Abstract
Importance
Various neuropsychiatric disorders, especially addictions, feature impairments in risky decision-making; clarifying the neural mechanisms underlying this problem can inform treatment.
Objective
To determine how methamphetamine-dependent and control subjects differ in brain activation during a risky decision-making task, resting-state functional connectivity within mesolimbic and executive control circuits, and the relationships between these measures.
Design
A case-control, functional magnetic resonance imaging study of methamphetamine-dependent and healthy comparison participants at rest and when performing the Balloon Analogue Risk Task, which involves the choice to pump a balloon or to cash out in the context of uncertain risk.
Setting
Clinical research center at an academic institution.
Participants
Twenty-five methamphetamine-dependent and 27 control subjects.
Main Outcomes and Measures
1) Parametric modulation of activation in the striatum and right dorsolateral prefrontal cortex, i.e., the degree to which activation changed as a linear function of risk and potential reward, both indexed by pump number; and 2) resting-state functional connectivity, measured in whole brain with seeds in the midbrain and right dorsolateral prefrontal cortex. Relationships between these outcomes were also tested.
Results
Parametric modulation of cortical and striatal activation by pump number during risk-taking differed with group. It was stronger in the ventral striatum but weaker in the right dorsolateral prefrontal cortex in methamphetamine-dependent participants than controls. Methamphetamine-dependent subjects also exhibited greater resting-state functional connectivity of the midbrain with the putamen, amygdala, and hippocampus. This connectivity was negatively related to modulation of right dorsolateral prefrontal cortex activation by risk level during risky decision-making. In controls, parametric modulation of right dorsolateral prefrontal cortex activation by risk during decision-making was positively related to resting-state functional connectivity of the right dorsolateral prefrontal cortex with the striatum.
Conclusions and Relevance
Maladaptive decision-making by methamphetamine users may reflect circuit-level dysfunction, underlying deficits in task-based activation. Heightened resting-state connectivity within the mesocorticolimbic system, coupled with reduced prefrontal cortical connectivity, may create a bias toward reward-driven behavior over cognitive control in methamphetamine users. Interventions to improve this balance may enhance treatments for stimulant dependence and other disorders that involve maladaptive decision-making.
Keywords: risk-taking, decision-making, prefrontal cortex, striatum, mesolimbic, fMRI, resting state
Deficits in decision-making have been linked with addiction, and likely contribute to addiction vulnerability and to the maintenance and severity of dependence1–5. Chronic methamphetamine use is associated with abnormalities in the neural circuits involved in risky decision-making6–9, including structural and functional deficits in the prefrontal cortex (PFC) and striatum10–12, and in striatal dopaminergic markers13–17. Little is known, however, about the links between these observations and problems with decision-making.
The mesocorticolimbic system, originating in the midbrain ventral tegmental area (VTA) and projecting to the nucleus accumbens, amygdala, hippocampus and medial PFC18, substantially influences goal-directed behaviors, and pathological drug-seeking behavior may result from drug-induced changes in this circuitry18, 19. Studies using resting-state functional connectivity (RSFC) to assess temporal correlations of spontaneous regional activity when participants are at rest20 have identified abnormalities in connectivity between nodes of the mesocorticolimbic system in cocaine and opiate users18. However, PFC and striatal dysfunction during risky decision-making by substance-dependent individuals21 has not been linked directly to network activity, nor has it yet been examined in methamphetamine users. We therefore used RSFC and task-based fMRI to clarify how circuit-level abnormalities may influence adaptive decision-making in methamphetamine users.
Functional magnetic resonance imaging (fMRI) was paired with the Balloon Analogue Risk Task (BART)22, which presents sequential choices – pumping a balloon to increase monetary gains while risking loss, or cashing out to retain earnings. Using a parametric modulation analysis, we tested for differences between methamphetamine-dependent and control subjects in modulation of right dorsolateral prefrontal cortex (rDLPFC) and striatal activation by risk and potential reward (both indexed by pump number) during decision-making. As chronic methamphetamine users exhibit ventral striatal hyper-responsivity to reward23 but rDLPFC hypoactivity during decision-making24, 25, we expected them to display greater modulation of striatal activation by pump number during risky decision-making but less modulation in the rDLPFC, and to earn less on the BART than controls. RSFC was assessed with seeds in the midbrain, because of its dopaminergic projections to limbic and cortical regions, and in rDLPFC, which exhibits risk-sensitivity while participants perform the BART7, 9, 26, 27. Because stimulants produce adaptations in the mesolimbic dopamine system, which are thought to underlie psychomotor sensitization in animals28–30, it was expected that midbrain RSFC would be greater in methamphetamine users than in controls.
Finally, because adaptations in mesolimbic and prefrontal cortical regions are thought to underlie addiction-related cognitive deficits31–34, the relationship between task-based activation and connectivity within mesocorticolimbic (midbrain seed) and corticostriatal circuits (rDLPFC seed) was tested. It was expected that modulation of rDLPFC activation would be positively related to rDLPFC RSFC in controls and negatively related to midbrain RSFC in methamphetamine users. Negative association of midbrain RSFC with modulation of rDLPFC activation would suggest that mesolimbic circuit dysfunction promotes maladaptive decision-making in methamphetamine users. As faulty decision-making is a target for addiction therapies, understanding its determinants might facilitate the development of more effective interventions.
METHODS
Participants
Fifty-three volunteers, recruited via newspaper and Internet advertisements, provided written informed consent, as approved by the UCLA Institutional Review Board. Exclusion criteria, determined by physical examination, medical history, and laboratory blood tests were systemic, neurological, cardiovascular, or pulmonary disease, or head trauma with loss of consciousness. They were assigned to two groups: Methamphetamine and Control. Current Axis I diagnoses, other than nicotine dependence for either group and methamphetamine dependence for the Methamphetamine group, assessed with the Structured Clinical Inventory for DSM-IV-TR, were exclusionary.
The Methamphetamine group included 26 non-treatment seeking subjects (13 men/women, 20 smokers, 35.68 ±1.64 years old), who provided a positive urine test for methamphetamine, and reported using 3.57 ± 1.04 g/week of methamphetamine and using methamphetamine, alcohol, and marijuana on 23.60 ± 1.29, 4.68 ± 1.64, and 1.68 ± 0.70 days of the month before enrollment, respectively (Table 1). Eleven participated on a residential basis, abstinent from methamphetamine for 4–7 days before scanning; 14 participated on a nonresidential basis, abstaining from methamphetamine for 5.78 ± 1.84 days before scanning. The Control group included twenty-seven subjects (11 women/16 men, 16 smokers, 33.88 ± 2.30 years old), partly overlapping with subjects from a previous study7. They reported alcohol and marijuana use on 4.36 ± 1.15 and 0.08 ± 0.08 days in the month before enrollment, respectively, but no other drug use. The groups differed in frequency of marijuana but not alcohol use (Table 1). Urine testing at intake and on test days verified abstinence from cocaine, methamphetamine, benzodiazepines, opiates, and cannabinoids.
Table 1.
Characteristics of Research Participants
| Healthy Control (n=27)a | MA-dependent (n=25)b | |
|---|---|---|
| Age (years)c | 33.88 ± 2.30 | 35.68 ± 1.64 |
| Sex (# male) | 16 | 12 |
| Education (years) | 13.62 ± 0.38 | 13.00 ± 0.38 |
| Alcohol Use | ||
| Days used in the last 30 d | 4.36 ± 1.15 | 4.68 ± 1.64 |
| Marijuana Use* | ||
| Days used in the last 30 d | 0.08 ± 0.08 | 1.68 ± 0.70 |
| Tobacco Use (# smokers) | 16 | 20 |
| Days used in the last 30 d | 17.57 ± 2.87 | 21.16 ± 2.54 |
| Methamphetamine Use | ||
| Days used in the last 30 d | 23.60 ± 1.29 | |
| Grams per week | 3.57 ± 1.04 | |
| Years of heavy use | 8.59 ± 1.37 |
n=18 and
n=15 for resting-state functional connectivity analysis
Data shown are means ± SEM
Significant differences between the groups by Student’s t-test (p = 0.033).
Balloon Analogue Risk Task
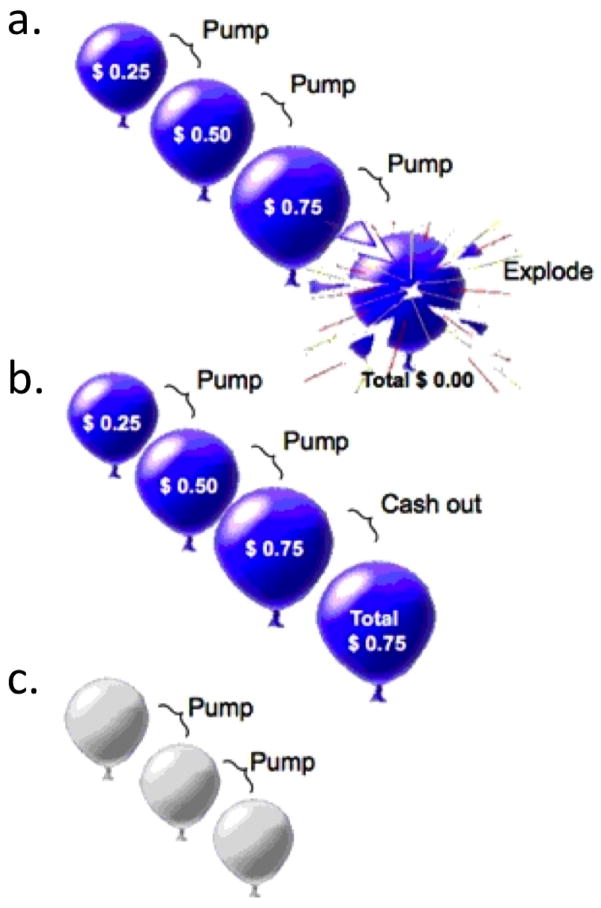
A event-related fMRI version of the BART22 was administered in two 10-min runs (Fig. 1). Active trials, presenting red or blue balloons, and control trials, presenting white balloons, were randomly dispersed throughout the task. On active trials, subjects chose between pumping a balloon to increase earnings ($0.25/pump) or to cash out, retaining accumulated earnings. Pumping either increased the balloon size or was followed by a 2-s display of an exploded balloon and the message, “Total=$0.00”. Each trial included all pumps before an explosion or cashing out, followed by a 2-s display of total earnings. Subjects were informed that the colored balloons were associated with monetary reward, with winnings distributed after scanning. They were unaware that the number of pumps before an explosion was pre-determined; and that it was selected from a uniform probability distribution, ranging from 1–8 and 1–12 pumps for red and blue balloons, respectively. Subjects were told that the white balloons did not explode and had no monetary value, and that they should pump each one until the trial ended. The number of white balloons in a trial varied randomly between 1–12, according to a uniform distribution. As the task was self-paced, the numbers of trials and pumps within a trial varied between subjects. The inter-stimulus interval for balloon presentations was 1–3 s, and the inter-trial interval was 1–14 s with a mean of 4 s.
Figure 1. Schematic of Balloon Analogue Risk Task.
a. Pumping the balloon increased potential earnings but carried the risk of the balloon exploding, resulting in a loss of accumulated earnings during the trial. b. If participants cashed out before the balloon exploded, they retained the earnings accumulated. c. In control trials, white balloons were presented. These balloons did not increase in size with pumping, did not explode, and were not associated with reward potential (see Methods).
fMRI
Task-based scans were collected from 26 Methamphetamine and 27 Control subjects. One Methamphetamine subject was excluded due to excessive head motion (> 2 mm translational displacement, > 1.5 degrees rotation), leaving a final sample of 25. Eighteen Control and 15 Methamphetamine subjects underwent resting-state fMRI in the same session while viewing a black screen for 5 min. Imaging was performed on a 3-T Siemens Trio MRI system, with 302 functional task-based and 152 resting-state T2*-weighted, echoplanar images (EPI) acquired (slice thickness = 4 mm; 34 slices; TR = 2 s; TE = 30 ms; flip angle = 90°; matrix = 64 x 64; fov = 200 mm). High-resolution, T2-weighted, matched-bandwidth (MBW) and magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scans were also acquired. The orientation for these scans was oblique axial to maximize brain coverage and to optimize signal from ventromedial PFC.
Data Analysis
A general linear mixed model (GLMM) was used to examine trial-by-trial, risk-taking behavior, accounting for individual subject variables. The model included trial number (across both runs), balloon color, and outcome of the immediately preceding trial, with pumps/trial as the dependent variable. Data were analyzed using the Statistical Package for the Social Sciences.
The rDLPFC region of interest (ROI) was sampled with a 10-mm sphere around the peak voxel (MNI coordinates: x = 30, y = 36, z = 20) from a cluster showing modulation of activation during balloon pumping on the BART9,7. A bilateral striatal ROI was derived from the Harvard-Oxford atlas (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). A 9-mm spherical midbrain ROI was created using the coordinates (MNI: x = 0, y = −15, z = 9) from a study examining the effect of methylphenidate on midbrain RSFC35.
Image analysis was performed using FSL 5.0.2.1 (www.fmrib.ox.ac.uk/fsl). Images were realigned to compensate for motion36, and high-pass temporal filtering was applied. Data were skull-stripped and spatially smoothed (5-mm FWHM Gaussian kernel). The EPI images were registered to the MBW image, then to the high-resolution MPRAGE image, and finally into standard Montreal Neurological Institute space, using 12-parameter affine transformation and FNIRT nonlinear registration37.
Four types of events were included in the general linear model (GLM): pumps on active balloons, cash outs, balloon explosions and pumps on control balloons. Two regressors for each of the four types of events were included to obtain estimates of parametric modulation38 of activation by pump number and of mean activation for each event type. As a trial progressed, the risk of balloon explosion increased with each pump, as did the amount earned with cashing out. Parametric regressors tested the linear relationship between pump number and activation (i.e., modulation of activation by pump number) by assigning greater weight to events that carried greater risk and potential reward. For example, within a trial, the second pump, for which twice the reward was at stake, was given twice the weight as the first. For regressors that estimated mean activation for each event, the escalation of risk was not considered, and each pump was assigned equal weight. To test for differences in overall activation during risky decision-making and for the modulation of activation with risk and reward levels, the contrasts of interest were mean pump events versus mean control-balloon events, and parametric pump events, respectively.
Regressors were created by convolving a set of delta functions, representing onset times of each event with a canonical (double-gamma) hemodynamic response function. The first temporal derivatives of the eight task-related regressors were included to capture variance associated with the temporal lag of the hemodynamic response along with six motion parameters estimated during motion correction.
Fixed-effects analyses were conducted for each imaging run of data from each participant, and again to combine contrast images across both runs. For within- and between-group mixed-effects analyses, all whole-brain fMRI statistics were corrected for multiple comparisons by using cluster-correction with voxel height threshold of Z > 2.3 and cluster significance of p italic> 0.05, unless otherwise noted. All analyses included sex, age, smoker status (smoker, non-smoker), and marijuana use (days used in preceding month) as nuisance covariates. Analyses of group differences in the modulation of activation by pump number were restricted to the rDLPFC and striatal ROIs (voxel height threshold of Z > 2.3 and cluster-corrected at p bold> 0.05). The interaction of group with the association of total earnings on the modulation of activation during risky decision-making in the rDLPFC ROI and whole-brain was also tested.
For resting-state analysis, images were further pre-processed to include additional nuisance regressors: average signal of cerebrospinal fluid, and two metrics of motion-related artifact, specifically frame-wise displacement and a combination of the temporal derivative of the time series and root mean squared variance over all voxels39. Global signal regression was not applied. The mean time series across all voxels within the rDLPFC and midbrain seeds from pre-processed images were used as covariates in separate whole-brain, voxel-wise correlation analyses.
Parameter estimates (average of β-values) corresponding to modulation of activation by pump number in the rDLPFC ROI were regressed against whole-brain voxel-wise maps of RSFC with rDLPFC and midbrain seeds between and within groups. First, the interaction of participant group with the associations between RSFC and modulation of activation was tested. Subsequently, the relationship between RSFC and modulation of activation during decision-making was examined within each group.
RESULTS
Task Performance
There was a significant main effect of active balloon color (red, blue) (F(1, 1,828.28)= 16.684, p < 0.001) on pumping, but no significant main effect of group (F(1, 62.413)= 0.043, p= 0.836) and no interactions. There were no significant group differences in the average number of pumps before cashing out (t = 1.342, p = 0.180: Control: 2.84 ± 1.518 (mean ± SD); Methamphetamine: 2.74 ± 1.544). A two-tailed t-test showed significant differences in overall performance (t(49) = 2.357, p = 0.022) with Controls (33.33 ± 3.83 USD) earning more than Methamphetamine subjects (30.15 ±6.65 USD).
Task-Based fMRI
During pumping, modulation of rDLPFC activation by pump number was greater in the Control group than the Methamphetamine group, but Methamphetamine subjects displayed greater modulation of ventral striatal activation than Controls (p < 0.05, cluster-corrected) (Fig. 2) in ROI analyses. In a whole-brain analysis, Controls exhibited greater modulation of activation than the Methamphetamine Group in a cluster that included and extended beyond the rDLPFC ROI (peak coordinates: x = 42, y = 40, z = 30; extent: 610 voxels; Z-statistic: 3.4, p < 0.001, whole-brain corrected). No other significant group differences in whole-brain or in mean activation were found.
Figure 2. Modulation of ventral striatal and right DLPFC activation by pump number during risky decision-making (ROI analysis).
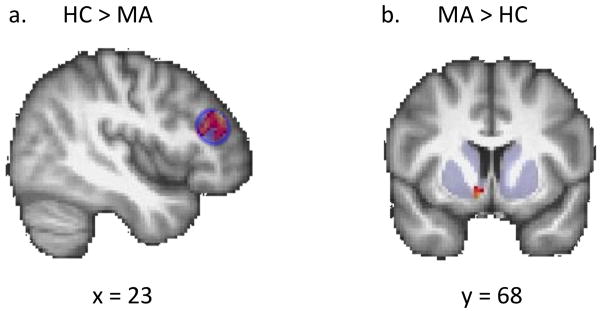
a. The Control Group exhibited greater modulation of activation by pump number in the right DLPFC during active balloon pumps compared to the Methamphetamine Group (see Methods for details of parametric modulation and ROI analyses). b. Compared to the Control Group, the Methamphetamine Group displayed greater modulation of ventral striatal activation by pump number during active balloon pumps. Statistical maps representing Z-statistic values are shown, masked by regions of interest in which statistical comparisons were confined (p < 0.05, cluster corrected). Results were controlled for age, sex, smoking status, and marijuana use.
A group interaction with monetary earnings on modulation of activation by risk was found in whole-brain but not ROI analysis. Post-hoc analyses showed a negative correlation between the amount earned and modulation of activation in bilateral anterior insula and right caudate in the Control group. Controls showed no positive correlations, and there were no positive or negative correlations in the Methamphetamine group (p < 0.05, whole-brain cluster-corrected).
RSFC and Relationship to Task-Based Activation
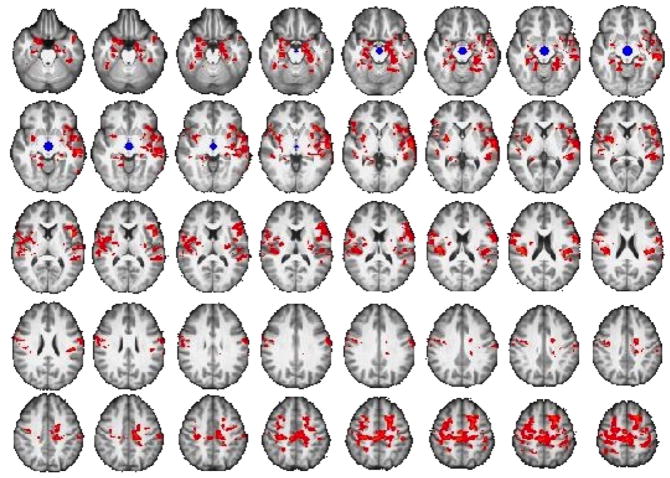
Compared with Controls, Methamphetamine subjects exhibited greater RSFC (midbrain seed) with the putamen, amygdala, hippocampus, insula, orbital, superior, and inferior frontal cortices, temporal cortices and parietal operculum (p < 0.05, whole-brain cluster-corrected) (Fig. 3, eTable 1). There were no regions where Controls exhibited greater midbrain RSFC than Methamphetamine subjects, nor were there any group differences in RSFC of the rDLPFC.
Figure 3. Comparison of mesocorticolimbic resting-state connectivity in Methamphetamine- and Control Group.
Connectivity maps show greater connectivity between midbrain seed (shown in blue) and putamen, amygdala, hippocampus, insula, and prefrontal cortex in the Methamphetamine Group compared to the healthy control group (p < 0.05, whole-brain cluster corrected) (see eTable 2 for list of regions). Results controlled for age, sex, smoking status and marijuana use.
A group interaction with the modulation of rDLPFC activation on RSFC between midbrain and putamen was found at p < 0.0005, uncorrected. Post-hoc analyses showed a negative correlation in the Methamphetamine group between modulation of rDLPFC activation during risk-taking and midbrain RSFC with orbitofrontal cortex, putamen, ventral striatum, amygdala, insula, hippocampus, anterior cingulate cortex, orbital medial and superior frontal cortices, and temporal and occipital cortices (p < 0.05, whole-brain cluster-corrected) (Fig. 4, eTable 2). Controls showed no correlations between modulation of rDLPFC activation and midbrain RSFC.
Figure 4. Relationship between resting-state connectivity of the midbrain and modulation of activation in DLPFC during risky decision-making in Methamphetamine Group.
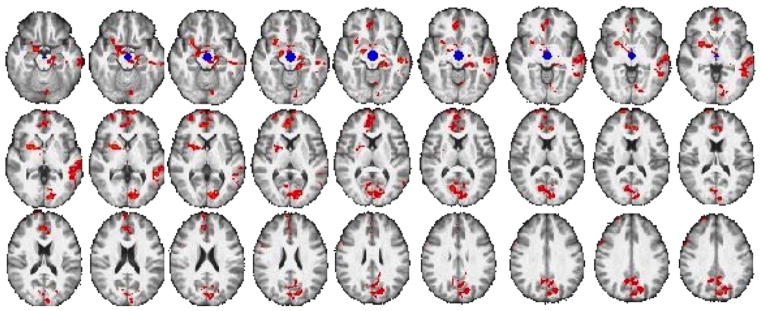
Connectivity maps show a negative correlation between modulation of activation in right DLPFC during balloon pumps and the connectivity between midbrain seed (shown in blue) and nucleus accumbens, putamen, amygdala, hippocampus, orbital frontal cortex, anterior cingulate, and superior frontal gyrus in the Methamphetamine Group (p < 0.05, whole-brain cluster corrected) (see eTable 3 for list of regions). Results controlled for age, sex, smoking status and marijuana use.
There was a significant group interaction with modulation of rDLPFC activation during risk-taking on RSFC between rDLPFC and nucleus accumbens, putamen, amygdala, hippocampus, thalamus, and orbital frontal cortex (p < 0.05, whole-brain, cluster-corrected) (Fig. 5A, eTable 3). In post-hoc analysis, modulation of rDLPFC activation during risk-taking in Controls was positively correlated with rDLPFC RSFC to ventral striatum, caudate, putamen, hippocampus, orbital, medial frontal and subcallosal cortices, insula, thalamus, paracingulate cortex, and the superior and inferior frontal gyri (p < 0.05, whole-brain cluster-corrected) (Fig. 5B, eTable 3). Methamphetamine subjects exhibited a negative correlation between modulation of rDLPFC activation during risk-taking and rDLFPC RSFC with the anterior cingulate cortex (p < 0.05, whole-brain cluster-corrected).
Figure 5. Relationship between resting-state connectivity of DLPFC and modulation of activation in DLPFC during risky decision-making.
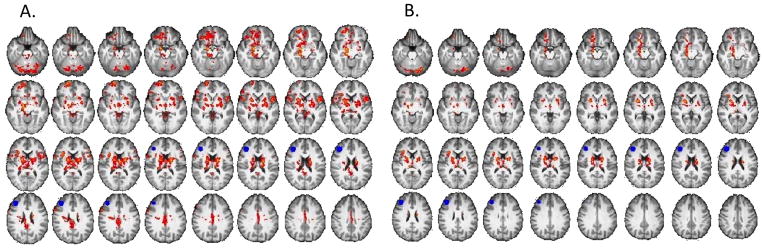
A. Brain regions where the relationship between resting-state connectivity with the DLPFC seed (shown in blue) and modulation of activation in right DLPFC by pump number varied by group. Connectivity maps show a group interaction between modulation of activation in right DLPFC during balloon pumps and RSFC of DLPFC with nucleus accumbens, putamen, amygdala, hippocampus, thalamus, orbital frontal cortex and cerebellum (p < 0.05, whole-brain cluster corrected) (see eTable 4 for list of regions). B. Post-hoc analysis within the Control Group showed a positive correlation between modulation of activation in right DLPFC during balloon pumps and RSFC of right DLFPC (show in blue) with caudate, putamen, nucleus accumbens, and orbital frontal cortex (p < 0.05, whole-brain cluster corrected) (see Table 4 for list of regions).
CONCLUSION
Methamphetamine users earned less than controls on the BART, and showed less sensitivity to risk and reward in the rDLPFC, greater sensitivity in ventral striatum, and greater mesocorticolimbic RSFC. Controls exhibited greater association between RSFC of the rDLPFC and sensitivity of the rDLPFC to risk during risky decision-making, suggesting that a deficit in rDLPFC connectivity contributes to dysfunction in methamphetamine users. These findings suggest that circuit-level abnormalities affect brain function during risky decision-making in stimulant users.
Methamphetamine users took fewer pumps than controls although this effect was not statistically significant. While risk-taking may be problematic, moderate risk-taking on the BART can be the adaptive40. Risk-aversive choices may reflect the preference for smaller but more immediate rewards over larger, later ones40, and therefore may be indicative of impulsive behavior. In line with this view, methamphetamine users previously exhibited greater temporal discounting of rewards41, 42 than controls, and reported greater impulsiveness on the Barratt Impulsiveness Scale (BIS-11)13, as did Methamphetamine subjects in this study (t = 4.491, p < 0.001 for BIS-11 total score: Control: 53.46 ± 10.24 (mean ± SD); Methamphetamine: 70.13 ± 9.27). Group differences in this study support this view, as rDLPFC activation has been related to selection of choices leading to large, future rewards despite small immediate losses whereas ventral striatal activation has been related to obtaining short-term reward43.
As modulation of activation was stronger in the ventral striatum but weaker in the rDLPFC of methamphetamine users than controls, decision-making in methamphetamine users may reflect the influence of immediate reward on behavior. Notably, the amount of earnings was negatively associated with modulation of striatal activation in control subjects. Moreover, deactivation of the medial PFC, the rodent analog of the DLPFC44, 45, promotes maladaptive risk-taking in animals46; and in humans, modulation of rDLPFC activation by risk was associated positively with earnings but negatively with striatal D2/D3 dopamine receptor availability7. The relationship between rDLPFC RSFC and modulation of rDLPFC activation in the Control but not Methamphetamine Group suggests that PFC deficits contribute to top-down impairments in stimulant dependence34. Computational models have indicated a modulatory effect of PFC on striatal activity47, 48, and that suggest PFC activity can override striatal representations of reinforcement value47. Dynamic causal modeling analyses also have shown a modulatory role of the DLPFC on nucleus accumbens activation during reward cues49. Repeated stimulant exposure, however, can alter corticostriatal synaptic activity, with reductions in extracellular glutamate50 and depression of activity in corticostriatal affents51. Taken together, these findings suggest that heightened ventral striatal but blunted rDLPFC sensitivity to risk and reward of methamphetamine subjects reflect dysregulated corticostriatal connectivity.
Greater midbrain RSFC in methamphetamine users than controls may reflect stimulant-induced sensitization as posited by the Incentive Sensitization Theory52, 53. Amphetamine-induced sensitization in rats increases neuronal firing within mesolimbic structures54, and in humans, amphetamine-induced sensitization of dopamine release can be long-lasting55. Heightened midbrain RSFC in methamphetamine users may reflect such sensitization even in the absence of reward-related stimuli. Sensitization has been studied primarily in terms of facilitating drug self-administration, conditioned place-preference and the motivation for drugs56–58. The present findings suggest more extensive effects on psychological processes, and support a link between neural dysfunction during decision-making and circuit-level abnormalities in methamphetamine dependence.
LIMITATIONS
The temporal resolution of fMRI with the BART did not completely isolate decision-making processes, such as evaluation, selection and anticipation, and tasks that provide finer resolution are needed59. This study had a priori hypotheses regarding the rDLPFC and striatum, and tested functionally connected networks, bolstering the view that the cognitive processes under study were in fact examined. Still caution is warranted to avoid making conclusions from reverse inference60. In this regard, anticipation of either reward or aversive stimuli can elicit striatal activation61,62. Therefore, the cognitive process underlying the modulation of ventral striatal activation is uncertain. Finally, as RSFC provides no directional information, it is unknown to what extent RSFC between rDLPFC and striatum reflects top-down control or spontaneous coherence of activation.
Footnotes
All authors have contributed to the scientific process leading up to the writing of the paper and have contributed to the critical review/revision of this manuscript. None of the authors have a financial relationship with any organization that sponsored this research.
References
- 1.Bechara A. Risky business: emotion, decision-making, and addiction. J Gambl Stud. 2003 Spring;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- 2.Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 3.Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992 Nov;87(11):1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 4.Gossop M, Griffiths P, Powis B, Strang J. Severity of heroin dependence and HIV risk. I. Sexual behaviour. AIDS Care. 1993;5(2):149–157. doi: 10.1080/09540129308258595. [DOI] [PubMed] [Google Scholar]
- 5.Rogers RD, Everitt BJ, Baldacchino A, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999 Apr;20(4):322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 6.Ernst M, Paulus MP. Neurobiology of Decision Making: A selective Review from a Neurocognitive and Clinical Perspective. Biological Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Kohno M, Ghahremani DG, Morales AM, et al. Risk-Taking Behavior: Dopamine D2/D3 Receptors, Feedback, and Frontolimbic Activity. Cereb Cortex. 2013 Aug 21; doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. NeuroImage. 2006 Aug 1;32(1):477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 9.Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: An fMRI Study of the Balloon Analog Risk Rask (BART) NeuroImage. 2008;42:902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.London ED, Simon SL, Berman SM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004 Jan;61(1):73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 11.Morales AM, Lee B, Hellemann G, O’Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 2012 Oct 1;125(3):230–238. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson PM, Hayashi KM, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004 Jun 30;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee B, London ED, Poldrack RA, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009 Nov 25;29(47):14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkow ND, MD, Chang L, MD, Wang G-J, MD, et al. Low Level of Brain Dopamine D2 Receptors in Methamphetamine Abusers: Association With Metabolism in the Orbitofrontal Cortex. American Journal of Psychiatry. 2001 Dec;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. 2001. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JM, Kalasinsky KS, Levey AI, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996 Jun;2(6):699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 16.Wang GJ, Smith L, Volkow ND, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2011 Sep;17(9):918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCann UD, Wong DF, Kyokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced Striatal Dopamine Transporter Density in Abstinent Methamphetamine and Methcathinone Users: Evidence from Positron Emission Tomgraphy Studies with [11C]WIN-35,428. The Journal of Neuroscience. 1998 Oct 15;18(20):8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feltenstein MW, See RE. Systems level neuroplasticity in drug addiction. Cold Spring Harb Perspect Med. 2013 May;3(5):a011916. doi: 10.1101/cshperspect.a011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007 Sep;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 21.Gowin JL, Mackey S, Paulus MP. Altered risk-related processing in substance users: imbalance of pain and gain. Drug Alcohol Depend. 2013 Sep 1;132(1–2):13–21. doi: 10.1016/j.drugalcdep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002 Jun;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 23.Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40(10):1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 24.Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002 Jan;26(1):53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 25.Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision Making by Methamphetamine-Dependent Subjects Is Associated with Error-Rate-Independent Decrease in Prefrontal and Parietal Activation. Biological Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- 26.Galvan A, Schonberg T, Mumford J, Kohno M, Poldrack RA, London ED. Greater risk sensitivity of dorsolateral prefrontal cortex in young smokers than in nonsmokers. Psychopharmacology (Berl) 2013 May 5; doi: 10.1007/s00213-013-3113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schonberg T, Fox CR, Mumford JA, Congdon E, Trepel C, Poldrack RA. Decreasing ventromedial prefrontal cortex activity during sequential risk-taking: an FMRI investigation of the balloon analog risk task. Front Neurosci. 2012;6:80. doi: 10.3389/fnins.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001 Feb;2(2):119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 29.Thomas M, Kalivas P, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British Journal of Pharmacology. 2008 Mar 17;154:327–342. doi: 10.1038/bjp.2008.77. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther. 1995 Apr;273(1):445–454. [PubMed] [Google Scholar]
- 31.Chen BT, Hopf FW, Bonci A. Synaptic plasticity in the mesolimbic system: therapeutic implications for substance abuse. Ann N Y Acad Sci. 2010 Feb;1187:129–139. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2012 Jan;38(2):259–274. doi: 10.1038/npp.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002 Oct;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999 Oct;146(4):373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 35.Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of Methylphenidate on Resting-State Functional Connectivity of the Mesocorticolimbic Dopamine Pathways in Cocaine Addiction. JAMA Psychiatry. 2013 Jun 26;:1–11. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002 Oct;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 37.Andersson J, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. FMRIB technical report. 2007 [Google Scholar]
- 38.Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. NeuroImage. 1998 Aug;8(2):140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- 39.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2011 Feb 1;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dean AC, Sugar CA, Hellemann G, London ED. Is all risk bad? Young adult cigarette smokers fail to take adaptive risk in a laboratory decision-making test. Psychopharmacology (Berl) 2011 Jun;215(4):801–811. doi: 10.1007/s00213-011-2182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006 Oct;188(2):162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 42.Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal Cortical Activity of Methamphetamine-Dependent and Comparison Subjects Performing a Delay Discounting Task. Human Brain Mapping. 2007 Aug 30;28:383–393. doi: 10.1002/hbm.20281. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004 Aug;7(8):887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 44.Preuss TM. Do rats have prefrontal cortex? The rose-woolsey-akert program reconsidered. J Cogn Neurosci. 1995 Winter;7(1):1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002 Jul;25(7):340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 46.Jentsch JD, Woods JA, Groman SM, Seu E. Behavioral characteristics and neural mechanisms mediating performance in a rodent version of the Balloon Analog Risk Task. Neuropsychopharmacology. 2010 Jul;35(8):1797–1806. doi: 10.1038/npp.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doll BB, Jacobs WJ, Sanfey AG, Frank MJ. Instructional control of reinforcement learning: a behavioral and neurocomputational investigation. Brain Res. 2009 Nov 24;1299:74–94. doi: 10.1016/j.brainres.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frank MJ. Computational models of motivated action selection in corticostriatal circuits. Curr Opin Neurobiol. 2011 Jun;21(3):381–386. doi: 10.1016/j.conb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci. 2011 Jul 13;31(28):10340–10346. doi: 10.1523/JNEUROSCI.0895-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker DA, McFarland K, Lake RW, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003 Jul;6(7):743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 51.Bamford NS, Zhang H, Joyce JA, et al. Repeated exposure to methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron. 2008 Apr 10;58(1):89–103. doi: 10.1016/j.neuron.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993 Sep-Dec;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 53.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008 Oct 12;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tindell AJ, Berridge KC, Zhang J, Pecina S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005 Nov;22(10):2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- 55.Boileau I, Dagher A, Leyton M, et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006 Dec;63(12):1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- 56.Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 1989;98(3):357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- 57.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004 Jan;27(8):827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Ward SJ, Lack C, Morgan D, Roberts DC. Discrete-trials heroin self-administration produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology (Berl) 2006 Apr;185(2):150–159. doi: 10.1007/s00213-005-0288-9. [DOI] [PubMed] [Google Scholar]
- 59.Schonberg T, Fox CR, Poldrack RA. Mind the gap: bridging economic and naturalistic risk-taking with cognitive neuroscience. Trends Cogn Sci. 2011 Jan;15(1):11–19. doi: 10.1016/j.tics.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci. 2006 Feb;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001 Aug 15;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003 Dec 18;40(6):1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]