Abstract
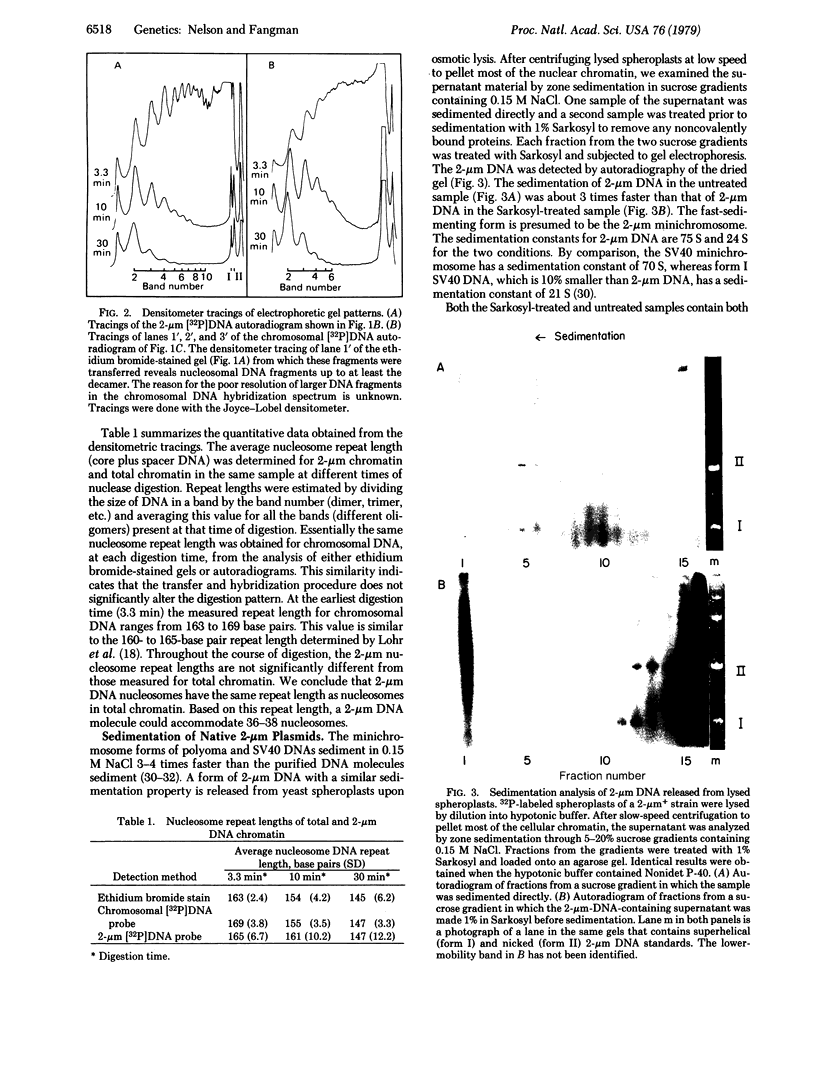
The eukaryotic microorganism Saccharomyces cerevisiae contains 50-100 copies per cell of a circular plasmid called 2-micrometer DNA. The intracellular structure of these molecules, which represent about 4% of the total DNA, was examined by digestion of total cellular chromatin with micrococcal nuclease (nucleate 3'-oligonucleotidohydrolase, EC 3.1.31.1). Nuclease-resistant DNA fragments were fractionated by gel electrophoresis and 2-micrometer DNA sequences were detected by hybridization. The 2-micrometer and chromosomal DNA digestion patterns were very similar indicating that both types of DNA are condensed into nucleosomes. An analysis of these digestion patterns showed that the kinetics of digestion of 2-micrometer chromatin and total chromatin are similar and that both have the same nucleosome repeat length of about 165 base pairs. Native 2-micrometer plasmids were examined by zone sedimentation in sucrose gradients containing 0.15 M NaCl and were found to have a sedimentation constant of 75 S, about 3 times the sedimentation constant of protein-free 2-micrometer DNA. This sedimentation property is what would be expected for a 2-micrometer DNA minichromosome. We conclude that within the cell 2-micrometer DNA molecules are organized in a chromatin structure very similar to that of the yeast chromosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs J. D., Guerineau M., Atkins J. F. A map of the restriction targets in yeast 2 micron plasmid DNA cloned on bacteriophage lambda. Mol Gen Genet. 1976 Nov 17;148(3):287–294. doi: 10.1007/BF00332903. [DOI] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. Mouse L cell mitochondrial DNA molecules are selected randomly for replication throughout the cell cycle. Cell. 1977 Aug;11(4):719–727. doi: 10.1016/0092-8674(77)90286-0. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Atkins J. F., McGill C., Chow L. Identification and mapping of the transcriptional and translational products of the yeast plasmid, 2mu circle. Cell. 1979 Apr;16(4):827–839. doi: 10.1016/0092-8674(79)90098-9. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Philippsen P., Davis R. W. Analysis of chromosomal integration and deletions of yeast plasmids. Nucleic Acids Res. 1977;4(5):1429–1448. doi: 10.1093/nar/4.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D. Isolation of circular DNA from a mitochondrial fraction from yeast. Proc Natl Acad Sci U S A. 1972 Feb;69(2):388–392. doi: 10.1073/pnas.69.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., Miklos G. L. Localization and quantification of circular DNA in yeast. Eur J Biochem. 1974 Jan 16;41(2):359–365. doi: 10.1111/j.1432-1033.1974.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Structure of chromatin. Nat New Biol. 1971 Jan 27;229(4):101–106. doi: 10.1038/newbio229101a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Feldman H. A., Shelton E. R., Wassarman P. M., DePamphilis M. L. Structure of simian virus 40 chromosomes. Appendix. J Mol Biol. 1978 Nov 15;125(4):511–514. doi: 10.1016/0022-2836(78)90313-3. [DOI] [PubMed] [Google Scholar]
- Forte M. A., Fangman W. L. Naturally occurring cross-links in yeast chromosomal DNA. Cell. 1976 Jul;8(3):425–431. doi: 10.1016/0092-8674(76)90155-0. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Griffith J. D., Christiansen G. The multifunctional role of histone H1, probed with the SV40 minichromosome. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):215–226. doi: 10.1101/sqb.1978.042.01.024. [DOI] [PubMed] [Google Scholar]
- Gubbins E. J., Newlon C. S., Kann M. D., Donelson J. E. Sequence organization and expression of a yeast plasmid DNA. Gene. 1977 May;1(3-4):185–207. doi: 10.1016/0378-1119(77)90045-2. [DOI] [PubMed] [Google Scholar]
- Guerineau M., Grandchamp C., Paoletti C., Slonimski P. Characterization of a new class of circular DNA molecules in yeast. Biochem Biophys Res Commun. 1971 Feb 5;42(3):550–557. doi: 10.1016/0006-291x(71)90406-2. [DOI] [PubMed] [Google Scholar]
- Guerineau M., Grandchamp C., Slonimski P. P. Circular DNA of a yeast episome with two inverted repeats: structural analysis by a restriction enzyme and electron microscopy. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3030–3034. doi: 10.1073/pnas.73.9.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. R., Meinke W., Goldstein D. A. Nucleoprotein complexes containing replicating Simian virus 40 DNA: comparison with polyoma nucleoprotein complexes. J Virol. 1973 Oct;12(4):901–908. doi: 10.1128/jvi.12.4.901-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Three additional genes required for deoxyribonucleic acid synthesis in Saccharomyces cerevisiae. J Bacteriol. 1973 Sep;115(3):966–974. doi: 10.1128/jb.115.3.966-974.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L. M., Hartwell L. H. Sequential gene function in the initiation of Saccharomyces cerevisiae DNA synthesis. J Mol Biol. 1974 Apr 15;84(3):445–461. doi: 10.1016/0022-2836(74)90451-3. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. P., Degelmann A., Kustermann-Kuhn B., Royer H. D. Characterization of 2-mum DNA of Saccharomyces cerevisiae by restriction fragment analysis and integration in an Escherichia coli plasmid. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2072–2076. doi: 10.1073/pnas.73.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W., Müller U., Eicken I., Wendel I., Zentgraf H. Biochemical and ultrastructural analysis of SV40 chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):227–244. doi: 10.1101/sqb.1978.042.01.025. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Inheritance of the 2 micrometer m DNA plasmid from Saccharomyces. Genetics. 1977 May;86(1):73–84. doi: 10.1093/genetics/86.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M., Klein H. L. Deoxyribonucleic acid sequence organization of a yeast plasmid. J Bacteriol. 1977 Jan;129(1):472–481. doi: 10.1128/jb.129.1.472-481.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M., Kupfer D. M. Control of Saccharomyces cerevisiae 2microN DNA replication by cell division cycle genes that control nuclear DNA replication. J Mol Biol. 1977 Oct 25;116(2):249–260. doi: 10.1016/0022-2836(77)90215-7. [DOI] [PubMed] [Google Scholar]
- Lohr D., Kovacic R. T., Van Holde K. E. Quantitative analysis of the digestion of yeast chromatin by staphylococcal nuclease. Biochemistry. 1977 Feb 8;16(3):463–471. doi: 10.1021/bi00622a020. [DOI] [PubMed] [Google Scholar]
- Lohr D., Van Holde K. E. Yeast chromatin subunit structure. Science. 1975 Apr 11;188(4184):165–166. doi: 10.1126/science.1090006. [DOI] [PubMed] [Google Scholar]
- Mackey J. K., Brackmann K. H., Green M. R., Green M. Preparation and characterization of highly radioactive in vitro labeled adenovirus DNA and DNA restriction fragments. Biochemistry. 1977 Oct 4;16(20):4478–4483. doi: 10.1021/bi00639a023. [DOI] [PubMed] [Google Scholar]
- Mathis D. J., Gorovsky M. A. Subunit structure of rDNA-containing chromatin. Biochemistry. 1976 Feb 24;15(4):750–755. doi: 10.1021/bi00649a005. [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Beltz W. R., Rill R. L. Chromatin subunits from baker's yeast: isolation and partial characterization. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1343–1347. doi: 10.1073/pnas.74.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlon C. S., Fangman W. L. Mitochondrial DNA synthesis in cell cycle mutants of Saccharomyces cerevisiae. Cell. 1975 Aug;5(4):423–428. doi: 10.1016/0092-8674(75)90061-6. [DOI] [PubMed] [Google Scholar]
- Piñon R., Salts Y. Isolation of folded chromosomes from the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2850–2854. doi: 10.1073/pnas.74.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. Nucleosome structure of Xenopus oocyte amplified ribosomal genes. Biochemistry. 1978 Nov 14;17(23):4908–4916. doi: 10.1021/bi00616a008. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. The nucleotide sequence of Saccharomyces cerevisiae 5.8 S ribosomal ribonucleic acid. J Biol Chem. 1973 Jun 10;248(11):3860–3875. [PubMed] [Google Scholar]
- Scheer U., Zentgraf H. Nucleosomal and supranucleosomal organization of transcriptionally inactive rDNA circles in Dytiscus oocytes. Chromosoma. 1978 Nov 22;69(2):243–254. doi: 10.1007/BF00329922. [DOI] [PubMed] [Google Scholar]
- Seebeck T., Stalder J., Braun R. Isolation of a minichromosome containing the ribosomal genes from Physarum polycephalum. Biochemistry. 1979 Feb 6;18(3):484–490. doi: 10.1021/bi00570a017. [DOI] [PubMed] [Google Scholar]
- Shaw J. E., Levinger L. F., Carter C. W., Jr Nucleosomal structure of Epstein-Barr virus DNA in transformed cell lines. J Virol. 1979 Feb;29(2):657–665. doi: 10.1128/jvi.29.2.657-665.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabak H. F. Absence of 2 micrometer DNA sequences in Saccharomyces cerevisiae Y 379-5D. FEBS Lett. 1977 Dec 1;84(1):67–70. doi: 10.1016/0014-5793(77)81058-2. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Moustacchi E. The synthesis of mitochondrial DNA during the cell cycle in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1971 Jan 22;42(2):195–201. doi: 10.1016/0006-291x(71)90087-8. [DOI] [PubMed] [Google Scholar]
- Zakian V. A., Brewer B. J., Fangman W. L. Replication of each copy of the yeast 2 micron DNA plasmid occurs during the S phase. Cell. 1979 Aug;17(4):923–934. doi: 10.1016/0092-8674(79)90332-5. [DOI] [PubMed] [Google Scholar]




