Abstract
Psychopathology is increasingly viewed from a circuit perspective in which a disorder stems not from circumscribed anomalies in discrete brain regions, but rather from impairments in distributed neural networks. This focus on neural circuitry has rendered resting state functional connectivity MRI (rs-fcMRI) an increasingly important role in the elucidation of pathophysiology including attention-deficit/hyperactivity disorder (ADHD). Unlike many other MRI techniques that focus on the properties of discrete brain regions, rs-fcMRI measures the coherence of neural activity across anatomically disparate brain regions, examining the connectivity and organization of neural circuits. In this review, we explore the methods available to investigators using rs-fcMRI techniques, including a discussion of their relative merits and limitations. We then review findings from extant rs-fcMRI studies of ADHD focusing on neural circuits implicated in the disorder, especially the default mode network, cognitive control network, and cortico-striato-thalmo-cortical loops. We conclude by suggesting future directions that may help advance subsequent rs-fcMRI research in ADHD.
Keywords: Attention-Deficit/Hyperactivity Disorder, Functional Connectivity, Default Mode Network, Cognitive Control Network, Striatum
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD), a common neuropsychiatric disorder characterized by developmentally inappropriate levels of inattention, impulsivity, and hyperactivity, affects 3 to 10% of school-aged children with approximately 30 to 50% of cases persisting into adulthood (Polanczyk et al. 2007; Kessler et al. 2006; Barkley et al. 2010). Associated with deficits across social, academic, occupational, and neuropsychiatric domains, including poor academic performance, underemployment, family dysfunction, substance abuse and incarceration, ADHD is a considerable burden to affected children, their families and society at large (Barkley 2002; Biederman et al. 2008). Whereas the diagnostic symptoms of ADHD are restricted to inattention, hyperactivity, and impulsivity, a wider range of symptoms are commonly associated with ADHD including poor frustration tolerance (Barkley 1997), emotional lability (Posner et al. 2013b), and defiant behaviors (Biederman et al. 1991). For some children with ADHD, these associated symptoms are as impairing as the diagnostic ADHD symptoms (Barkley and Fischer 2010). Thus studies of ADHD have considered, and should continue to investigate, the neurobiological underpinning not only of the diagnostic symptoms of ADHD, but also of the associated symptoms (Posner et al. 2013b).
In an effort to stimulate novel preventive and treatment strategies for the disorder, a large body of research over the past two decades has used neuroimaging to examine the neurobiological substrates that underlie ADHD. Here we review an emerging neuroimaging technique, resting-state functional connectivity MRI (rs-fcMRI), focusing on the neural circuits implicated in the disorder. We examine the methods available to investigators using rs-fcMRI, including their relative merits and potential limitations. We then review findings from extant rs-fcMRI studies of ADHD. Lastly, we conclude by suggesting future directions that may help advance subsequent rs-fcMRI research in ADHD.
Neuroimaging Modalities
Neuroimaging studies of ADHD have traditionally focused on brain regions thought to subserve cognitive, motor, and attentional functions (Bush et al. 2005). The large majority of neuroimaging studies of ADHD have been conducted with structural MRI (which focuses on the size and shape of various brain regions), task-based functional MRI (fMRI) (which correlates patterns of fMRI signal with participants' performance on a cognitive task), and Positron Emission Tomography (PET) (which examines metabolic activity, cerebral perfusion, and receptor binding potentials within examined brain regions or receptor systems). These neuroimaging modalities focus primarily on the properties of discrete brain regions and have left the interactions between brain regions largely unexplored in ADHD. This, however, has changed dramatically over the past decade with the development of the connectivity measures provided by rs-fcMRI, which analyzes temporal correlations in neural activity between brain regions. Though prior reviews (Castellanos et al. 2009; Konrad and Eickhoff 2010) have discussed the extant rs-fcMRI literature in ADHD, rapid advances in this field render an updated review desirable.
Methodological Considerations
First described in 1995 by Biswal et al (1995), rs-fcMRI focuses on spontaneous fluctuations in neural activity, as indexed by fMRI signal, present during the resting condition—that is, in the absence of overt task performance or stimulation (Fox and Greicius 2010). Brain regions that demonstrate strong coherence of neural activity (i.e., fMRI signal that is highly correlated over time) are considered “functionally connected.” (Fox and Raichle 2007; Posner et al. 2013a) When the fMRI signal across multiple brain regions is correlated, this is termed a “resting state network.” These networks (e.g. the cognitive control network) consist of brain regions that are known to co-activate during task-based fMRI studies. For example, using task-based fMRI, task-related activations within regions associated with cognitive control such as the dorsolateral prefrontal cortex, supplementary motor area and the anterior insular cortex can be detected as participants engage in a task with cognitive control demands, such as the Go/No Go (Casey et al. 1997) or Stroop (Whalen et al. 2006) Tasks. Using rs-fcMRI, functional connections are reliably detected across these same regions, and thus the cognitive control network can be termed a “resting state network.” (Posner et al. 2013b; Sheline et al. 2010) The function of resting state activity remains an area of active investigation but may reflect an endogenous mechanism of the brain to self-organize (Fox et al. 2005). Spontaneous neural activity strengthens synaptic connections across neural networks and thereby may maintain the coherence, or architecture, of these neural networks (Fair et al. 2007; Fair et al. 2009).
Resting-state functional connectivity MRI typically relies upon two approaches: seed-based and independent component analysis. In seed-based correlations, the fMRI signal from a single or cluster of voxels is extracted from a specific neural region of interest and a map of the brain is created by calculating the correlations between the designated seed region and all other voxels of the brain (Biswal et al. 1995; Fox and Raichle 2007). A second method, independent component analysis (ICA), is a data-driven approach that considers all voxels simultaneously and separates a dataset into spatially distinct maps of four-dimensional fMRI signal (i.e., three spatial dimensions and a fourth dimension indexing time) (Calhoun et al. 2003; Wang and Peterson 2008). Conceptually and computationally more intuitive than ICA, seed-based analyses have been used in most rs-fcMRI studies of ADHD. However, as noted elsewhere (Power et al. 2011; Fox and Greicius 2010), seed-based approaches are susceptible to investigator biases. For example, investigators must decide upon the specific seed region for a given analysis, as well as the anatomical definitions to characterize the seed regions. Each of these decisions can, in turn, influence the rs-fcMRI findings. If an investigator chooses, for example, to examine connectivity based on the dorsolateral prefrontal cortex (DLPFC) as the seed region, any potential findings will de facto be limited to regions with connectivity to the DLPFC (i.e., connections between non-DLPFC regions will not be examined). This limits the scope of inquiry, and unanticipated findings are unlikely to emerge. Moreover, the DLPFC is a large, functionally heterogeneous region, and thus connectivity may not be independent of the subregions, or anatomical definitions, chosen to delineate the DLPFC. One option to curtail the potentially confounding influence of anatomically defining a seed region is to use meta-analytically defined anatomical regions. That is, an investigator can use a priori stereotactic coordinates for a given brain region that have been described in meta-analyses of the functional neuroimaging literature. Although this circumvents problems arising from investigator derived anatomical definitions, it, nonetheless, introduces a related limitation in that it assumes uniform anatomical definitions across all study participants. In the example of the DLFPC, the meta-analytic approach would utilize the same stereotactic coordinates for the DLPFC for all study participants, obscuring probable inter-subject anatomical variability. An alternative is to define, on a subject-by-subject basis, the stereotactic coordinates of a seed region based on the activation of that region during an fMRI task. For example, rather than anatomically defining a seed region based on a priori stereotactic coordinates, the DLPFC, which is associated with cognitive and inhibitory control, could be anatomically defined based on findings from an inhibitory control fMRI task, such as the Simon Spatial Interference Task, that reliably engages the DLPFC in healthy individuals (Peterson et al. 2002). In this example, task-related activations would then define the anatomy of the DLPFC for subsequent seed-based connectivity analyses (Posner et al. in press). This, of course, necessitates running parallel experiments to ascertain both task-based and resting fMRI data, which may limit study feasibility.
Independent component analysis is a data-driven statistical approach that avoids some limitations inherent to seed-based analysis (Wang and Peterson 2008; Beckmann et al. 2005). Based on blind source separation, (i.e., disentangling a mixture of signals that emerge from distinct but unknown sources), ICA is able to decompose a set of linearly mixed independent source signals from a complex dataset (Kisilev et al. 2003; Konrad and Eickhoff 2010). For example, it can separate signals unrelated to the components being analyzed, such as motion artifacts embedded in the data (Beckmann et al. 2005). An important advantage of ICA is that it does not require previous information about the source signals or the mixing process itself (Wenger and Schuster 2007). This enables the derivation of hidden and unknown relationships underlying sets of random variables and measures (Ejaz 2008). However, ICA can yield variable results depending on the given dataset and whether the statistical independence analysis is correct. While arguably less subject to investigator bias than seed-based approaches, ICA nonetheless requires investigators to determine how many components to isolate, which components likely represent artifactual signal, how to correctly identify the correspondence of components between subjects at the individual and group level (i.e., the first component for subject A may not correspond to the first component for subject B). Simply stated, though ICA can identify distinct functional networks without relying on a prior hypotheses, it is not exclusively a blind algorithm.
Regardless of the methodological approach implemented, an important consideration when analyzing rs-fcMRI data is the confounding effect that head motion during MRI scanning can have upon connectivity measures. Recent studies suggest that movement of the head as small as 0.1 mm can distort connectivity measures (Van Dijk et al. 2012; Power et al. 2011). Scanning techniques such as the use of fitted pillows, taping of the head, and training sessions in a mock MRI scanner can help minimize the degree to which a study participant moves his/her head during the scanning session. Analytic techniques are also available to investigators to help curtail head motion as a potential confound. For example, investigators often covary for head motion by incorporating motion parameters into multiple regression models. Resting MRI data can also be “scrubbed” by either removing images acquired during a period of excessive head motion, or adding nuisance regressors to specific time points (Power et al. 2011). More sophisticated techniques involve assessing frame-by-frame head displacements as well as frame-by-frame changes in mean fMRI signal (large, sudden changes in fMRI signal are often attributable to head motion). A related concern, and one that is particularly germane to ADHD, is that head motion may be inherent to the disorder. That is, by covarying for excessive head motion, investigators may risk inadvertently covarying for the effects of ADHD itself. Whenever possible group matching participants based on the degree of head motion is desirable, but the optimal approach for addressing the effects of head motion on connectivity measures remains an active area of debate and investigation (Fair et al. 2007).
Systematic Review of the Literature
To review the literature, we queried PubMed and Google Scholar using the following search terms: ADHD, attention-deficit/hyperactivity disorder, resting state MRI, rs-fcMRI, and functional connectivity. Manually, we then reviewed the reference lists provided by each of the identified articles. Our search included all years through 2013 inclusive, and was restricted to peer-reviewed English language articles. Study samples had to consist of participants for whom ADHD was the primary diagnosis. Comorbidities were permitted based on the following considerations. First, comorbid disorders are extremely common in ADHD with research suggesting that up to 50-70% of individuals with ADHD have a comorbid disorder (Pliszka 2009; Biederman et al. 1991). It is thus extremely uncommon for neuroimaging studies of ADHD to restrict samples to individuals who are unaffected by a comorbid disorder. Second, given that the majority of individuals with ADHD have a comorbid disorder, restricting a sample to those without comorbidities (and restricting our review likewise) would severely curtain the generalizability of a study of ADHD. Based on our search criteria, we found a total of 24 studies. Sample sizes ranged from N = 16 to N = 648 participants with ADHD and included children, adolescents, and adults. Studies that examined neural connectivity within the context of an fMRI task (J. Posner et al. 2011b) were not included. The studies reviewed are summarized in Tables 1 – 3.
Table 1. Connectivity between the Default Mode and Cognitive Control Networks and within the Default Mode Network.
| Authors | Sample | Methods (seeds used) | Medication Status | Findings |
|---|---|---|---|---|
| Castellanos et al. 2008 | N = 40 with 20 ADHD adults and 20 controls; 31.2-34.9 years. | Seed-based (frontal regions) | 9 patients discontinued for at least 24 hours before scanning | Decreased inverse cognitive control-DMN connectivity in adults with ADHD. Secondary analyses revealed ADHD-related decreases in connectivity within the DMN itself (mPFC and PCC). |
| Cao et al. 2009 | N = 42 with 19 ADHD children and 23 controls; 11-16 years. | Seed-based (putamen) | Medication-naïve | Decreased inverse connectivity between putamen and DMN regions and decreased connectivity between putamen and frontolimbic regions in children with ADHD. |
| Sun et al. 2012 | N = 42 with 19 ADHD boys and 23 controls; 11-16 years. | Seed-based (dACC) | Medication-naïve | Decreased inverse cognitive control-DMN connectivity in boys with ADHD. PCC and dACC connectivity associated with age in healthy controls, but not in ADHD. |
| Sato et al. 2012 | N = 63 with 21 ADHD adults, 21 age-matched controls, and 21 young controls; 20-50 years & 9-22 years. | Seed-based (PCC and dACC) | Medication discontinued 24 hours before scanning | Higher abnormality index for dACC and PCC connectivity in ADHD adults, with ADHD connectivity patterns more akin to younger healthy controls than age-matched peers. |
| Hoekzema et al. 2013 | N = 45 with 22 male ADHD adults and 23 controls; 29.26-32.82 years. | ICA, post-hoc seed-based | Medication-naïve | Healthy controls exhibited inverse connectivity between the DLPFC and DMN components; adults with ADHD displayed positive connectivity between these regions. |
| Fair et al. 2010 | N = 46 with 23 ADHD youth and 23 controls; 7-16 years. | Seed-based (DMN seeds) | Medication washout of five half-lives | Reduced connectivity between multiple regions within the DMN including between the PCC and mPFC in children and adolescents with ADHD. Pattern of DMN connectivity in ADHD suggestive of delayed neuromaturation. |
| Fair et al. 2012 | N = 648 with 193 ADHD (combined and inattentive-subtype) youths and 455 controls; 7-14 years. | Seed-based, 160 a priori ROIs based on Dosenbach et al. (2010) | Medication discontinued 24 to 48 hours before scan | Overlap in connectivity patterns detected for ADHD-C and ADHD-I subtypes in the sensorimotor systems. However, in contrast to the prominent atypical connectivity in midline DMN components, as well as insular cortex for ADHD-C, children with ADHD-I exhibited atypical patterns within dlPFC and cerebellum. |
| Qiu et al. 2011 | N = 30 with 15 ADHD (inattention-subtype) patients and 15 controls; 10.5-15.0 years. | ICA | Medication-free for at least half a year | Combined rs-fcMRI and DTI. Rs-fcMRI data suggest DMN hypoconnectivity in regions including PCC and precuneus and hyperconnectivity in bilateral posterior frontal cortex in ADHD. |
ADHD, attention-deficit/hyperactivity disorder; ACC, anterior cingulate cortex; DMN, default mode network; dACC, dorsal anterior cingulate cortex; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; ICA, independent component analysis; ROI, region of interest; ADHD-C, ADHD-combined subtype; ADHD-I, ADHD-predominantly inattentive subtype. rs-fcMRI, resting state functional connectivity MRI; DTI, diffusion tensor imaging.
Table 3. Additional Resting MRI Measurements.
| Authors | Sample | Methods | Medication status | Findings |
|---|---|---|---|---|
| Wang et al. 2013 | N = 46 with 23 ADHD adults and 23 controls; 32.04-35.14 years. | ReHo; Support Vector Machines | 11 discontinued half a year before scan; 1 discontinued 48 hours before scan | Increased activity in bilateral occipital and left frontal lobe in adults with ADHD. |
| Cao et al. 2006 | N = 44 with 23 ADHD boys and 21 controls; 11-16.5 years. | ReHO | 19 medication-naïve; 4 discontinued 48 hours before scan | Reduced ReHo in the frontal-striatal-cerebellar circuits and increased ReHo in the occipital cortex of boys with ADHD. |
| Uddin et al. 2008 | N = 40 with 20 ADHD adults and 20 controls; 31.2-34.9 years. (Same sample as Castellanos et al. 2008) | Network homogeneity | Medication-naive or discontinued 24 hours before scan | Reduced network homogeneity within the DMN of adults with ADHD. |
| Yu-Feng et al. 2007 | N = 25 with 13 ADHD boys and 12 controls; 13.0-13.1 years. | ALFF | 11 medication-naïve; 2 discontinued 48 hours before scan | Increased ALFF in the right anterior cingulate cortex, left sensorimotor cortex, and bilateral brainstem in boys with ADHD; decreased ALFF in right inferior frontal cortex, left sensorimotor cortex and bilateral cerebellum. |
| Yang et al. 2011 | N = 34 with 17 ADHD boys and 17 controls; 7.5 – 9.7 years. | ALFF | Medication-naïve | Increased ALFF in the left superior frontal gyrus and sensorimotor cortex in boys with ADHD; reduced ALFF in bilateral anterior and middle cingulate cortex and right middle frontal gyrus. |
| Tian et al. 2008 | N = 16 with 8 ADHD and 8 controls; 11-15 years. | RSAI | 7 discontinued half a year before scan; 1 discontinued 48 hours before scan | Elevated RSAI in primary sensory and sensory-related cortices in adolescents with ADHD. |
| Wang et al. 2009 | N = 39 with 19 ADHD boys and 20 controls; 13.32-13.59 years. | Small world topology. | Not specified | Increased local efficiencies and decreased global efficiencies found in boys with ADHD. |
| Cocchi et al. 2012 | N = 31 with 16 ADHD adults and 15 controls; 22.4-23.8 years. | Multivariate approaches (complex network measures); bivariate (network-based statistic); univariate (ReHo). | Medication-naïve | Abnormal local connections found in frontal, temporal and occipital cortices in adults with ADHD, as well as altered connectivity in frontal amygdala-occipital and frontal temporal-occipital networks. |
ADHD, attention-deficit/hyperactivity disorder; ReHo, regional homogeneity; DMN, default mode network; ALFF, amplitude of low-frequency fluctuation; RSAI, resting-state activity index.
Interactions between the Default Mode Network and Cognitive Control Network
The default mode network (DMN) has been the target of many rs-fcMRI studies (Posner et al. 2013a; Whitfield-Gabrieli and Ford 2012). A readily identified interconnected network of brain regions demonstrating strongly correlated neural activity during rest, the DMN encompasses the precuneus/posterior cingulate cortex, the medial prefrontal cortex and the lateral and inferior parietal cortex (Fig 1A) (Fox et al. 2005). Functionally, the DMN is characterized as a network of brain regions associated with task-irrelevant mental processes, mind-wandering, self-referential cognitions, and ruminations (Buckner et al. 2008; Raichle and Snyder 2007). The DMN shows higher activity when individuals are at wakeful rest, engaged in internal tasks such as daydreaming, recovering memories, and assessing others' perspectives (Buckner et al. 2008). Conversely, when individuals transition from internally-focused cognitions to external, goal-directed tasks, the DMN becomes deactivated, with stronger deactivations corresponding to increasing attentional demands (Raichle and Snyder 2007; Buckner et al. 2008). Interestingly, failure to suppress, or deactivate, the DMN is associated with momentary lapses in attention (Weissman et al. 2006) and greater errors in task performance such as the stop signal task (Li et al. 2007). This suggests that the inappropriate persistence of DMN activity may interfere with sustained attention, particularly on attentionally demanding tasks.
Figure 1.
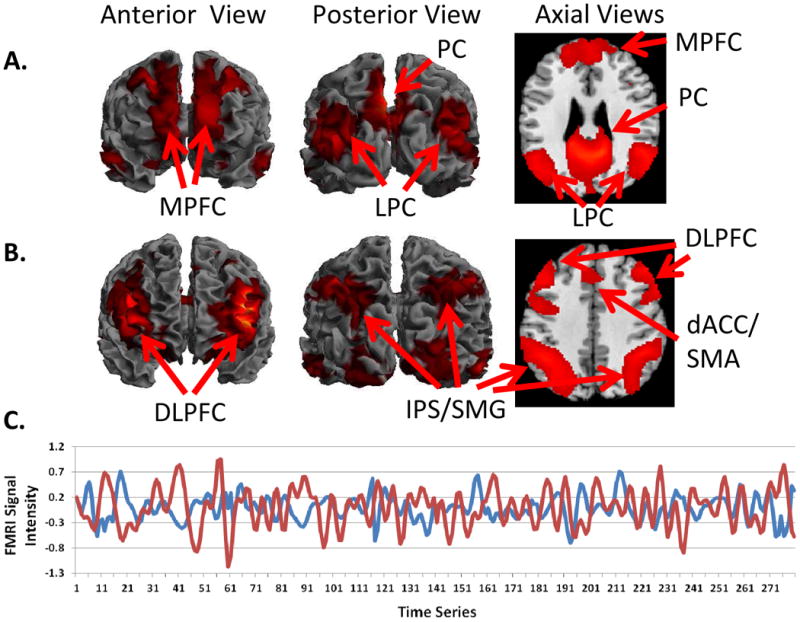
Whole-brain resting state functional connectivity maps with seed region in the (A) posterior cingulate cortex and (B) dorsal anterior cingulate cortex. A, The default mode network derived from a seed in the posterior cingulate cortex (PCC) encompasses the precuneus (PC), medial prefrontal cortex (MPFC), and lateral parietal lobules (LPC). B, The cognitive control network derived from a seed in the dorsal anterior cingulate cortex (dACC) encompasses the supplemental motor area (SMA), dorsolateral prefrontal cortex (DLPFC), and the intraparietal sulcus/supramarginal gyrus (IPS/SMG). C, Time series data extracted from the PCC (in red) and the dACC (in blue) demonstrate inversely correlated fMRI signal intensity between the default mode and cognitive control networks.
Several rs-fcMRI studies have focused on the connectivity of the DMN in ADHD with the hypothesis that dysconnectivity of the DMN may reflect an inability to properly modulate DMN activity (Table 1). Likewise, interactions between the DMN and the cognitive control network have also been a focus for investigation. The cognitive control network, which encompasses the dorsal anterior cingulate cortex/supplementary motor area, dorsolateral prefrontal cortex, inferior frontal junction, anterior insular cortex, and posterior parietal cortex, is involved in high-level cognitive processes such as working memory, inhibitory control, and set shifting (Fig 1B) (Cole and Schneider 2007). The DMN and cognitive control networks habitually work in opposing directions in relation to attentional demands – as attentional demands increase, activation of the cognitive control network increases, whereas DMN activation is attenuated; conversely, during periods of ‘rest’ or internally focused cognitions, activation in the cognitive control network is reduced, and DMN activation increases (Fig 1C) (Fox et al. 2005; Raichle et al. 2001; Grady et al. 2010).
In the first of a series of related studies, Castellanos et al (Castellanos et al. 2008) used seed-based rs-fcMRI analysis to examine the connectivity between the DMN and three frontal regions within the cognitive control network: the dorsal anterior cingulate cortex (dACC), the right inferior frontal gyrus, and the right medial frontal gyrus. Based on a sample of 20 adults with ADHD and 20 healthy control participants, the authors found that activity in the frontal cognitive control regions was inversely correlated with activity in a region spanning the precuneus and posterior cingulate cortex, central hubs of the DMN. This inverse frontal cognitive control-DMN relationship was detected in both the ADHD and healthy controls participants. However, the magnitude of the inverse correlation was weaker in ADHD vs healthy control participants, particularly between the dACC and precuneus/posterior cingulate cortex. This finding points to a disruption in the normal negative, or inverse, relationship between the cognitive control network and the DMN in ADHD. The authors postulate that focused, goal-directed attention mediated by the cognitive control network may become disrupted, leading to attentional lapses, as the mind-wandering function of the DMN intrudes upon the normal functioning of the cognitive control network.
Analogous findings were subsequently reported in a sample of medication naïve children with ADHD (Cao et al. 2009). Based on seeds in the putamen, negative connectivity between the putamen and nodes within the DMN were detected in healthy control children (Cao et al. 2009). The magnitude of these putamen-DMN anticorrelations was attenuated in ADHD. Relative to the dACC, the mediating role of the putamen in attention is less established; nonetheless, the authors suggest that interference from the DMN may impair normal attentional functioning in ADHD.
Continuing to build off this work, three additional studies have mirrored many of the findings described by Castellanos et al (Castellanos et al. 2008). The first used a sample of 19 medication naïve adolescents with ADHD and 23 healthy control participants. The authors found that healthy control adolescents demonstrated an inverse cognitive control-DMN relationship with inversely correlated connectivity between the dACC and regions within the DMN including the posterior cingulate cortex (Sun et al. 2012). This inverse cognitive control-DMN relationship was attenuated in adolescents with ADHD, paralleling findings in adults with ADHD (Castellanos et al. 2008). A second study used a machine-learning algorithm to classify adults with ADHD based on an abnormality index derived from examining connectivity between the dACC and the posterior cingulate cortex (Sato et al. 2012). The degree of abnormality of this connection was significantly greater in ADHD compared with healthy control participants. Moreover, connectivity between the dACC and the posterior cingulate cortex in adults with ADHD was more similar to the connectivity detected in typically developing children than healthy control adults. This developmental finding suggests a potential delay in neuromaturation in ADHD, at least in terms of the dACC-DMN connectivity. A third study used ICA in a sample of adults with ADHD and found that whereas the DMN was anticorrelated with the left dorsolateral prefrontal cortex (DLPFC) in healthy control adults, the DMN and left DLPFC were positively correlated in ADHD (Hoekzema et al. 2013). Though the authors did not replicate the DMN-dACC findings reported by others, the DLPFC is a central component of the cognitive control network as is the dACC.
In sum, five separate studies including children, adolescents, and adults with ADHD, both with and without prior medication exposure, have found that anti-correlations between the cognitive control network and the DMN are either reduced, or absent, in ADHD. The lack of temporal segregation (i.e. anticorrelations) between the cognitive control network and the DMN in ADHD offers a neurocognitive model of attentional lapses – that is, mind-wandering mediated by the DMN impedes sustained attention by disrupting or interfering with the normal functioning of the cognitive control network. This neurocognitive model, though conceptually appealing, requires additional empirical testing. Indeed, behavioral correlates of cognitive control-DMN dysconnectivity are largely absent from the aforementioned studies. Thus the functional significance of cognitive control-DMN dysconnectivity remains speculative. Though its biological plausibility and apparent reliability make it intriguing, further testing with an eye toward functional and behavioral correlates is needed.
Connectivity within the Default Mode Network
In addition to interactions between the DMN and the cognitive control network, connectivity within the DMN itself has also been examined in ADHD. In the aforementioned Castellanos et al (2008) study, the authors conducted a secondary analysis in which the precuneus/posterior cingulate cortex was used as the seed region. They found that adults with ADHD showed abnormally reduced connectivity between two hubs of the DMN: the precuneus/posterior cingulate cortex and the medial prefrontal cortex. In a related study, Fair et al (2010) examined connectivity within the DMN in a sample of children with ADHD using a seed-based analysis with the posterior cingulate cortex as the selected seed. Connectivity between the posterior cingulate cortex and other nodes within the DMN were detected in both the ADHD and healthy control children. However, consistent with the adult ADHD finding of hypoconnectivity of the DMN (Castellanos et al. 2008), Fair et al found hypoconnectivity of the DMN in children with ADHD as well. Specifically, both studies found reduced connectivity in ADHD between two central DMN nodes: the posterior cingulate cortex and the medial prefrontal cortex. Taking a neurodevelopmental approach, Fair et al (2010) then examined 14 specific connections within the DMN for which developmental trajectories had previously been described (i.e., DMN connections known to either increase or decrease in strength with age) (Fair et al. 2008). The authors found that DMN connections that tend to increase with development were weaker in participants with ADHD, and conversely, a DMN connection known to decrease with development was abnormally increased in ADHD. This developmental analysis of the DMN is consistent with neurocognitive models of ADHD suggesting that the disorder is characterized by delays in neuromaturation. Structural MRI studies have similarly suggested delayed cortical maturation of the temporal and frontal lobes in ADHD (Shaw et al. 2007; Shaw et al. 2006). In a second paper, Fair et al (2012) demonstrated that node strength (indexing the aggregated connections of a given brain region) of regions within the DMN was atypical in children with ADHD-Combined Type, again suggesting atypical DMN connectivity in ADHD. Using ICA to examine the connectivity of the DMN, Qiu et al (2011) similarly reported DMN hypoconnectivity in ADHD. Interestingly, the sample of ADHD participants in this study was exclusively patients with ADHD-Primarily Inattentive Subtype, suggesting that DMN hypoconnectivity may be detectable irrespective of ADHD subtype.
To summarize, resting state connectivity studies have examined interactions between the DMN and cognitive control network, as well as connectivity within the DMN itself. These studies have been consistent in supporting three interrelated themes: cognitive control-DMN anticorrelations are attenuated in ADHD, connectivity within the DMN itself is reduced in ADHD, and connectivity patterns, at least based on the DMN and cognitive control-DMN anticorrelations, parallel structural MRI findings in ADHD suggesting delayed neuromaturation. The neuropsychological correlates of these patterns of dysconnectivity, however, remain sparsely explored.
Cortico-striato-thalamo-cortical loops
Cortico-striato-thalamo-cortical (CSTC) loops are a series of parallel neural circuits that project from the cortex to the striatum and thalamus, and then back to the cortex again (Alexander et al. 1986; Alexander and Crutcher 1990; Maia et al. 2008; Wang et al. 2011). There are numerous CSTC loops that involve different cortical regions with each loop believed to mediate different neurocognitive functions. Whereas the precise number of CSCT loops is subject to debate (Middleton and Strick 2001), a common partition is into three principal loops: sensorimotor, cognitive, and limbic (or affective), involving the sensorimotor cortices, dorsolateral prefrontal cortex, and orbitofrontal and anterior cingulate cortices, respectively (Yin and Knowlton 2006; Lehéricy et al. 2004; Di Martino et al. 2008). Multiple lines of evidence, including human neuroimaging studies and studies of animal models, suggest that impulsivity involves functional and anatomical abnormalities within the CSTC loops, particularly the cognitive and limbic CSTC loops (Casey et al. 1997; Cardinal et al. 2004; Marsh et al. 2009). In line with this literature, several rs-fcMRI studies of ADHD have examined connectivity between regions within the CSTC loops (Table 2). In the first of such studies, Cao et al (2009) examined connectivity in a sample of medication naïve adolescents with ADHD using seed-based analyses with bilateral putamen seeds. The authors found that relative to the healthy controls participants, children with ADHD have reduced connectivity between the left putamen and the superior frontal gyrus, regions within the cognitive CSTC loop. This differed from a prior finding suggesting hyperconnectivity between regions within this loop in ADHD (Tian et al. 2006). The prior study, however, consisted of a limited sample of only eight children with ADHD (Tian et al. 2006). In a larger sample of 67 children with ADHD, altered thalamic-putamen connectivity was reported, again suggesting dysconnectivity within the cognitive CSTC loop in ADHD (Mills et al. 2012). Cao et al (2009) also found that children with ADHD have hypoconnectivity between the left putamen and the ventral striatum (encompassing the nucleus accumbens, a region associated with reward processing), implicating the limbic, in addition to the cognitive, CSTC loop. Reduced putamen-nucleus accumbens connectivity was associated with more severe ADHD symptoms (Cao et al. 2009).
Table 2. Connectivity within the Cortico-striato-thalamo-cortical loops.
| Authors | Sample | Methods (seeds) | Medication Status | Findings |
|---|---|---|---|---|
| Cao et al. 2009 | N = 42 with 19 ADHD children and 23 controls; 11-16 years. | Seed-based (putamen) | Medication-naïve | Hypoconnectivity between left putamen and NAcc in children with ADHD correlated with ADHD symptom severity. (See Table 1 for additional results.) |
| Tian et al. 2006 | N = 16 with 8 ADHD and 8 controls; 11-15 years. | Seed-based (dACC) | 7 patients medication-free for at least half a year; 1 off medication for 48 hours | Hyperconnectivity between dACC and bilateral thalamus, bilateral cerebellum, bilateral insula and bilateral brainstem in ADHD youth. |
| Mills et al. 2012 | N = 67 with 24 ADHD children and 43 controls; 7-11 years. | Seed-based; (thalamus) | Most patients discontinued 24 to 48 hours before scan | Connectivity of five thalamic regions was correlated with spatial span working memory in children with and without ADHD. Connections between the thalamus and basal ganglia were altered in ADHD and correlated to spatial working memory. |
| Posner et al. 2013 | N = 42 with 22 ADHD children and 20 controls; 7-12 years. | Seed-based; (DLPFC and VS) | Medication-naïve | Hypoconnectivity between VS and anterior prefrontal cortex and between the DLPFC and dorsal caudate in children with ADHD children. Hypoconnectivity between VS and anterior prefrontal cortex correlated with increased emotional lability. |
| Costa Dias et al. 2013 | N = 99 with 35 ADHD children and 64 controls; 7-12 years. | Seed-based; (NAcc) | Medication washout of five-half lives | Hyperconnectivity between NAcc and anterior prefrontal cortex in ADHD children correlated with impulsivity as measured by delayed discounting. |
| An et al. 2013 | N = 55 with 23 ADHD boys and 32 controls; 8-15 years. | ReHo; voxelwise analysis | Medication discontinued one month before scan | Methylphenidate normalized ReHo in fronto-parieto-cerebellar regions. |
ADHD, attention-deficit/hyperactivity disorder; dACC, dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; VS, ventral striatum; NAcc, nucleus accumbens; ReHo, regional homogeneity.
Involvement of reward and affective systems has been implicated in two other rs-fcMRI studies of ADHD. Based on a sample of medication naïve children with ADHD, Posner et al (2013b) found that children with ADHD have reduced connectivity between the ventral striatum and the anterior prefrontal cortex. The detected hypoconnectivity within regions of the limbic CSTC loop correlated with increased emotional lability. A second study involving children with ADHD also found altered connectivity between the ventral striatum (with a nucleus accumbens seed) and the anterior prefrontal cortex (Costa Dias et al. 2012). However, the direction of dysconnectivity differed: whereas the two prior studies (Cao et al. 2009; Posner et al. 2013b) reported hypoconnectivity within the limbic CSTC loop in ADHD, the third study found hyperconnectivity of this loop (Costa Dias et al. 2012). A potential explanation for this discrepancy is that the studies reporting hypoconnectivity within the limbic CSTC loop were based on medication naïve samples, whereas the study reporting hyperconnectivity was based on a sample of children with prior psychostimulant exposure. Psychostimulants, such as methylphenidate and amphetamine salts, can alter resting state fMRI signal in children with ADHD (An et al. 2013), and indeed, changes in connectivity specifically at the nucleus accumbens have been reported after a single dose of methyphenidate, albeit in a different clinical population (Konova et al. 2013).
Taken together, rs-fcMRI studies suggest atypical connectivity in both the cognitive and limbic CSTC loops in ADHD. Such findings lend support to a neuropsychological model of ADHD termed the “dual pathway model.” (Sonuga-Barke et al. 2008). This neuropsychological model suggests that for some children with ADHD, the principal neurocognitive deficit may reside in neural substrates underlying executive functions (such as the cognitive CSTC loop). However, for others, affective and motivational systems (such as the limbic CSTC loop) may play a more central role in the pathophysiology of the disorder (Posner et al. 2011a; Sonuga-Barke et al. 1992). Interestingly, at least one study (Posner et al. 2013b) suggests that the behavioral correlates of anomalies in the cognitive and limbic CSTC loops may be dissociable. That is, in ADHD, impairments in executive functioning, but not emotional regulation, correlate with dysconnectivity in the cognitive CSTC loop. Conversely, impairments in emotional regulation, but not executive functioning, correlate with dysconnectivity in the limbic CSTC loop. Such findings raise important questions for future research such as the effects of treatment on these two dissociable circuits and whether disturbances in one loop vs the other have prognostic significance (Posner et al. 2013b).
Resting state MRI Signal: Additional Properties
Whereas rs-fcMRI studies examine the correlations in fMRI signal across brain regions, additional properties of resting state fMRI activity have also been explored in ADHD (Table 3). For example, investigators have examined the coherence of neural activity of a specific brain region with its neighboring, or adjacent, brain regions – a resting state measurement termed regional homogeneity (Wang et al. 2013; Cao et al. 2006). Regional homogeneity may reflect regional metabolism similar to measurements provided in PET imaging (Cao et al. 2006). Studies of regional homogeneity have suggested reduced homogeneity within regions of the cognitive CSCT loop including the inferior frontal gyrus and dorsal caudate (Cao et al. 2006). Using a similar approach, network homogeneity within the DMN was found to be reduced in ADHD (Uddin et al. 2008), complimenting prior findings of DMN hypoconnectivity in ADHD (Fair et al. 2010). Another measurement of resting state activity is termed amplitude of low-frequency fluctuations (ALFF) and reflects the magnitude of fMRI signal oscillations in a given brain region. Regions with greater ALFF may have greater baseline neural activity than regions with lower ALFF. Studies of ALFF in ADHD thus far have reported inconsistent findings with one study suggesting that individuals with ADHD have increased ALFF in the anterior cingulate cortex (ACC) (Yu-Feng et al. 2007), whereas another study reporting the opposite finding – reduced ALFF in the ACC of ADHD participants (Yang et al. 2011). Despite some inconsistent findings, additional and novel resting MRI measurements, such as the resting-state activity index (Tian et al. 2008), small-world topology (Wang et al. 2009), and multivariate approaches (Cocchi et al. 2012) remain an active area of inquiry and underscore the increasing interest in and methodological sophistication of the field.
Of note, the relationship between these novel non-connectivity resting state measurements and underlying biological processes remains obscure. Whereas resting connectivity is interpreted as neural signaling across a neural circuit, the biological connotations of non-connectivity resting state measurements, such as ALFF, is less clear. This limitation notwithstanding, these resting state measurements may prove to be of considerable scientific merit. For example, whereas biological markers of a disorder need marked reliability, as well as high sensitivity and specificity, they do not need to elucidate or reflect a biological mechanism of a disorder (Castellanos et al. 2013). Though considerable research is still needed, non-connectivity resting state measurements may prove more serviceable for exploring biomarkers than for explaining the biological mechanisms of ADHD.
Conclusions
Superficially, the resting brain would seem an unlikely source of information about a disorder of hyperactivity. This, however, has proven to be far from the truth. Its limitations notwithstanding, rs-fcMRI provides an important index of spontaneous brain activity, neural organization, and circuit architecture. In particular, rs-fcMRI has underscored the importance of the DMN in attentional regulation, and the potential role of interactions between the DMN and the cognitive control network in sustained attention. In doing so, rs-fcMRI studies have helped establish a working neurobiological model of attentional lapses in ADHD, implicating a deficiency in the segregation of the DMN and cognitive control network.
Such studies have also suggested maturational delays in circuit organization, complimenting existing structural MRI studies suggesting delays in synaptic pruning and myelination in ADHD particularly in the prefrontal, cingulate, and temporal cortices (Shaw et al. 2006; Shaw et al. 2007). Finally, rs-fcMRI studies support the view of heterogeneity of ADHD demonstrating that altered connectivity in ADHD is not limited to neural systems implicated in attentional control and executive functions. Rather, altered connectivity is also noted in affective and motivational circuits as well. Consistent with the neuropsychological literature on ADHD, rs-fcMRI studies support moving away from earlier hypotheses suggesting that a single core neurocognitive deficit is likely in all children with ADHD. Conversely, the clinical phenotype of ADHD may instead be the shared outcome of anomalies in either attentional or affective/motivational systems or the combined effects of anomalies in both systems (Posner et al. 2013b). Demonstrating dissociable anomalies in attentional and affective/motivational systems, rs-fcMRI research has provided neurobiological support for heterogeneity in the neuropsychological basis of ADHD (Sonuga-Barke 2005).
Despite the significant advances that rs-fcMRI studies have provided the field of ADHD, plenty of work still needs to be done. Indeed, treatment studies incorporating rs-fsMRI have scarcely been conducted. The reliability of rs-fcMRI in characterizing neural networks makes this technology well suited for longitudinal studies. Studies that incorporate pre- and post-treatment MRI scanning, for example, could use rs-fcMRI to identify treatment effects on the organization of neural circuits. Advancing our understanding of the mechanisms by which existing treatments exert their therapeutic effects, such studies could pave the way to developing more focused and effective pharmacotherapies. An additional research avenue that is emerging, but nonetheless remains underappreciated, is data sharing (Biswal et al. 2010; Nooner et al. 2012). Resting state networks can reliably be detected across individuals regardless of the MRI scanner used to collect the data (Biswal et al. 2010). Moreover, unlike task-based fMRI in which uniform behavioral paradigms across institutions are uncommon, data acquisition with rs-fcMRI can readily be made comparable between one institution and the next. Because of the potential for uniformity, rs-fcMRI datasets from disparate institutions can be shared and jointly analyzed creating pooled samples substantially larger than those obtainable by one institution alone (Biswal et al. 2010). Though efforts at data sharing, such as the ADHD 200 Consortium (Milham et al. 2012), are underway, a considerable hurdle for data sharing is the quality of data acquisition. Investigators must feel confident, for example, that diagnostic and behavioral measurements from disparate institutions are reliably and homogeneously administered – a considerable, but not insurmountable, obstacle. In conclusion, rs-fcMRI has proven to be a valuable tool for exploring the neuropsychological underpinnings of ADHD and given the reliability and uniformity of rs-fcMRI, future research is likely to capitalize on this approach through endeavors such as longitudinal MRI studies and inter-institutional data sharing.
Acknowledgments
This study was supported in part by NIMH grants: K23-MH091249 (JP) and R01-MH101172 (JP) and by funding from the Edwin S. Webster Foundation. Dr. Posner is a principal investigator on an investigator-initiated grant from Shire Pharmaceuticals.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in neurosciences. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9(1):357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- An L, Cao XH, Cao QJ, Sun L, Yang L, Zou QH, et al. Methylphenidate Normalizes Resting-State Brain Dysfunction in Boys With Attention Deficit Hyperactivity Disorder. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley R, Fischer M. The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. Journal of the American Acadamy of Child and Adolescent Psychiatry. 2010;49(5):503–513. doi: 10.1097/00004583-201005000-00011. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Major life activity and health outcomes associated with attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry. 2002;63(Suppl 12):10–15. [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Fischer M. ADHD in adults: What the science says. Guilford Press; 2010. [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. American Journal of Psychiatry. 1991;148(5):564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Fried R, Kaiser R, Dolan CR, Schoenfeld S, et al. Educational and occupational underattainment in adults with attention-deficit/hyperactivity disorder: A controlled study. Journal of Clinical Psychiatry. 2008 doi: 10.4088/jcp.v69n0803. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R, Andrews-Hanna J, Schacter D. The Brain's Default Network. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry. 2005;57(11):1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Hansen LK, Larsen J, Pekar JJ. ICA of functional MRI data: an overview 2003 [Google Scholar]
- Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, et al. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17(10):1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, et al. Abnormal resting-state functional connectivity patterns of the putamen in medication-naive children with attention deficit hyperactivity disorder. Brain research. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Annals of the New York Academy of Sciences. 2004;1021(1):33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- Casey B, Trainor R, Orendi J, Schubert A, Nystrom L, Giedd J, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(3):374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Di Martino A, Craddock RC, Mehta AD, Milham MP. Clinical applications of the functional connectome. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Kelly C, Milham MP. The restless brain: attention-deficit hyperactivity disorder, resting-state functional connectivity, and intrasubject variability. Canadian journal of psychiatry. Revue canadienne de psychiatrie. 2009;54(10):665. doi: 10.1177/070674370905401003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L, Bramati IE, Zalesky A, Furukawa E, Fontenelle LF, Moll J, et al. Altered functional brain connectivity in a non-clinical sample of young adults with Attention-Deficit/Hyperactivity Disorder. The Journal of Neuroscience. 2012;32(49):17753–17761. doi: 10.1523/JNEUROSCI.3272-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, et al. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. European Neuropsychopharmacology. 2012 doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies D, Kelly A, Uddin L, Shehzad Z, et al. Functional connectivity of human striatum: a resting state FMRI study. Cerebral cortex. 2008;18(12):2735. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Ejaz M. A Framework for Implementing Independent Component Analysis Algorithms. ProQuest; 2008. [Google Scholar]
- Fair D, Cohen A, Dosenbach N, Church J, Miezin F, Barch D, et al. The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences. 2008;105(10):4028. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D, Dosenbach N, Church J, Cohen A, Brahmbhatt S, Miezin F, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences. 2007;104(33):13507. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, et al. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Frontiers in systems neuroscience. 2012;6 doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TGC, Mills KL, et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Raichle M. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Frontiers in systems neuroscience. 2010;4 doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, et al. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cerebral cortex. 2010;20(6):1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos-Quiroga JA, Richarte Fernández V, Bosch R, Soliva JC, et al. An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Human brain mapping. 2013 doi: 10.1002/hbm.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. The American journal of psychiatry. 2006;163(4):716. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisilev P, Zibulevsky M, Zeevi YY. A multiscale framework for blind separation of linearly mixed signals. The Journal of Machine Learning Research. 2003;4:1339–1363. [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of Methylphenidate on Resting-State Functional Connectivity of the Mesocorticolimbic Dopamine Pathways in Cocaine AddictionEffects of Methylphenidate in Cocaine AddictionEffects of Methylphenidate in Cocaine Addiction. JAMA Psychiatry. 2013;70(8):857–868. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human brain mapping. 2010;31(6):904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van De Moortele PF, Francois C, Thivard L, Poupon C, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of neurology. 2004;55(4):522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Li CSR, Yan P, Bergquist KL, Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage. 2007;38(3):640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Dev Psychopathol. 2008;20(4):1251–1283. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. American Journal of Psychiatry. 2009;166(6):664. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. A revised neuroanatomy of frontal-subcortical circuits. Frontal-subcortical circuits in psychiatric and neurological disorders. 2001:44–58. [Google Scholar]
- Milham MP, Fair D, Mennes M, Mostofsky SH. The ADHD-200 consortium: a model to advance the translational potential of neuroimaging in clinical neuroscience. Frontiers in systems neuroscience. 2012;6:62. doi: 10.3389/fnsys.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Bathula D, Dias TGC, Iyer SP, Fenesy MC, Musser ED, et al. Altered cortico-striatal–thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Frontiers in Psychiatry. 2012;3 doi: 10.3389/fpsyt.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, et al. The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Frontiers in neuroscience. 2012;6 doi: 10.3389/fnins.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B, Kane M, Alexander G, Lacadie C, Skudlarski P, Leung H, et al. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cognitive Brain Research. 2002;13(3):427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Pliszka S. Treating ADHD and Comorbid Disorders: Psychosocial and Psychopharmacological Interventions. New York: Guilford Press; 2009. [Google Scholar]
- Polanczyk G, de Lima M, Horta B, Biederman J, Rohde L. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. American Journal of Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, et al. Antidepressants Normalize the Default Mode Network in Patients With DysthymiaAntidepressants and the Default Mode Network. JAMA Psychiatry. 2013a;70(4):373–382. doi: 10.1001/jamapsychiatry.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Maia TV, Fair D, Peterson BS, Sonuga-Barke EJ, Nagel BJ. The attenuation of dysfunctional emotional processing with stimulant medication: An fMRI study of adolescents with ADHD. Psychiatry Research: Neuroimaging. 2011a doi: 10.1016/j.pscychresns.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Marsh R, Maia T, Peterson B, Gruber A, Simpson H. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults wth Obsessive-Compulsive Disorder. Human brain mapping. doi: 10.1002/hbm.22371. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Nagel BJ, Maia TV, Mechling A, Oh M, Wang Z, et al. Abnormal Amygdalar Activation and Connectivity in Adolescents With Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2011b;50(8):828–837. e823. doi: 10.1016/j.jaac.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Rauh V, Gruber A, Gat I, Wang Z, Peterson BS. Dissociable attentional and affective circuits in medication-naïve children with attention-deficit/hyperactivity disorder. Psychiatry Research: Neuroimaging. 2013b doi: 10.1016/j.pscychresns.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Mg, Ye Z, Li Qy, Liu Gj, Xie B, Wang J. Changes of brain structure and function in ADHD children. Brain topography. 2011;24(3-4):243–252. doi: 10.1007/s10548-010-0168-4. [DOI] [PubMed] [Google Scholar]
- Raichle M, MacLeod A, Snyder A, Powers W, Gusnard D, Shulman G. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M, Snyder A. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Sato JR, Hoexter MQ, Castellanos XF, Rohde LA. Abnormal brain connectivity patterns in adults with ADHD: a coherence study. PloS one. 2012;7(9):e45671. doi: 10.1371/journal.pone.0045671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of general psychiatry. 2006;63(5):540. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences. 2010;107(24):11020. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Sergeant JA, Nigg J, Willcutt E. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: nosologic and diagnostic implications. Child Adolesc Psychiatr Clin N Am. 2008;17(2):367–384. ix. doi: 10.1016/j.chc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion--I. The effect of delay on choice. J Child Psychol Psychiatry. 1992;33(2):387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Sun L, Cao Q, Long X, Sui M, Cao X, Zhu C, et al. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naïve boys with attention deficit hyperactivity disorder. Psychiatry Research: Neuroimaging. 2012;201(2):120–127. doi: 10.1016/j.pscychresns.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Tian L, Jiang T, Liang M, Zang Y, He Y, Sui M, et al. Enhanced resting-state brain activities in ADHD patients: a fMRI study. Brain and Development. 2008;30(5):342–348. doi: 10.1016/j.braindev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, et al. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2006;400(1-2):39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly A, Biswal BB, Margulies DS, Shehzad Z, Shaw D, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. Journal of neuroscience methods. 2008;169(1):249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu C, He Y, Zang Y, Cao Q, Zhang H, et al. Altered small world brain functional networks in children with attention deficit/hyperactivity disorder. Human brain mapping. 2009;30(2):638–649. doi: 10.1002/hbm.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jiao Y, Tang T, Wang H, Lu Z. Altered regional homogeneity patterns in adults with attention-deficit hyperactivity disorder. European journal of radiology. 2013 doi: 10.1016/j.ejrad.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS. The neural circuits that generate tics in Tourette's syndrome. American Journal of Psychiatry. 2011;168(12):1326–1337. doi: 10.1176/appi.ajp.2011.09111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Peterson BS. Partner matching for the automated identification of reproducible ICA components from fMRI datasets: Algorithm and validation. Human brain mapping. 2008;29(8):875–893. doi: 10.1002/hbm.20434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D, Roberts K, Visscher K, Woldorff M. The neural bases of momentary lapses in attention. Nature neuroscience. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wenger MJ, Schuster C. Statistical and process models for cognitive neuroscience and aging. Psychology Press; 2007. [Google Scholar]
- Whalen PJ, Bush G, Shin LM, Rauch SL. The emotional counting Stroop: a task for assessing emotional interference during brain imaging. Nat Protoc. 2006;1(1):293–296. doi: 10.1038/nprot.2006.45. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Yang H, Wu QZ, Guo LT, Li QQ, Long XY, Huang XQ, et al. Abnormal spontaneous brain activity in medication-naive ADHD children: a resting state fMRI study. Neuroscience letters. 2011;502(2):89–93. doi: 10.1016/j.neulet.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yu-Feng Z, Yong H, Chao-Zhe Z, Qing-Jiu C, Man-Qiu S, Meng L, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain and Development. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]


