2C12 cells before and after serum starvation in the presence or absence of siRNA against CycT1. The interaction between endogenous MEF2 and P-TEFb was first confirmed by coimmunoprecipitation (Fig. 6a). It is to be noted that Cdk9 was also detected in the MEF2 immunocomplex, which indicates that the active P-TEFb complex interacts with MEF2 (Fig. 6a, top panel). Knockdown of the endogenous CycT1 was confirmed by Western blot analysis (Fig. 6b). Neither control siRNA nor siRNA against CycT1 altered the expression levels of the endogenous MEF2, as assessed by Western blot analysis (data not shown). C2C12 cells transfected with MCK-Luc reporter plasmid and siRNAs were cultured with a lower concentration of serum (0.5%), and luciferase activity was measured at 0, 6, and 24 h after serum starvation. As shown in Fig. 6c, luciferase activity was increased after serum starvation, reaching its peak at 6 h after starvation (7.8-fold) and then declining gradually (Fig. 6c, filled circle). The starvation-induced MEF2 activity was abolished in the presence of siRNA against CycT1 (Fig. 6c, filled triangle) but not control siRNA (filled circle). Interestingly, serum starvation induced a rapid dissociation of P-TEFb from HEXIM1 within 30 min (Fig. 6d, upper panel). The interaction between P-TEFb and HEXIM1 was restored 2 h after serum starvation (Fig. 6d, upper panel). The amount of Cdk9 associated with CycT1 remained unchanged during serum starvation (Fig. 6d, lower panel). These results further confirm that P-TEFb mediates MEF2-dependent transcription in myocyte-derived C2C12 cells.
Fig. 6.
P-TEFb mediates MEF2-dependent transcription induced by serum starvation of C2C12 cells. (a) Interaction between the endogenous MEF2 and CycT1 was detected by coimmunoprecipitation. Upper panel: NEs from C2C12 cells were incubated with rabbit IgG (rIgG), rabbit anti-CycT1, or mouse anti-Cdk9 and protein–A Sepharose beads. MEF2 proteins retained in the bead fractions were detected by Western blot analysis. Lower panel: C2C12 cell lysates were incubated with rIgG or rabbit anti-MEF2 and protein A–Sepharose beads. CycT1 proteins retained in the bead fractions were detected by Western blot analysis. (b) The expression of CycT1 in the presence or absence of the specific siRNA was detected by Western blot analysis, using α-tubulin as a loading control. (c) Differentiation of C2C12 cells induces MCK-Luc expression in a P-TEFb-dependent manner. C2C12 cells were transfected with MCK-Luc reporter plasmid with 20 nM of a control oligoribonucleotide (filled circles) or siRNA against CycT1 (filled triangles). Forty-eight hours after transfection, cells were washed and cultured with low (0.5%) serum-containing media to induce differentiation. At various time points (0, 6, and 24 h) after the induction of differentiation, cells were harvested and luciferase activities were measured. Luciferase activity is shown as fold transactivation relative to the activity obtained with undifferentiated cells. (d) The interaction between P-TEFb and HEXIM1 after serum starvation was examined by coimmunoprecipitation assays. C2C12 cells were serum-starved for 0, 30, and 120 min prior to NE preparation. HEXIM1 and Cdk9 proteins coimmunoprecipitated with CycT1 were detected by Western blot analyses.
P-TEFb and MEF2 co-occupy the promoter region of MEF2 target genes in skeletal muscle cells
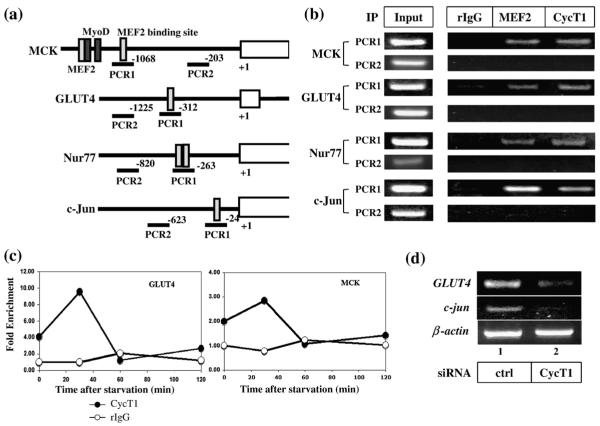
Finally, we performed chromatin immunoprecipitation (ChIP) analyses to examine whether P-TEFb associates with the promoters of MEF2 target genes. Four known endogenous MEF2 target genes (MCK, GLUT4, Nur77, and c-Jun) were tested. The promoter regions of these genes contain one or two MEF2 binding sites (Fig. 7a).28,42,43,56,57 By using a combination of ChIP assays and DNA microarray analysis (ChIP on chip) for three major muscle-specific transcription factors (MEF2, myogenin, and MyoD), Blais et al. have demonstrated that the expression of c-Jun and Nur77 is regulated by MEF2 but not by myogenin or MyoD.54 Thus, we tested for co-occupancy of CycT1 and MEF2 to the MEF2 binding sites of MCK, GLUT4, Nur77, and c-Jun genes by ChIP analyses in C2C12 cells. Since the MCK gene contains two MEF2 binding sites, each site was amplified separately. As shown in Fig. 7b, the MEF2 binding regions in the promoter of GLUT4, MCK (the down-stream MEF2 binding site), c-Jun, and Nur77 were detected in the immunocomplex with anti-MEF2 and anti-CycT1 but not with normal immunoglobulin G (IgG). As a control, a region in the promoter of each MEF2 target gene that is distant from the MEF2 binding site was also amplified (PCR2, Fig. 7a). Neither MEF2 nor CycT1 was detected in this region, confirming that the recruitment of CycT1 is specific to the MEF2 binding site. Quantification of the DNA by real-time PCR analyses indicated 7.4- and 3.3-fold enrichment of MEF2 and CycT1, respectively, on the MEF2 binding site in the c-Jun promoter (Fig. S2a). For the upstream MEF2 binding site in the MCK gene, stronger signals were obtained with anti-MEF2 than with anti-CycT1 (Fig. S2). This is consistent with the previous observation by Giacinti et al. that the MyoD/CycT2 complex is preferentially recruited to this region.28 Similar results were obtained with the myogenin gene that also contains MEF2 and MyoD sites next to each other (Fig. S2b). We further examined the time-dependent enrichment of CycT1 on the MEF2 binding sites in GLUT4 and MCK promoters after serum starvation. As shown in Fig. 7c, the highest amount of CycT1 was detected at 30 min after starvation (10-fold in GLUT4 promoter and 3-fold in the MCK promoter), which coincides with the dissociation of P-TEFb from HEXIM1 (Fig. 6d). Similar results were obtained with c-Jun and Nur77 promoters (data not shown). To confirm that the endogenous MEF2 target genes are regulated by P-TEFb in C2C12 cells, we measured the mRNA levels of two MEF2 target genes, GLUT4 and c-Jun,54,55,58 by RT-PCR when endogenous CycT1 was knocked down. The siRNA against CycT1 reduced the mRNA levels of GLUT4 and c-Jun but not β-actin (Fig. 7d). These results indicate that P-TEFb activates MEF2 target gene expression through an association with MEF2 binding sites in these genes.
Fig. 7.
CycT1 and MEF2 are recruited to MEF2 target gene promoters. (a) Schematic presentation of the promoter regions of MEF2 target genes (MCK, GLUT4, Nur77, and c-Jun) tested for ChIP assays. The MEF2 binding sites (light gray box) were amplified by PCR using primers indicated by solid lines below the gene. Two MyoD binding sites flanking the upstream MEF2 site in the MCK gene are indicated by dark gray boxes. Two primer sets (PCR1 and PCR2) were used to amplify the promoter region of each MEF2 target gene. The primer set PCR1 amplifies the region encompassing MEF2 binding site, whereas PCR2 amplifies the region distal to the MEF2 binding site. (b) ChIP analyses were performed using C2C12 cells as described in Materials and Methods. The endogenous MEF2 proteins and P-TEFb were immunoprecixpitated with anti-MEF2 and anti-CycT1 polyclonal antibody, respectively. (c) CycT1 is recruited to the MEF2 binding site immediately after serum starvation. C2C12 cells were serum-starved and harvested for ChIP analysis at the indicated time points. The DNA fragments containing MEF2 binding sites in GLUT4 (left panel) and MCK (right panel) coimmunoprecipitated with rIgG (open circles) or anti-CycT1 (filled circles) were quantified by real-time PCR analysis using primers indicated in (a). (d) CycT1 is required for the transcription of endogenous MEF2 target genes. C2C12 cells were transfected with 20 nM of a control siRNA (lane 1) or siRNA against CycT1 (lane 2). Twenty-four hours after transfection, the mRNA for GLUT4, c-Jun, and β-actin was amplified from the total cellular RNA by RT-PCR.
Discussion
P-TEFb stimulates the elongation step of transcription by hyperphosphorylating the carboxyterminal domain of RNAPII. Thus, one of the key questions is how P-TEFb is recruited to its target genes. At least two distinct mechanisms for the recruitment of P-TEFb are possible: (1) gene-specific recruitment through an interaction between P-TEFb and transactivators and (2) less specific recruitment via the interaction between P-TEFb and Brd4. To date, it is unclear whether these two apparently distinct pathways occur cooperatively or separately. Although we could not completely rule out the latter pathway in MEF2-dependent transcription in this study, we favor the model in which P-TEFb is recruited to these genes through interaction with MEF2 according to the following observations: (i) MEF2 protein interacts with P-TEFb in vitro and in vivo (Figs. 4 and 6) in a similar manner as it does with other transactivators, (ii) overexpression of CycT1 failed to activate the c-Jun promoter that contains a mutated MEF2 binding site (Fig. 1c), (iii) hCycT1(1–227) that interacts with MEF2C but not with the endogenous Cdk9 is incapable of activating MCK-Luc expression (Figs. 3 and 4), and (iv) P-TEFb can be recruited to the regions harboring MEF2 binding elements in MEF2 target genes (Fig. 7). Indeed, CycT1 was not detected at a promoter region distal to the MEF2 binding site in the MEF2 target gene promoters (Fig. 7, PCR 2), which further supports our hypothesis that recruitment of P-TEFb by MEF2 would stimulate transcriptional elongation of the MEF2 target genes via hyperphosphorylation of RNAPII mediated by the Cdk9 subunit of P-TEFb.
In murine myocytes, it has been demonstrated that P-TEFb mediates MyoD-dependent transcription through an interaction with CycT2 subunits. In the MCK promoter, there are two MEF2 binding sites (Fig. 7a). The upstream MEF2 binding site is flanked by two MyoD binding sites whereas the downstream MEF2 binding site is not accompanied with MyoD binding sites. Our ChIP data indicate that more CycT1 proteins were detected at the downstream site (Fig. 7a and Fig. S2b). This is consistent with the observation by Giacinti et al. in which CycT2 was preferentially recruited to the upstream MEF2 binding region, through the interaction with MyoD.28 Perhaps, when MyoD is induced, MyoD/CycT2 complexes are sufficient to recruit the P-TEFb complex to this region. Thus, it is of interest to quantify the occupancy of this site by MEF2/CycT1 and MyoD/CycT2 upon various cellular stimuli, which would provide important information about the regulation of transcriptional elongation by the two myocyte-specific transcription factors. In the present study, we have not excluded the possibility that CycT2 also interacts with MEF2; however, since siRNA against CycT1 almost completely abolished MEF2-dependent transcription, the contribution of CycT2 would be minor. To this end, we speculate that, in muscle cells, P-TEFb with different cyclins (Cdk9: CycT1, Cdk9:CycT2, and/or Cdk9/CycK) regulates transcription elongation through interactions with different transcription factors. Recently, Blais et al. elegantly identified the target genes activated by three major transcription factors (MyoD, MEF2, and myogenin) solely or in combination, during myocyte differentiation in C2C12 cells.54 A similar pattern of the distinct promoter selections might be obtained with different P-TEFb complexes.
Our ChIP data also indicate that at least two subpopulations of MEF2 (MEF2 with and without P-TEFb) bind to the promoter region. This is consistent with the results that overexpression of P-TEFb stimulates MEF2-dependent transcription (Figs. 1, 2 and 5). It is possible that promoters occupied with MEF2 without P-TEFb are in an inactive state in terms of transcriptional elongation, with the transcription prematurely paused, as is the case for HIV-1 transcription21,22,59 unless P-TEFb is brought by another factor such as MyoD. The recruitment of P-TEFb through interaction with MEF2 may therefore be the rate-limiting step of activation of transcription from these MEF2 target genes.
P-TEFb has been demonstrated to be actively involved in cardiac hypertrophy;23,27 however, the mechanism by which P-TEFb dissociates from HEXIM1/7SK upon hypertrophic signals remains largely unidentified. In Drosophila, the Lis laboratory has demonstrated that upon heat shock stimulation, P-TEFb is rapidly recruited to heat shock loci, suggesting an active mechanism of P-TEFb recruitment by cellular signaling.60,61 In C2C12 cells, serum starvation stimulates MEF2-dependent transcription in a P-TEFb-dependent manner. P-TEFb dissociates from HEXIM1 within 30 min after serum starvation and reassociates with HEXIM1 2 h after starvation, which would explain the spike of MEF2-dependent transcription observed at 6 h after starvation (Fig. 6) and the recruitment of P-TEFb to MEF2 sites at 30 min after starvation (Fig. 7). Also, a similar process of P-TEFb/HEXIM1 dissociation and MEF2 upregulation is observed when cells are treated with HMBA, a potent inducer of cell differentiation (Fig. 5), which is consistent with an observation by He et al. using murine erythroleukemia cells.16 P-TEFb/HEXIM1 interaction is perhaps strictly controlled by the status of cell differentiation, and different sets of genes are activated in its early and late stages though P-TEFb activation. It is therefore of interest whether other MEF2-inducing stimuli such as endothelin 1 or insulinlike growth factor stimulation promote the dissociation of HEXIM1/7SK from P-TEFb and the subsequent recruitment of P-TEFb to MEF2 target genes.62,63
Gene expression during muscle development is precisely programmed. Hence, aberrant transcription leads to a serious failure in muscle functions. In this study, we present evidence that MEF2, one of the major transcription factors in muscle cells, utilizes P-TEFb for optimal transcriptional activity. It is of interest whether other muscle-specific transactivators also require P-TEFb. Further studies are required to elucidate the precise mechanism by which P-TEFb is recruited to each of its target genes. Understanding of the cross talk between myocyte-specific transcription factors and P-TEFb will provide vital information for the development of therapeutic strategies for manipulating muscle growth and differentiation.
Materials and Methods
Materials
HeLa cells were maintained in Dulbecco's modified Eagle's medium including 10% fetal bovine serum at 37 °C with 5% CO2. C2C12 cells were maintained with a higher concentration (20%) of serum. The reporter plasmid pGL2-MEF2-Luc (MCK-Luc); MEF2 expression plasmid pCMX-1F-MEF2A, -C, and -D; and GST-MEF2 expression vector, pGEX-2TK-MEF2C, have been described previously.64 The c-Jun reporter plasmids containing intact and mutated MEF2 binding sites (pJC6-GL3 and pJSX-GL3, respectively) were a generous gift from Dr. Ron Prywes.42 The plasmids encoding the full-length (amino acids 1–726) and C-terminally truncated (amino acids 1–300, 1–250, 1–227, and 1–150) CycT1, pEF-HA-CycT1, pEF-HA-CycT1(1–300), pEF-HA-CycT1(1–250), pEF-HA-CycT1(1–227), and pEF-HA-CycT1(1–150), respectively, were constructed by inserting the corresponding PCR fragments into the pEF-HA vector using BamHI and EcoRI sites. The pCMV-Luc was constructed by inserting a PCR fragment encoding a luciferase gene amplified from pGL-basic (Promega, Madison, WI, USA) luciferase reporter plasmid into pCDNA3.1 (Invitrogen, Carlsbad, CA, USA). The antibodies against HA (Y-11 and F-7), CycT1 (H-245), and MEF2 (C-21, and B-4) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The siRNA against CycT1 and MEF2D was also purchased from Santa Cruz Biotechnology. The mouse monoclonal anti-Flag-M2 mouse and anti-α-tubulin antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA), and EMD Biosciences (San Diego, CA, USA), respectively. Rabbit anti-HEXIM1 antibodies were obtained from Dr. Monica Montano.36
Luciferase assays
Cells (1 × 105) were transfected with luciferase reporter gene construct (0.1 μg) in the presence or absence of various amounts (as indicated in the figure legends) of expression plasmids for MEF2, CycT1 and Cdk9, and/or siRNA using Lipofectamine 2000 (Invitrogen). The total amount of DNA was adjusted to be constant between samples using empty vectors. Forty-eight hours after transfection, luciferace activities in the cell lysates were measured using a Lumimark Plus microplate reader (Bio-Rad, Hercules, CA, USA) by standard procedures described elsewhere.7
Serum starvation
C2C12 cells were transfected with MCK-Luc reporter genes with or without CycT1/Cdk9 expression plasmids and/or siRNAs using Lipofectamine 2000. Twenty-four to 48 hours after transfection, cells were washed once with phosphate-buffered saline and cultured in Dulbecco's modified Eagle's medium containing a reduced concentration (0.5%) of serum to induce cellular differentiation for up to 72 h. Cells were harvested at indicated time points after serum starvation for luciferase assays, coimmunoprecipitation, ChIP, RT-PCR, and Western blot analysis.
Coimmunoprecipitation
The HA-epitope-tagged full-length and C-terminally truncated CycT1 and/or the Flag-tagged MEF2 proteins were expressed in 293T cells using calcium phosphate transfection. Twenty-four hours after transfection, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer [50 mM Tris–HCl, 0.15 M NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% sodium deoxycholate, 1% NP-40, 1% SDS, and 1 mM DTT (pH 7.4)] in the presence of protease inhibitors (Sigma-Aldrich). Cell lysates were incubated with 5 μg of rabbit polyclonal anti-CycT1 antibody, 1 μg of rabbit polyclonal anti-HA antibody (Y-11), or 5 μg of normal rabbit IgG (rIgG) overnight at 4 °C. Reaction mixtures were then incubated with protein A– Sepharose beads (GE Healthcare, Piscataway, NJ, USA) at 4 °C for 2 h. The beads were washed three times with RIPA buffer and subjected to SDS-PAGE, followed by Western blot analysis with anti-Flag monoclonal antibody. Coimmunoprecipitation of endogenous proteins (Figs. 5 and 6) was carried out using the nuclear extract (NE) of C2C12 cells and anti-CycT1, anti-CDk9, and anti-MEF2 antibodies (Santa Cruz Biotechnology). NE was prepared from C2C12 cells (1 × 108) by a standard protocol.16
GST pull-down assays
Cell lysates of 293T cells overexpressing the full-length HA-CycT1 were incubated with GST-MEF2 fusion proteins or GST proteins purified from Escherichia coli (BL21DE3; Promega), coupled with glutathione–Sepharose beads (GE Healthcare), in RIPA buffer at 4 °C for 60 min. The beads were washed three times with RIPA buffer to remove unbound proteins. HA-CycT1 proteins associated with the bead fractions were detected by Western blot analyses with anti-HA antibody.
RT-PCR
Cells (5 × 105) were grown in a 6-well plate and transfected with siRNA against CycT1 or randomized oligoribonucleotide and cultured in media containing a low serum concentration (0.5%) for 72 h. Total RNA was extracted by using PureLink Total RNA Purification System (Invitrogen) and quantified by measuring optical density at 260/280 nm. The cDNA was synthesized by SuperScript II reverse transcriptase with random priming (Invitrogen). The cDNA of GLUT4, c-Jun, and β-actin was amplified by PCR (5 min at 94 °C followed by 25 to 30 cycles of 30 s at 94 °C, 30 s at 58 °C, and 1 min at 72 °C, followed by a 10-min extension at 72 °C). The sequence of the primers is available upon request.
ChIP assays
C2C12 cells (5 × 107) were cultured in media containing a low serum concentration (0.5%) for the indicated lengths of time. Cells were then incubated with formaldehyde (1%) for 10 min to cross-link proteins and DNA and lysed in CE buffer (10 mM Hepes-KOH, pH 7.9, 60 mM KCl, 1 mM EDTA, 0.5% NP-40, 1 mM DTT, and 1 mM PMSF). Nuclei were collected by low-speed centrifugation (2000 rpm for 10 min) and resuspended in SDS lysis buffer (50 mM Tris–HCl, pH 8.0, 10 mM EDTA, and 1% SDS) containing protease inhibitors. Chromosomal DNA was fragmented (0.2–1 kbp) by sonication. The endogenous MEF2 proteins and P-TEFb were then immunoprecipitated with 10 μg of anti-MEF2, anti-CycT1, or rIgG as a negative control and protein A–Sepharose. After the beads were extensively washed , the protein/DNA complexes were eluted by incubating with elution buffer (1% SDS and 0.1 M NaHCO3). After reverse cross-linking by incubating at 65 °C overnight, DNA was extracted by phenol and precipitated by ethanol. The MEF2 binding sites in the promoter regions of four MEF2 target genes, MCK, GLUT4, Nur77, and c-Jun, as indicated in Fig. 7a, were amplified by PCR. Real-time PCR analyses were performed to quantify the DNA fragments including the promoter region in c-Jun associated with rIgG, anti-MEF2, and anti-CycT1, using the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad). The sequence of the primers is shown in Supplementary Table 1.
Acknowledgements
We thank Lindsey McGowen, Adam Heath, Satsumi Roos, Laura Paszkowsky, Michael Zane, and Renee Vanek for technical assistance; Drs. Monica Montano and Ron Prywes for reagents; Erin Reineke and Drs. Jonathan Karn, Matija Peterlin, Erik Andrulis, and Ran Taube and the members of the Fujinaga lab for helpful discussions; and Antonia Fraser Fujinaga for proofreading the manuscripts. This work was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases [R01 DK62985 (H.-Y.K.)], the American Cancer Society [IRG-91-022-10 (K.F.)], and the University Hospital of Cleveland.
Abbreviations
- P-TEFb
positive transcription elongation factor b
- MEF2
myocyte enhancer factor 2
- N-TEF
negative transcription elongation factor
- RNAPII
RNA polymerase II
- Cdk9
cyclin-dependent kinase 9
- CycT1
cyclin T1
- CycT2
cyclin T2
- CycK
cyclin K
- Brd4
bromodomain protein 4
- HIV-1
human immunodeficiency virus type 1
- siRNA
small interfering RNA
- MCK
muscle creatine kinase
- GLUT4
glucose transporter protein 4
- RT
reverse transcription
- GST
glutathione S-transferase
- HMBA
N′,N′-hexamethylene bis-acetamide
- ChIP
chromatin immunoprecipitation
- IgG
immunoglobulin G
- RIPA
radioimmunoprecipitation assay
- EDTA
ethylenediaminetetraacetic acid
- rIgG
rabbit IgG
- NE
nuclear extract
Footnotes
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jmb.2008.07.017
References
- 1.Conaway JW, Shilatifard A, Dvir A, Conaway RC. Control of elongation by RNA polymerase II. Trends Biochem. Sci. 2000;25:375–380. doi: 10.1016/s0968-0004(00)01615-7. [DOI] [PubMed] [Google Scholar]
- 2.Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Mol. Cell. 2006;23:297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Price DH. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell. Biol. 2000;20:2629–2634. doi: 10.1128/mcb.20.8.2629-2634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei P, Garber ME, Fang SM, Fischer WH, Jones KA. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 5.Ivanov D, Kwak YT, Guo J, Gaynor RB. Domains in the SPT5 protein that modulate its transcriptional regulatory properties. Mol. Cell. Biol. 2000;20:2970–2983. doi: 10.1128/mcb.20.9.2970-2983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourgeois CF, Kim YK, Churcher MJ, West MJ, Karn J. Spt5 cooperates with human immunodeficiency virus type 1 Tat by preventing premature RNA release at terminator sequences. Mol. Cell. Biol. 2002;22:1079–1093. doi: 10.1128/MCB.22.4.1079-1093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujinaga K, Irwin D, Huang Y, Taube R, Kurosu T, Peterlin BM. Dynamics of human immunodeficiency virus transcription: P-TEFb phosphorylates RD and dissociates negative effectors from the transactivation response element. Mol. Cell. Biol. 2004;24:787–795. doi: 10.1128/MCB.24.2.787-795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada T, Takagi T, Yamaguchi Y, Watanabe D, Handa H. Evidence that P-TEFb alleviates the negative effect of DSIF on RNA polymerase II-dependent transcription in vitro. EMBO J. 1998;17:7395–7403. doi: 10.1093/emboj/17.24.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryser S, Fujita T, Tortola S, Piuz I, Schlegel W. The rate of c-fos transcription in vivo is continuously regulated at the level of elongation by dynamic stimulus-coupled recruitment of P-TEFb. J. Biol. Chem. 2007;282:5075–5084. doi: 10.1074/jbc.M607847200. [DOI] [PubMed] [Google Scholar]
- 10.Napolitano G, Majello B, Lania L. Role of cyclinT/Cdk9 complex in basal and regulated transcription (review) Int. J. Oncol. 2002;21:171–177. [PubMed] [Google Scholar]
- 11.Ni Z, Schwartz BE, Werner J, Suarez JR, Lis JT. Coordination of transcription, RNA processing, and surveillance by P-TEFb kinase on heat shock genes. Mol. Cell. 2004;13:55–65. doi: 10.1016/s1097-2765(03)00526-4. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen VT, Kiss T, Michels AA, Bensaude O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature. 2001;414:322–325. doi: 10.1038/35104581. [DOI] [PubMed] [Google Scholar]
- 13.Yang Z, Zhu Q, Luo K, Zhou Q. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature. 2001;414:317–322. doi: 10.1038/35104575. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, Zhou Q. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell. 2005;19:535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell. 2005;19:523–534. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 16.He N, Pezda AC, Zhou Q. Modulation of a P-TEFb functional equilibrium for the global control of cell growth and differentiation. Mol. Cell. Biol. 2006;26:7068–7076. doi: 10.1128/MCB.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garriga J, Grana X. Cellular control of gene expression by T-type cyclin/CDK9 complexes. Gene. 2004;337:15–23. doi: 10.1016/j.gene.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Lin X, Taube R, Fujinaga K, Peterlin BM. P-TEFb containing cyclin K and Cdk9 can activate transcription via RNA. J. Biol. Chem. 2002;277:16873–16878. doi: 10.1074/jbc.M200117200. [DOI] [PubMed] [Google Scholar]
- 19.Taube R, Lin X, Irwin D, Fujinaga K, Peterlin BM. Interaction between P-TEFb and the C-terminal domain of RNA polymerase II activates transcriptional elongation from sites upstream or downstream of target genes. Mol. Cell. Biol. 2002;22:321–331. doi: 10.1128/MCB.22.1.321-331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napolitano G, Majello B, Licciardo P, Giordano A, Lania L. Transcriptional activity of positive transcription elongation factor b kinase in vivo requires the C-terminal domain of RNA polymerase II. Gene. 2000;254:139–145. doi: 10.1016/s0378-1119(00)00278-x. [DOI] [PubMed] [Google Scholar]
- 21.Taube R, Fujinaga K, Wimmer J, Barboric M, Peterlin BM. Tat transactivation: a model for the regulation of eukaryotic transcriptional elongation. Virology. 1999;264:245–253. doi: 10.1006/viro.1999.9944. [DOI] [PubMed] [Google Scholar]
- 22.Karn J. Tackling Tat. J. Mol. Biol. 1999;293:235–254. doi: 10.1006/jmbi.1999.3060. [DOI] [PubMed] [Google Scholar]
- 23.Sano M, Schneider MD. Cyclins that don't cycle—cyclin T/cyclin-dependent kinase-9 determines cardiac muscle cell size. Cell Cycle. 2003;2:99–104. [PubMed] [Google Scholar]
- 24.De Falco G, Giordano A. CDK9: from basal transcription to cancer and AIDS. Cancer Biol. Ther. 2002;1:342–347. [PubMed] [Google Scholar]
- 25.De Falco M, De Luca A. Involvement of cdks and cyclins in muscle differentiation. Eur. J. Histochem. 2006;50:19–23. [PubMed] [Google Scholar]
- 26.Sano M, Abdellatif M, Oh H, Xie M, Bagella L, Giordano A, et al. Activation and function of cyclin T-Cdk9 (positive transcription elongation factor-b) in cardiac muscle-cell hypertrophy. Nat. Med. 2002;8:1310–1317. doi: 10.1038/nm778. [DOI] [PubMed] [Google Scholar]
- 27.Espinoza-Derout J, Wagner M, Shahmiri K, Mascareno E, Chaqour B, Siddiqui MA. Pivotal role of cardiac lineage protein-1 (CLP-1) in transcriptional elongation factor P-TEFb complex formation in cardiac hypertrophy. Cardiovasc. Res. 2007;75:129–138. doi: 10.1016/j.cardiores.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giacinti C, Bagella L, Puri PL, Giordano A, Simone C. MyoD recruits the cdk9/cyclin T2 complex on myogenic-genes regulatory regions. J. Cell. Physiol. 2006;206:807–813. doi: 10.1002/jcp.20523. [DOI] [PubMed] [Google Scholar]
- 29.Simone C, Stiegler P, Bagella L, Pucci B, Bellan C, De Falco G, et al. Activation of MyoD-dependent transcription by cdk9/cyclin T2. Oncogene. 2002;21:4137–4148. doi: 10.1038/sj.onc.1205493. [DOI] [PubMed] [Google Scholar]
- 30.Barboric M, Nissen RM, Kanazawa S, Jabrane-Ferrat N, Peterlin BM. NF-kappaB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol. Cell. 2001;8:327–337. doi: 10.1016/s1097-2765(01)00314-8. [DOI] [PubMed] [Google Scholar]
- 31.Eberhardy SR, Farnham PJ. c-Myc mediates activation of the cad promoter via a post-RNA polymerase II recruitment mechanism. J. Biol. Chem. 2001;276:48562–48571. doi: 10.1074/jbc.M109014200. [DOI] [PubMed] [Google Scholar]
- 32.Kanazawa S, Okamoto T, Peterlin BM. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity. 2000;12:61–70. doi: 10.1016/s1074-7613(00)80159-4. [DOI] [PubMed] [Google Scholar]
- 33.Kanazawa S, Soucek L, Evan G, Okamoto T, Peterlin BM. c-Myc recruits P-TEFb for transcription, cellular proliferation and apoptosis. Oncogene. 2003;22:5707–5711. doi: 10.1038/sj.onc.1206800. [DOI] [PubMed] [Google Scholar]
- 34.Lee DK, Duan HO, Chang C. Androgen receptor interacts with the positive elongation factor P-TEFb and enhances the efficiency of transcriptional elongation. J. Biol. Chem. 2001;276:9978–9984. doi: 10.1074/jbc.M002285200. [DOI] [PubMed] [Google Scholar]
- 35.Simone C, Bagella L, Bellan C, Giordano A. Physical interaction between pRb and cdk9/cyclinT2 complex. Oncogene. 2002;21:4158–4165. doi: 10.1038/sj.onc.1205511. [DOI] [PubMed] [Google Scholar]
- 36.Wittmann BM, Fujinaga K, Deng H, Ogba N, Montano MM. The breast cell growth inhibitor, estrogen down regulated gene 1, modulates a novel functional interaction between estrogen receptor alpha and transcriptional elongation factor cyclin T1. Oncogene. 2005;24:5576–5588. doi: 10.1038/sj.onc.1208728. [DOI] [PubMed] [Google Scholar]
- 37.Iankova I, Petersen RK, Annicotte JS, Chavey C, Hansen JB, Kratchmarova I, et al. Peroxisome proliferator-activated receptor gamma recruits the positive transcription elongation factor b complex to activate transcription and promote adipogenesis. Mol. Endocrinol. 2006;20:1494–1505. doi: 10.1210/me.2005-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolodziejczyk SM, Wang L, Balazsi K, DeRepentigny Y, Kothary R, Megeney LA. MEF2 is upregulated during cardiac hypertrophy and is required for normal postnatal growth of the myocardium. Curr. Biol. 1999;9:1203–1206. doi: 10.1016/S0960-9822(00)80027-5. [DOI] [PubMed] [Google Scholar]
- 39.Czubryt MP, Olson EN. Balancing contractility and energy production: the role of myocyte enhancer factor 2 (MEF2) in cardiac hypertrophy. Recent Prog. Horm. Res. 2004;59:105–124. doi: 10.1210/rp.59.1.105. [DOI] [PubMed] [Google Scholar]
- 40.Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TA, et al. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J. Clin. Invest. 2000;105:1395–1406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Gong NL, Bodi I, Aronow BJ, Backx PH, Molkentin JD. Myocyte enhancer factors 2A and 2C induce dilated cardiomyopathy in transgenic mice. J. Biol. Chem. 2006;281:9152–9162. doi: 10.1074/jbc.M510217200. [DOI] [PubMed] [Google Scholar]
- 42.Han TH, Prywes R. Regulatory role of MEF2D in serum induction of the c-jun promoter. Mol.Cell. Biol. 1995;15:2907–2915. doi: 10.1128/mcb.15.6.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thai MV, Guruswamy S, Cao KT, Pessin JE, Olson AL. Myocyte enhancer factor 2 (MEF2)-binding site is required for GLUT4 gene expression in transgenic mice. Regulation of MEF2 DNA binding activity in insulin-deficient diabetes. J. Biol. Chem. 1998;273:14285–14292. doi: 10.1074/jbc.273.23.14285. [DOI] [PubMed] [Google Scholar]
- 44.Garber ME, Wei P, KewalRamani VN, Mayall TP, Herrmann CH, Rice AP, et al. The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev. 1998;12:3512–3527. doi: 10.1101/gad.12.22.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujinaga K, Taube R, Wimmer J, Cujec TP, Peterlin BM. Interactions between human cyclin T, Tat, and the transactivation response element (TAR) are disrupted by a cysteine to tyrosine substitution found in mouse cyclin T. Proc. Natl. Acad. Sci. USA. 1999;96:1285–1290. doi: 10.1073/pnas.96.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bieniasz PD, Grdina TA, Bogerd HP, Cullen BR. Recruitment of a protein complex containing Tat and cyclin T1 to TAR governs the species specificity of HIV-1 Tat. EMBO J. 1998;17:7056–7065. doi: 10.1093/emboj/17.23.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujinaga K, Irwin D, Geyer M, Peterlin BM. Optimized chimeras between kinase-inactive mutant Cdk9 and truncated cyclin T1 proteins efficiently inhibit Tat transactivation and human immunodeficiency virus gene expression. J. Virol. 2002;76:10873–10881. doi: 10.1128/JVI.76.21.10873-10881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwak YT, Ivanov D, Guo J, Nee E, Gaynor RB. Role of the human and murine cyclin T proteins in regulating HIV-1 tat-activation. J. Mol. Biol. 1999;288:57–69. doi: 10.1006/jmbi.1999.2664. [DOI] [PubMed] [Google Scholar]
- 49.Garber ME, Wei P, Jones KA. HIV-1 Tat interacts with cyclin T1 to direct the P-TEFb CTD kinase complex to TAR RNA. Cold Spring Harbor Symp. Quant. Biol. 1998;63:371–380. doi: 10.1101/sqb.1998.63.371. [DOI] [PubMed] [Google Scholar]
- 50.Hoque M, Young TM, Lee CG, Serrero G, Mathews MB, Pe'ery T. The growth factor granulin interacts with cyclin T1 and modulates P-TEFb-dependent transcription. Mol. Cell. Biol. 2003;23:1688–1702. doi: 10.1128/MCB.23.5.1688-1702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young TM, Wang Q, Pe'ery T, Mathews MB. The human I-mfa domain-containing protein, HIC, interacts with cyclin T1 and modulates P-TEFb-dependent transcription. Mol. Cell. Biol. 2003;23:6373–6384. doi: 10.1128/MCB.23.18.6373-6384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores O, Lee G, Kessler J, Miller M, Schlief W, Tomassini J, Hazuda D. Host-cell positive transcription elongation factor b kinase activity is essential and limiting for HIV type 1 replication. Proc. Natl. Acad. Sci. USA. 1999;96:7208–7213. doi: 10.1073/pnas.96.13.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chao SH, Fujinaga K, Marion JE, Taube R, Sausville EA, Senderowicz AM, et al. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J. Biol. Chem. 2000;275:28345–28348. doi: 10.1074/jbc.C000446200. [DOI] [PubMed] [Google Scholar]
- 54.Blais A, Tsikitis M, Acosta-Alvear D, Sharan R, Kluger Y, Dynlacht BD. An initial blueprint for myogenic differentiation. Genes Dev. 2005;19:553–569. doi: 10.1101/gad.1281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paris J, Virtanen C, Lu Z, Takahashi M. Identification of MEF2-regulated genes during muscle differentiation. Physiol. Genomics. 2004;20:143–151. doi: 10.1152/physiolgenomics.00149.2004. [DOI] [PubMed] [Google Scholar]
- 56.Cserjesi P, Lilly B, Hinkley C, Perry M, Olson EN. Homeodomain protein MHox and MADS protein myocyte enhancer-binding factor-2 converge on a common element in the muscle creatine kinase enhancer. J. Biol. Chem. 1994;269:16740–16745. [PubMed] [Google Scholar]
- 57.Youn HD, Liu JO. Cabin1 represses MEF2-dependent Nur77 expression and T cell apoptosis by controlling association of histone deacetylases and acetylases with MEF2. Immunity. 2000;13:85–94. doi: 10.1016/s1074-7613(00)00010-8. [DOI] [PubMed] [Google Scholar]
- 58.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 59.Garber ME, Jones KA. HIV-1 Tat: coping with negative elongation factors. Curr. Opin. Immunol. 1999;11:460–465. doi: 10.1016/S0952-7915(99)80077-6. [DOI] [PubMed] [Google Scholar]
- 60.Lis JT, Mason P, Peng J, Price DH, Werner J. P-TEFb kinase recruitment and function at heat shock loci. Genes Dev. 2000;14:792–803. [PMC free article] [PubMed] [Google Scholar]
- 61.Andrulis ED, Guzman E, Doring P, Werner J, Lis JT. High-resolution localization of Drosophila Spt5 and Spt6 at heat shock genes in vivo: roles in promoter proximal pausing and transcription elongation. Genes Dev. 2000;14:2635–2649. doi: 10.1101/gad.844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akazawa H, Komuro I. Roles of cardiac transcription factors in cardiac hypertrophy. Circ. Res. 2003;92:1079–1088. doi: 10.1161/01.RES.0000072977.86706.23. [DOI] [PubMed] [Google Scholar]
- 63.McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division differentiation and death. Trends Biochem. Sci. 2002;27:40–47. doi: 10.1016/s0968-0004(01)02031-x. [DOI] [PubMed] [Google Scholar]
- 64.Kao HY, Verdel A, Tsai CC, Simon C, Juguilon H, Khochbin S. Mechanism for nucleocytoplasmic shuttling of histone deacetylase 7. J. Biol. Chem. 2001;276:47496–47507. doi: 10.1074/jbc.M107631200. [DOI] [PubMed] [Google Scholar]