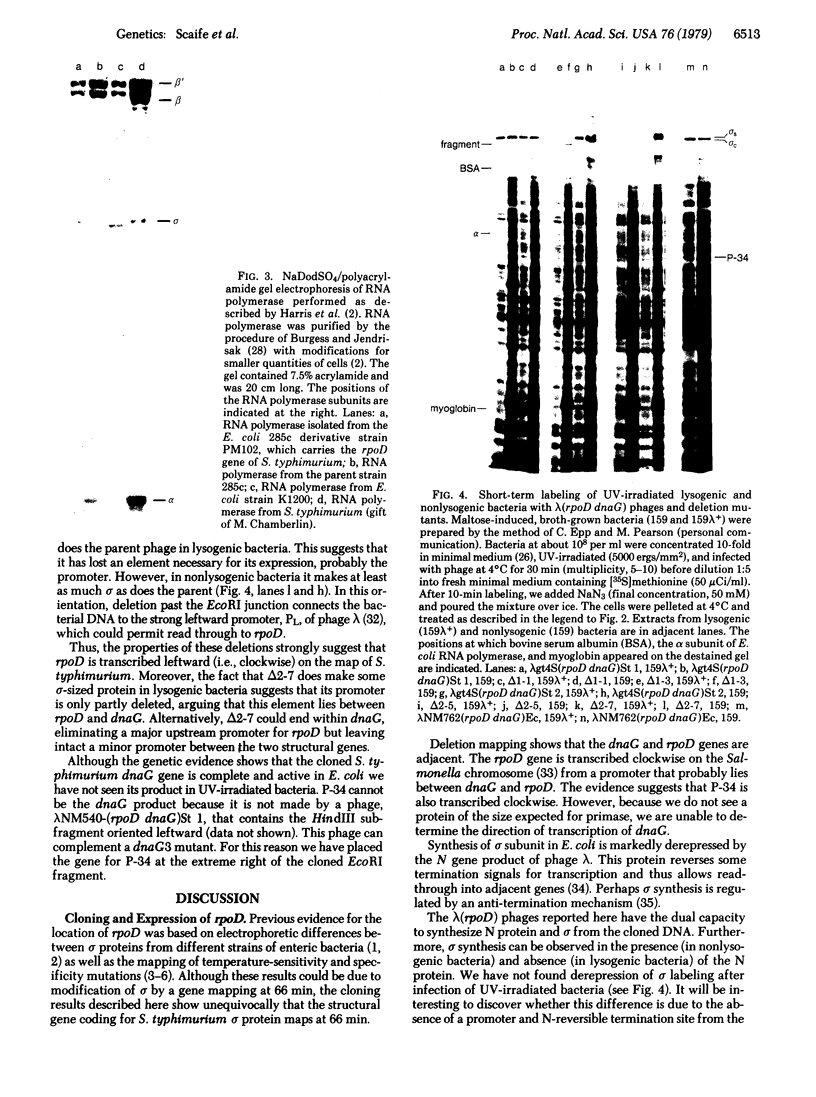
Abstract
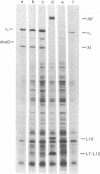
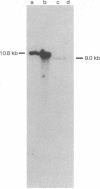
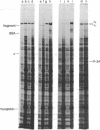
The genes for the RNA polymerase sigma subunit (rpoD) and DNA primase (dnaG) of Salmonella typhimurium have been cloned into lambda vectors. Combined restriction, deletion and functional analysis of the cloned fragment allows us to map the genes precisely on the fragment, establishes the direction in which rpoD is transcribed, and reveals the existence of at least one new gene in the vicinity. A closely homologous, smaller fragment of Escherichia coli DNA, also cloned into lambda, contains rpoD and at least part of dnaG.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Bowden D. W., Twersky R. S., Calendar R. Escherichia coli deoxyribonucleic acid synthesis mutants: their effect upon bacteriophage P2 and satellite bacteriophage P4 deoxyribonucleic acid synthesis. J Bacteriol. 1975 Oct;124(1):167–175. doi: 10.1128/jb.124.1.167-175.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., Falkow S. Genetics of the Enterobacteriaceae. C. Molecular relationships among members of the Enterobacteriaceae. Adv Genet. 1971;16:81–118. doi: 10.1016/s0065-2660(08)60355-7. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Campbell A. The steric effect in lysogenization by bacteriophage lambda. I. Lysogenization of a partially diploid strain of Escherichia coli K-12. Virology. 1965 Nov;27(3):329–339. doi: 10.1016/0042-6822(65)90112-1. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Parkinson J. S. Deletion mutants of bacteriophage lambda. 3. Physical structure of att-phi. J Mol Biol. 1971 Mar 14;56(2):403–423. doi: 10.1016/0022-2836(71)90473-6. [DOI] [PubMed] [Google Scholar]
- Dürwald H., Hoffmann-Berling H. Endonuclease-I-deficient and ribonuclease I-deficient Escherichia coli mutants. J Mol Biol. 1968 Jul 14;34(2):331–346. doi: 10.1016/0022-2836(68)90257-x. [DOI] [PubMed] [Google Scholar]
- Echols H., Green L. Establishment and maintenance of repression by bacteriophage lambda: the role of the cI, cII, and c3 proteins. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2190–2194. doi: 10.1073/pnas.68.9.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L. W., Weisberg R. A. The red plaque test: a rapid method for identification of excision defective variants of bacteriophage lambda. Virology. 1976 Jul 1;72(1):147–153. doi: 10.1016/0042-6822(76)90319-6. [DOI] [PubMed] [Google Scholar]
- Gross C., Hoffman J., Ward C., Hager D., Burdick G., Berger H., Burgess R. Mutation affecting thermostability of sigma subunit of Escherichia coli RNA polymerase lies near the dnaG locus at about 66 min on the E. coli genetic map. Proc Natl Acad Sci U S A. 1978 Jan;75(1):427–431. doi: 10.1073/pnas.75.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. D., Calendar R. Transcription map of satellite coliphage P4. Virology. 1978 Apr;85(2):343–358. doi: 10.1016/0042-6822(78)90443-9. [DOI] [PubMed] [Google Scholar]
- Harris J. D., Heilig J. S., Martinez I. I., Calendar R., Isaksson L. A. Temperature-sensitive Escherichia coli mutant producing a temperature-sensitive sigma subunit of DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6177–6181. doi: 10.1073/pnas.75.12.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. D., Martinez I. I., Calendar R. A gene from Escherichia coli affecting the sigma subunit of RNA polymerase. Proc Natl Acad Sci U S A. 1977 May;74(5):1836–1840. doi: 10.1073/pnas.74.5.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S., Uchida H. Deletion mutations and the replication of DNA in bacteriophage lambda. Virology. 1977 Dec;83(2):277–286. doi: 10.1016/0042-6822(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Linn T., Scaife J. Identification of a single promoter in E. coli for rplJ, rplL and rpoBC. Nature. 1978 Nov 2;276(5683):33–37. doi: 10.1038/276033a0. [DOI] [PubMed] [Google Scholar]
- Messer W., Bergmans H. E., Meijer M., Womack J. E., Hansen F. G., von Meyenburg K. Mini-chromosomes: plasmids which carry the E. coli replication origin. Mol Gen Genet. 1978 Jul 4;162(3):269–275. doi: 10.1007/BF00268852. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Osawa T., Yura T. Chromosomal location of a structural gene for the RNA polymerase sigma factor in Escherichia coli. Proc Natl Acad Sci U S A. 1977 May;74(5):1831–1835. doi: 10.1073/pnas.74.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y. RNA polymerase mutant with altered sigma factor in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):1–6. doi: 10.1007/BF00270369. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Induction of sigma factor synthesis in Escherichia coli by the N gene product of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4405–4409. doi: 10.1073/pnas.73.12.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko S. M., Cameron J. R., Davis R. W., Lehman I. R. Five hundredfold overproduction of DNA ligase after induction of a hybrid lambda lysogen constructed in vitro. Science. 1977 Apr 8;196(4286):188–189. doi: 10.1126/science.322281. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Huskey R. J. Deletion mutants of bacteriophage lambda. I. Isolation and initial characterization. J Mol Biol. 1971 Mar 14;56(2):369–384. doi: 10.1016/0022-2836(71)90471-2. [DOI] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Buckland R., Goman M., Le Grice S. S., Scaife J. G. A mutation affecting the sigma subunit of RNA polymerase changes transcriptional specificity. Nature. 1978 Jun 1;273(5661):354–358. doi: 10.1038/273354a0. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wiggs J. L., Bush J. W., Chamberlin M. J. Utilization of promoter and terminator sites on bacteriophage T7 DNA by RNA polymerases from a variety of bacterial orders. Cell. 1979 Jan;16(1):97–109. doi: 10.1016/0092-8674(79)90191-0. [DOI] [PubMed] [Google Scholar]