Abstract
Chronic pain is a major symptom in patients with endometriosis, a common gynecologic condition affecting women in their reproductive years. While many pro-algesic substances are produced by endometriosis lesions, experimental evidence supporting their relative roles is still lacking. Furthermore, it is unclear whether these pro-algesic agents directly activate nociceptors to induce endometriosis pain. To determine their relative contribution to pain associated with endometriosis we evaluated the intrathecal administration of oligodeoxynucleotides antisense to mRNA for receptors for three pro-nociceptive mediators known to be produced by ectopic endometrium. Two weeks after the implant of autologous uterine tissue onto the gastrocnemius muscle, local mechanical hyperalgesia was observed in operated rats. Intrathecal antisense oligodeoxynucleotides (AS ODN) targeting mRNA for the interleukin 6 receptor-signaling complex subunit glycoprotein 130 and the NGF tyrosine kinase receptor A (TrkA), but not their mismatch ODNs, reversibly attenuated mechanical hyperalgesia at the implant site. In contrast, intrathecal AS ODN targeting the tumor necrosis factor receptor 1 (TNFR1), at a dose that markedly inhibited intramuscularly injected TNFα had only a small antihyperalgesic effect in this model. These results indicate the relative contribution of pronociceptive mediators produced by ectopic endometrial tissue to endometriosis pain. The experimental approach presented here provides a novel method to evaluate for the differential contribution of mediators produced by other painful lesions as well as endometriosis solutions lesions as targets for novel treatment of pain syndromes.
Perspective
This article presents evidence for the relative contribution of pro-algesic mediators to primary hyperalgesia displayed by rats submitted to a model of endometriosis pain. This approach can be used to identify potential targets for the treatment of endometriosis pain.
Keywords: Antisense, hyperalgesia, chronic pain models, nociceptor
Introduction
Endometriosis is a common and very disabling clinical condition affecting women in their reproductive years which is characterized by the presence of endometrial tissue and glands outside of the uterine cavity 24. Chronic pelvic pain is the main symptom reported by women with endometriosis, typically evoked by mechanical stimuli, (e.g., dysmenorrhea, dyschezia, dysuria and dyspareunia) 24, 54. This pain is often resistant to available analgesic treatments, presumably because they are not directed to specific mediators that induce endometriosis pain.
Given the complex pathophysiology of endometriosis, several mediators appear as possible candidates underlying endometriosis pain 24. While many attempts have been made to unveil the role of these pro-algesic mediators in endometriosis pain, the available studies are correlative, direct evidence for the involvement of candidate mediators as well as side-by-side comparison of their contribution is still lacking. Furthermore, it is unclear whether these pro-nociceptive mediators directly activate nociceptors innervating endometriosis lesions or act indirectly on other cell types to induce endometriosis pain. For instance, pro-inflammatory cytokines such as tumor necrosis alpha (TNFα), interleukin 6 (IL-6) and monocyte chemoattractant protein 1 have been observed in plasma and peritoneal fluid of patients suffering endometriosis pain 42, 50, 56 and all of them also induce primary mechanical hyperalgesia after local injection 12, 18, 19, 47. Neurotrophins such as nerve growth factor (NGF) are also produced in endometriosis lesions 4, 6-8 and NGF putative receptors, namely tyrosine kinase receptor A (TrkA) and p75, are expressed in nerve fibers innervating endometriosis lesions 62. The TrkA receptor plays a well-established role in mechanical inflammatory and neuropathic hyperalgesia 39.
Reversible knock-down of receptors in nociceptors by intrathecal administration of antisense (AS) oligodeoxynucleotides (ODN) has been shown to be a reliable tool to assess their role in the processing of nociceptive information 57. Injected intrathecally, AS ODN reach the soma of sensory neurons 11, 31, 32, 57 and selectively inhibit the expression of proteins in these cells 28, 30, 59 and peripheral fibers 29, 30, 59. Taking advantage of this approach, we assessed the contribution of IL-6, TNFα and NGF, which are pronociceptive mediators produced by endometriosis lesions 4, 6-8 and surgical rat models of endometriosis 68, by intrathecally administering to rats previously submitted to a model of endometriosis pain AS ODNs targeting mRNA of the TNF receptor 1 (TNFR1), the IL-6 receptor-signaling complex subunit glycoprotein 130 (gp130) and the tyrosine kinase receptor A (TrkA). Since the putative ligands for these receptors have been reported to induce a local mechanical hyperalgesia which is sensitive to intrathecal antisense treatment 18, 19, 39, 47, we evaluated their contribution to mechanical hyperalgesia observed in a surgical model of endometriosis by activating nociceptors innervating the endometriosis-like lesion.
Material and methods
Animals
Adult female Sprague Dawley rats (220–240 g; Charles River, Hollister, CA) were used in these experiments. They were housed in the Animal Care Facility at the University of California San Francisco, under environmentally controlled conditions (lights on 07:00–19:00 h; room temperature 21–23°C) with food and water available ad libitum. Upon completion of experiments, rats were killed by pentobarbital overdose followed by cervical dislocation. Animal care and use conformed to NIH guidelines (NIH Guide for the Care and Use of Laboratory Animals). The University of California San Francisco Committee on Animal Research approved all experimental protocols. Concerted effort was made to minimize number and suffering of experimental animals, in accordance with the principle of the minimal sample size stated in the Ethical Guidelines for investigations of Experimental Pain in Conscious Animals 69.
Surgical induction of endometriosis
Details of the model of surgically-induced muscle endometriosis used here have been previously described 2. We have previously provided evidence that the implant of autologous uterine tissue onto the gastrocnemius muscle, but not control surgical procedures, induces long lasting mechanical hyperalgesia 1, 2. We used the same surgical procedure to implant the ectopic uterine tissue and behavioral nociceptive evaluation here. We 2 and others 10, 41 have provided evidence that lesions developed from ectopic uterine implants are innervated by nociceptors arising from the receptor tissue. Furthermore, these nociceptors display markers typically observed in nociceptors of the receptor tissue, indicating a neo-innervation of the ectopic tissue by these primary nociceptive afferents 2, 10, 41. Briefly, female rats were pre-medicated with a mixture of ketamine hydrochloride and xylazine (80 and 6 mg /kg, s.c., respectively) and anesthesia was maintained with isoflurane (1 -1.5% in 99-98.5% oxygen). The right dorsal paravertebral area was infiltrated with 0.25% bupivacaine (Marcaine®, Hospira, Lake Forest, IL) and, under aseptic conditions, an incision approximately 2 cm in length was performed and to expose and isolate the right uterine horn. After ligature of uterine blood vessels, a 1 cm segment was removed and immediately placed in a Petri dish containing 0.9% NaCl. The musculature of the dorsal abdominal wall was closed with single crossed stitches and the skin incision closed with horizontal mattress stitches. The excised uterine tissue was measured with a millimeter scale and opened longitudinally; a full thickness 3 × 3 mm square of uterine tissue was then removed and kept in physiologic saline. The implant was performed through an incision in the biceps femoris muscle allowing exposure of the underlying gastrocnemius muscle. The square of uterine tissue was sutured to the surface of the gastrocnemius muscle applying three to four single stitches using 5-0 nylon with the endometrial portion of the uterine tissue contacting the gastrocnemius muscle. After checking for hemostasis, the b. femoris muscle and the skin incisions were sutured separately with single stitches. The sham surgical procedure was similar but the uterus was left intact and not implanted on the surface of the gastrocnemius. Surgical procedures were performed regardless of the estrous cycle status of the rats.
Determination of estrous cycle phases
The phase of the estrous cycle was assessed pre-operatively, as reported previously (Alvarez et al., 2012). Immediately after the induction of anesthesia for surgical implant of ectopic endometrium, 30 μl of NaCl 0.9% was flushed 3-4 times into the vaginal cavity. The resulting fluid was then placed onto a slide and observed unstained at 100× magnification. The diagnostic criteria used to determine the phase of the estrous cycle was based on cellular type predominance, as previously described 40.
Measurement of hyperalgesia at the site of endometriosis lesion
Mechanical nociceptive threshold in the site of surgical intervention was quantified using a digital force transducer (Chatillon DFI2; Amtek Inc., Largo, FL, USA) with a custom-made 7 mm-diameter probe 3. The use of a probe with a tip diameter ≥ 2.6 mm allows reliable measurements of mechanical nociceptive threshold in subcutaneous tissue, even when overlying cutaneous hyperalgesia is present 45. Rats were lightly restrained in a cylindrical acrylic holder with lateral slats that allows for easy access to the hind limb and application of the force transducer probe to the site of implantation, in the belly of the gastrocnemius muscle. The nociceptive threshold was defined as the force, in milliNewtons, required to produce a flexion reflex in the hind leg. Baseline withdrawal threshold was defined as the mean of 3 readings taken at 5-min intervals.
Intrathecal injections
Rats were briefly anesthetized with 2.5% isoflurane in 97.5% O2. Then, a 30-gauge hypodermic needle was inserted into the subarachnoid space, on the midline, between the L4 and L5 vertebrae and the injection of ODN performed (20 μl). Proper intrathecal injections were systematically confirmed by checking for a sudden flicking of the tail 43.
Antisense oligodeoxynucleotides
To determine the contribution of IL-6 produced by the endometriosis lesion, its effect on nociceptors was disrupted by attenuating the expression of the signal transducing molecule glycoprotein 130 (gp130), a subunit of the IL-6 receptor signaling complex, which is necessary for IL-6 receptor function 59. The AS ODN sequence, 5’-TCC TTCCCACCTTCTTCT G-3’, was directed against a unique sequence of rat gp130 mRNA. The corresponding GenBank accession number and ODN position within the cDNA sequence are M92340 and 1834–1852, respectively 63. The mismatch (MM) ODN sequence, 5’- TACTACTCACATTCATCA G-3’, corresponds to the gp130 subunit antisense sequence with six mismatched bases (denoted by bold letters). The dose of ODN (80 μg) was based on prior studies showing decreases in gp130 protein in nociceptors 59 and completely attenuated the IL-6 induced mechanical hyperalgesia 18.
To determine the contribution to endometriosis pain of TNFα produced by the endometriosis lesion, knockdown of the expression of TNF receptor type-1 (TNFR1) in nociceptors was performed. The antisense oligodeoxynucleotide (AS ODN) sequence 5’-ACACGGTGTTCTGTTTCTCC-3’ directed against a unique sequence of rat TNFR1 was used. The mismatch ODN (MM ODN) sequence, 5’- ACCCGTTGTTCGGTTGCTCC-3’, with four bases mismatched (denoted by bold face). We have previously shown that this AS ODN against TNFR1, at a dose of 80 μg, markedly decreases TNFR1 protein in nociceptors 47 and completely prevents mechanical hyperalgesia induced by administration of TNFα into peripheral tissues 19.
To determine the contribution of NGF produced by the endometriosis lesion, its effect on nociceptors was disrupted by attenuating the expression of TrkA. The antisense ODN sequence, 5′-CATCAACGAAGTCACCAGACCG-3′ was directed against a unique sequence of rat TrkA. The corresponding GenBank accession number and ODN position within the cDNA sequence are M85214 and 121–142, respectively. The mismatch ODN sequence, 5′-CAACATCGAAGTGACGAGACCG-3′, corresponds to the TrkA subunit antisense sequence with four bases mismatched (denoted by bold). A search of the NCBI database to Rattus norvegicus identified no other sequences homologous to that used in this experiment. We have previously shown that intrathecal administration of TrkA AS completely eliminated NGF-induced mechanical hyperalgesia 39.
Rats were intrathecally injected daily, with either AS or MM ODN (80 μg) against TNFR1, gp130 or TrkA mRNA for 3 consecutive days. The AS- and MM ODN primers were purchased from Invitrogen (San Francisco, CA).
Statistical analysis
A paired Student’s t-test (two tailed) was used to compare pre and post-operative mechanical nociceptive threshold. The analysis of the effect of intrathecal treatment was made by means of a two-way repeated measures analysis of variance (ANOVA), with one within subjects-factor (time) and one between-subjects factor (intrathecal treatment with two levels, AS ODN or MM ODN). If the ANOVA showed a significant interaction, Bonferroni’s multiple comparisons tests were performed to determine the basis of the differences. Graph Pad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA) was used to plot the graphics and to perform the statistical analysis. Data were plotted as mean ± S.E.M and statistical significance was set at P < 0.05.
Results
Surgical induction of endometriosis-like lesions
All rats recovered uneventfully from the surgery to implant uterine tissue onto the gastrocnemius muscle and developed cystic lesions, by our previously reported technique 2. These changes were observed regardless of their estrus cycle status at the time of surgery. Fourteen days after surgery, the mechanical nociceptive threshold at the site of implanted ectopic uterine tissue was markedly decreased by 56.7 ± 0.8% compared to pre-surgical baseline (1100 ± 19.6 mN versus 2541 ± 5.6 mN, respectively; n = 38, P < 0.0001, Fig. 1A-C).
Figure 1.
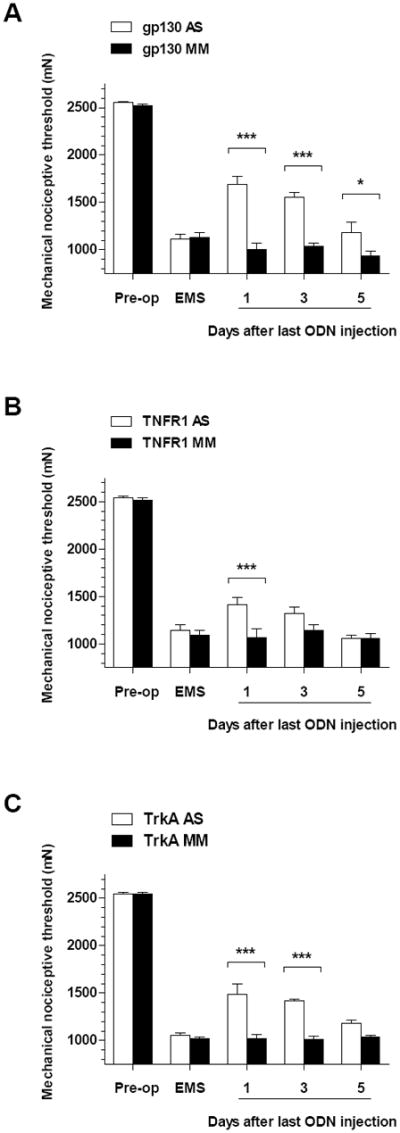
Effect of intrathecally (i.t.) administered antisense (AS, open bars) or mismatch (MM, solid bars) oligodeoxynucleotides (ODN) on mechanical hyperalgesia exhibited by rats submitted to a model of endometriosis pain induced by implanting uterine tissue on the gastrocnemius muscle (Alvarez et al., 2012). Two weeks after surgical implant of autologous uterine tissue, rats displayed a marked mechanical hyperalgesia (EMS, endometriosis pain model), compared to pre-operative baseline (Pre-op). The effects of i.t. AS or MM ODN (80 μg) on mechanical hyperalgesia at the site of surgical implant were assessed every other day up to day 5 after last ODN injection. (A) Effect of AS (n = 8) or MM (n = 6) ODN directed against mRNA for the glycoprotein 130 (gp130) subunit of the IL-6 receptor signaling complex. (B) Effect of AS (n = 8) or MM (n = 6) ODN directed against mRNA for the tumor necrosis receptor 1 (TNFR1). (C) Effect of AS (n = 5) or MM (n = 5) ODN directed against mRNA for the NGF tyrosine kinase receptor A (TrkA). ***P < 0.001; *P < 0.05.
Effect of antisense ODN against IL-6 receptor subunit gp130
The contribution of the IL-6 released by ectopic uterine tissue in the endometriosis pain model was assessed by intrathecal injection of the AS (or the respective MM) ODN targeting the gp130 signaling subunit of the IL-6 receptor, for 3 consecutive days. Two-way repeated measures ANOVA showed significant effects for ODN treatment × time interaction (F = 21.23; DFn = 4, DFd = 48, P < 0.0001), main effect of the ODN treatment (F = 20.17; DFn = 1, DFd = 48, P = 0.0007) and time (F = 329.7; DFn = 4, DFd =48, P <0.0001). Multiple comparisons analysis showed that mechanical hyperalgesia at the implant site was significantly attenuated by the AS-compared to the MM-ODN treatment on days 1 (1693 ± 77.1 mN [n=8] versus 1004.3 ± 62.8 mN [n=6], respectively; P < 0.001) through 5 (1182 ± 109.3 mN [n=8] versus 937 ± 49.5 mN [n=6], respectively; P < 0.05) after last injection of ODN (Fig. 1A).
Effect of antisense ODN against TNFR1
The contribution of TNFα released by ectopic uterine tissue in the endometriosis pain model was evaluated by injecting intrathecally the AS (or the respective MM) ODN targeting the TNFR1 for 3 consecutive days. The two-way repeated measures ANOVA showed a significant group x time interaction (F = 5.28; DFn = 4, DFd = 48, P = 0.0013) and time (F = 383.13; DFn = 4, DFd =48, P <0.0001), but not for main effect of ODN treatment (F = 3.85; DFn = 1, DFd = 48, P < 0.0734). Multivariate analysis showed that mechanical hyperalgesia at the site of implant was significantly attenuated by the AS, but not by MM, only at the day 1 time point (1416 ± 60.7 mN [n=8] versus 1068 ± 91.4 mN [n=6], respectively; P < 0.05) after the last ODN injection (Fig. 1B).
Effect of antisense ODN against TrkA
Finally, the contribution of NGF at the site of the ectopic uterine tissue was evaluated by injecting intrathecally an AS (or the respective MM) ODN targeting the TrkA receptor, for 3 consecutive days. The two way repeated measures ANOVA showed a significant group x time interaction (F = 11.70; DFn = 4, DFd = 32, P < 0.0001), main effect of group (F = 54.56; DFn = 1, DFd = 32, P < 0.0001) and time (F = 402.96; DFn = 4, DFd = 32, P < 0.0001). Multivariate analysis showed that mechanical hyperalgesia at the site of implant was significantly attenuated by the AS, but not by MM, treatment on days 1 (1483.6 ± 115.6 mN [n=5] versus 1020.4 ± 45.6 mN [n=5], respectively; P < 0.001) through 3 (1419 ± 20 mN [n=8] versus 1012.4 ± 29.9 mN [n=5], respectively; P < 0.001) after last ODN injection (Fig. 1C).
Discussion
The pain associated with the growth of ectopic endometrium in patients with endometriosis is extremely common and can be severely disabling. Moreover, it is often resistant to available analgesic treatments, which in part reflects our lack of knowledge of underlying mechanisms mediating the pain of endometriosis. Therefore, in the present study we evaluated the involvement of mediators produced by endometriotic lesions in a preclinical model of endometriosis pain 2, to determine inhibitors of which mediators might be useful leads for the development of novel mechanism-based analgesic strategies.
The clinical characteristics of endometriosis pain suggest that mechanical hyperalgesia is the main underlying mechanism mediating pain in patients with this condition characterized by the presence of dysmenorrhea, dyspareunia, dysuria and dyschezia 24, 54, consistent with mechanically-evoked pain observed during intraoperatory exploration 34. Surgical removal of endometriosis lesions provides an important degree of pain relief 66, indicating an important role for primary mechanical hyperalgesia in endometriosis pain. In our initial description of the endometriosis pain model 2 we described the marked similarity between lesions derived from our ectopic uterine tissue model and clinical lesions of endometriosis. Indeed, these explants exhibit not only epithelial and stromal endometrial cells, and typical uterine structures (endometrial glands), but also display molecular markers (cytokeratin 18, vimentin) reported in human cases of endometriosis 2. Furthermore, clinical cases of endometriosis affecting skeletal muscle are well reported 13, 22, 23, 35.
Therefore, the assessment of local mechanical hyperalgesia in our model of endometriosis pain is a pertinent approach, allowing the exploration of mediators and mechanisms underlying pain at the site of lesion induced by implanting uterine tissue at an ectopic site 2.
Nociceptors as key players in endometriosis pain
Nociceptors are posited to play an important role in persistent pain by interacting with the sources of proalgesic mediators 53. Importantly, lesions developing from ectopic uterine tissue are innervated by local nociceptors 2, 41, 68, which express many receptors for mediators involved in mechanical nociception 62, 68. And, surgical excision of lesions, whose stimulation exacerbates pain, produces clinical alleviation of pain 66.
Since most of the proteins required by nociceptors are synthetized in their somas 49, the intrathecal administration of AS ODN can be used to inhibit, reversibly, the expression of specific receptors 57 and prevents its peripheral role in nociception 19, 29, 30, 59. In the present experiments we used this approach to assess the role of pronociceptive mediators released from endometriosis-like lesions, in the mechanical hyperalgesia of a rodent model of endometriosis 2.
Involvement of IL-6 in endometriosis pain
Increased levels of IL-6 in peritoneal fluid of endometriosis patients 20, 51, 64, and in lesions 61 or peritoneal fluid 15 of rodents submitted to models of endometriosis have been reported. These observations are in good agreement with our finding that knock-down of the gp130 subunit of the IL-6 receptor signaling complex produced a marked inhibition of the mechanical hyperalgesia exhibited in rats submitted to our model of endometriosis pain. Indeed, IL-6 plays a well-established role in mechanical nociception by acting at its receptor on nociceptors to sensitize them, in many different tissues 18, 37, 59, 67. Furthermore, the signaling subunit gp130 is critical for the sensitizing effect of IL-6 on nociceptors 5, 52, which is consistent with the antihyperalgesic effect produced by the intrathecal administration of AS directed against gp130 observed here.
Involvement of TNFα in endometriosis pain
Increased levels of TNFα in peritoneal fluid of patients with endometriosis have also been observed 9, 21, 56, which have been reported to be correlated with pain 56 but not with the type of endometriosis lesions 16, suggesting a role in endometriosis pain. Moreover, local injections of TNFα produce persistent mechanical hyperalgesia in rodents 47 and marked inflammatory reaction in humans 25. Thus, the finding that AS ODNs targeting TNFR1 produced only mild and short lasting inhibition of the mechanical hyperalgesia in our model of endometriosis pain is somewhat surprising. An insufficient knockdown of TNFR1 is unlikely to explain such a lack of efficacy, since previous studies using the same sequence at doses even lower have consistently shown the inhibition of the expression of TNFR1 in peripheral nociceptors 47 and marked attenuation of mechanical hyperalgesia induced by injection of TNFα into peripheral tissue 19. It is still possible that TNFα produces pro-algesic effects in endometriosis by indirect mechanisms, such as increasing the release of NGF 65. Of note, clinical trials using anti-TNFα monoclonal antibodies have also shown a lack of efficacy for the relief of endometriosis pain 33, 38 but, since clinical experience targeting TNFα is still limited, it cannot be excluded as a player in endometriosis pain.
Involvement of NGF in endometriosis pain
Transient knock-down of the high affinity NGF receptor TrkA produced a marked attenuation of mechanical hyperalgesia at the site of endometriosis-like lesions. This indicates that NGF released from the endometriosis-like lesion, likely contributes to the mechanical hyperalgesia exhibited by rats submitted to our endometriosis pain model. This is in line with previous reports about the expression of TrkA in nerve fibers innervating both, endometriotic lesions in humans 62 and endometriosis-like lesions in rats 68. On the other hand, increased levels of NGF in peritoneal fluid of women affected by endometriosis have been reported 7, 8. And, exposure to peritoneal fluid of endometriosis patients produces neurite outgrowth in isolated dorsal root ganglion preparations, which is inhibited by an anti-NGF antibody 7. While local injection of NGF has consistently shown to produce mechanical hyperalgesia in humans 17, 55, 60 and animals 26, 39, 44, the expression of NGF in the peritoneal fluid of patients with endometriosis seems not to be proportional to pain levels 8. Such a lack of correlation between pain and local NGF levels has been observed in humans 46 and animals 27 suffering from osteoarthritis, a condition where the pain is sensitive to monoclonal antibodies directed against NGF 14. This might indicate that NGF acts synergistically with other pro-algesic mediators, its local levels not necessarily being representative of its full involvement in endometriosis pain. Equally, the potential role of non-canonical ligands acting on TrkA receptor cannot be ruled out in the nociceptive responses explored and its importance in the effect observed in the antisense TrkA knock-down experiments. For instance, it has been shown that some structurally diverse ligands generated in vivo can specifically interact with the same receptor to regulate inflammation 48. Indeed, other neurotrophins than NGF, such as neurotrophins 3 and 4, can elicit some activation of TrkA 36.
In summary, changes in mechanical hyperalgesia observed after the knock-down of gp130, TrkA and TNFR1 in nociceptors of rats submitted to a model of endometriosis pain suggest that IL-6 and NGF, but not TNFα, play a major role in endometriosis pain. This experimental approach might be useful for the identification of the contribution of additional putative putative proalgesic mediators in endometriosis pain, as well as of proalgesic mediators in other pain syndromes in which there is thought to be a major contribution of pronociceptive mediators generated at sites at which patients experience pain (e.g., arthritis, colitis, cystitis and myositis). Finally, this approach could be used to validate variations on an established model such as comparing the important pronociceptive mediators in the present novel model of endometriosis in which the ectopic endometrial lesion is growing extraperitoneally in skeletal muscle with mediators responsible for pain the well-established intraperitoneal endometriosis model 41, 58.
Acknowledgments
This study was funded by the National Institutes of Health (NIH).
Abbreviations
- AS
antisense
- gp130
glycoprotein 130 signal transducing subunit of the IL-6 receptor signaling complex
- IL-6
interleukin 6
- i.t.
intrathecal
- MM
mismatch
- NGF
nerve growth factor
- ODN
oligodeoxynucleotide
- TNFα
tumor necrosis alpha
- TNFR1
tumor necrosis receptor type 1
- TrkA
tyrosine kinase receptor A
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alvarez P, Bogen O, Chen X, Giudice LC, Levine JD. Ectopic endometrium-derived leptin produces estrogen-dependent chronic pain in a rat model of endometriosis. Neuroscience. 2014;258:111–120. doi: 10.1016/j.neuroscience.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez P, Chen X, Hendrich J, Irwin JC, Green PG, Giudice LC, Levine JD. Ectopic uterine tissue as a chronic pain generator. Neuroscience. 2012;225:269–282. doi: 10.1016/j.neuroscience.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. The European journal of neuroscience. 2010;32:819–825. doi: 10.1111/j.1460-9568.2010.07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anaf V, Simon P, El Nakadi I, Fayt I, Simonart T, Buxant F, Noel JC. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002;17:1895–1900. doi: 10.1093/humrep/17.7.1895. [DOI] [PubMed] [Google Scholar]
- 5.Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, Quarta S, Vogl C, Sailer CA, Uceyler N, Brockhaus J, Martini R, Sommer C, Zeilhofer HU, Müller W, Kuner R, Davis JB, Rose-John S, Kress M. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13473–13483. doi: 10.1523/JNEUROSCI.1822-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barcena de Arellano ML, Arnold J, Lang H, Vercellino GF, Chiantera V, Schneider A, Mechsner S. Evidence of neurotrophic events due to peritoneal endometriotic lesions. Cytokine. 2013;62:253–261. doi: 10.1016/j.cyto.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Barcena de Arellano ML, Arnold J, Vercellino F, Chiantera V, Schneider A, Mechsner S. Overexpression of nerve growth factor in peritoneal fluid from women with endometriosis may promote neurite outgrowth in endometriotic lesions. Fertility and sterility. 2011;95:1123–1126. doi: 10.1016/j.fertnstert.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Barcena de Arellano ML, Arnold J, Vercellino GF, Chiantera V, Ebert AD, Schneider A, Mechsner S. Influence of nerve growth factor in endometriosis-associated symptoms. Reprod Sci. 2011;18:1202–1210. doi: 10.1177/1933719111410711. [DOI] [PubMed] [Google Scholar]
- 9.Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, Agarwal A. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002;17:426–431. doi: 10.1093/humrep/17.2.426. [DOI] [PubMed] [Google Scholar]
- 10.Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11094–11098. doi: 10.1073/pnas.0403663101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilsky EJ, Bernstein RN, Pasternak GW, Hruby VJ, Patel D, Porreca F, Lai J. Selective inhibition of [D-Ala2, Glu4]deltorphin antinociception by supraspinal, but not spinal, administration of an antisense oligodeoxynucleotide to an opioid delta receptor. Life sciences. 1994;55:PL37–43. doi: 10.1016/0024-3205(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 12.Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 induced hyperalgesia on the isolectin B4-binding protein versican. Neuroscience. 2009;159:780–786. doi: 10.1016/j.neuroscience.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Botha AJ, Halliday AE, Flanagan JP. Endometriosis in gluteus muscle with surgical implantation. A case report. Acta orthopaedica Scandinavica. 1991;62:497–499. doi: 10.3109/17453679108996657. [DOI] [PubMed] [Google Scholar]
- 14.Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. The journal of pain : official journal of the American Pain Society. 2012;13:790–798. doi: 10.1016/j.jpain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Cao X, Yang D, Song M, Murphy A, Parthasarathy S. The presence of endometrial cells in the peritoneal cavity enhances monocyte recruitment and induces inflammatory cytokines in mice: implications for endometriosis. Fertility and sterility. 2004;82(Suppl 3):999–1007. doi: 10.1016/j.fertnstert.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 16.D’Hooghe TM, Xiao L, Hill JA. Cytokine profiles in autologous peritoneal fluid and peripheral blood of women with deep and superficial endometriosis. Archives of gynecology and obstetrics. 2001;265:40–44. doi: 10.1007/s004040000126. [DOI] [PubMed] [Google Scholar]
- 17.Deising S, Weinkauf B, Blunk J, Obreja O, Schmelz M, Rukwied R. NGF-evoked sensitization of muscle fascia nociceptors in humans. Pain. 2012;153:1673–1679. doi: 10.1016/j.pain.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibration-induced chronic musculoskeletal pain analyzed in the rat. The journal of pain : official journal of the American Pain Society. 2010;11:369–377. doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drosdzol-Cop A, Skrzypulec-Plinta V. Selected cytokines and glycodelin A levels in serum and peritoneal fluid in girls with endometriosis. The journal of obstetrics and gynaecology research. 2012;38:1245–1253. doi: 10.1111/j.1447-0756.2012.01860.x. [DOI] [PubMed] [Google Scholar]
- 21.Eisermann J, Gast MJ, Pineda J, Odem RR, Collins JL. Tumor necrosis factor in peritoneal fluid of women undergoing laparoscopic surgery. Fertility and sterility. 1988;50:573–579. doi: 10.1016/s0015-0282(16)60185-1. [DOI] [PubMed] [Google Scholar]
- 22.Fambrini M, Andersson KL, Campanacci DA, Vanzi E, Bruni V, Buccoliero AM, Pieralli A, Livi L, Scarselli G. Large-muscle endometriosis involving the adductor tight compartment: case report. Journal of minimally invasive gynecology. 2010;17:258–261. doi: 10.1016/j.jmig.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Giannella L, La Marca A, Ternelli G, Menozzi G. Rectus abdominis muscle endometriosis: case report and review of the literature. The journal of obstetrics and gynaecology research. 2010;36:902–906. doi: 10.1111/j.1447-0756.2010.01236.x. [DOI] [PubMed] [Google Scholar]
- 24.Giudice LC. Clinical practice. Endometriosis. The New England journal of medicine. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groves RW, Allen MH, Ross EL, Barker JN, MacDonald DM. Tumour necrosis factor alpha is proinflammatory in normal human skin and modulates cutaneous adhesion molecule expression. The British journal of dermatology. 1995;132:345–352. doi: 10.1111/j.1365-2133.1995.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 26.Hoheisel U, Reuter R, de Freitas MF, Treede RD, Mense S. Injection of nerve growth factor into a low back muscle induces long-lasting latent hypersensitivity in rat dorsal horn neurons. Pain. 2013 doi: 10.1016/j.pain.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Isola M, Ferrari V, Miolo A, Stabile F, Bernardini D, Carnier P, Busetto R. Nerve growth factor concentrations in the synovial fluid from healthy dogs and dogs with secondary osteoarthritis. Veterinary and comparative orthopaedics and traumatology: VCOT. 2011;24:279–284. doi: 10.3415/VCOT-10-04-0051. [DOI] [PubMed] [Google Scholar]
- 28.Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132:67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Joseph EK, Green PG, Bogen O, Alvarez P, Levine JD. Vascular endothelial cells mediate mechanical stimulation-induced enhancement of endothelin hyperalgesia via activation of P2X2/3 receptors on nociceptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2849–2859. doi: 10.1523/JNEUROSCI.3229-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khasar SG, Gold MS, Dastmalchi S, Levine JD. Selective attenuation of mu-opioid receptor-mediated effects in rat sensory neurons by intrathecal administration of antisense oligodeoxynucleotides. Neuroscience letters. 1996;218:17–20. doi: 10.1016/0304-3940(96)13111-6. [DOI] [PubMed] [Google Scholar]
- 32.Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neuroscience letters. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- 33.Koninckx PR, Craessaerts M, Timmerman D, Cornillie F, Kennedy S. Anti-TNF-alpha treatment for deep endometriosis-associated pain: a randomized placebo-controlled trial. Hum Reprod. 2008;23:2017–2023. doi: 10.1093/humrep/den177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koninckx PR, Renaer M. Pain sensitivity of and pain radiation from the internal female genital organs. Hum Reprod. 1997;12:1785–1788. doi: 10.1093/humrep/12.8.1785. [DOI] [PubMed] [Google Scholar]
- 35.Lipscomb GH, Givens VM, Smith WE. Endometrioma occurring in abdominal wall incisions after cesarean section. The Journal of reproductive medicine. 2011;56:44–46. [PubMed] [Google Scholar]
- 36.Longo FM, Massa SM. Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nature reviews Drug discovery. 2013;12:507–525. doi: 10.1038/nrd4024. [DOI] [PubMed] [Google Scholar]
- 37.Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. The journal of pain : official journal of the American Pain Society. 2007;8:127–136. doi: 10.1016/j.jpain.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Lu D, Song H, Shi G. Anti-TNF-alpha treatment for pelvic pain associated with endometriosis. The Cochrane database of systematic reviews. 2013;3 doi: 10.1002/14651858.CD008088.pub3. CD008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. The European journal of neuroscience. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- 40.Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Brazilian journal of biology = Revista brasleira de biologia. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 41.McAllister SL, Dmitrieva N, Berkley KJ. Sprouted innervation into uterine transplants contributes to the development of hyperalgesia in a rat model of endometriosis. PloS one. 2012;7:e31758. doi: 10.1371/journal.pone.0031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKinnon B, Bersinger NA, Wotzkow C, Mueller MD. Endometriosis-associated nerve fibers, peritoneal fluid cytokine concentrations, and pain in endometriotic lesions from different locations. Fertility and sterility. 2012;97:373–380. doi: 10.1016/j.fertnstert.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. Journal of pharmacological and toxicological methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 44.Mills CD, Nguyen T, Tanga FY, Zhong C, Gauvin DM, Mikusa J, Gomez EJ, Salyers AK, Bannon AW. Characterization of nerve growth factor-induced mechanical and thermal hypersensitivity in rats. Eur J Pain. 2013;17:469–479. doi: 10.1002/j.1532-2149.2012.00202.x. [DOI] [PubMed] [Google Scholar]
- 45.Nasu T, Taguchi T, Mizumura K. Persistent deep mechanical hyperalgesia induced by repeated cold stress in rats. EurJ Pain. 2010;14:236–244. doi: 10.1016/j.ejpain.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Orita S, Koshi T, Mitsuka T, Miyagi M, Inoue G, Arai G, Ishikawa T, Hanaoka E, Yamashita K, Yamashita M, Eguchi Y, Toyone T, Takahashi K, Ohtori S. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC musculoskeletal disorders. 2011;12:144. doi: 10.1186/1471-2474-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. The European journal of neuroscience. 2003;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- 48.Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nature medicine. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petho G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiological reviews. 2012;92:1699–1775. doi: 10.1152/physrev.00048.2010. [DOI] [PubMed] [Google Scholar]
- 50.Pizzo A, Salmeri FM, Ardita FV, Sofo V, Tripepi M, Marsico S. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecologic and obstetric investigation. 2002;54:82–87. doi: 10.1159/000067717. [DOI] [PubMed] [Google Scholar]
- 51.Punnonen J, Teisala K, Ranta H, Bennett B, Punnonen R. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. American journal of obstetrics and gynecology. 1996;174:1522–1526. doi: 10.1016/s0002-9378(96)70600-2. [DOI] [PubMed] [Google Scholar]
- 52.Quarta S, Vogl C, Constantin CE, Uceyler N, Sommer C, Kress M. Genetic evidence for an essential role of neuronally expressed IL-6 signal transducer gp130 in the induction and maintenance of experimentally induced mechanical hypersensitivity in vivo and in vitro. Molecular pain. 2011;7:73. doi: 10.1186/1744-8069-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reichling DB, Green PG, Levine JD. The fundamental unit of pain is the cell. Pain. 2013 doi: 10.1016/j.pain.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roman H, Vassilieff M, Gourcerol G, Savoye G, Leroi AM, Marpeau L, Michot F, Tuech JJ. Surgical management of deep infiltrating endometriosis of the rectum: pleading for a symptom-guided approach. Hum Reprod. 2011;26:274–281. doi: 10.1093/humrep/deq332. [DOI] [PubMed] [Google Scholar]
- 55.Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148:407–413. doi: 10.1016/j.pain.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Scholl B, Bersinger NA, Kuhn A, Mueller MD. Correlation between symptoms of pain and peritoneal fluid inflammatory cytokine concentrations in endometriosis. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2009;25:701–706. doi: 10.3109/09513590903159680. [DOI] [PubMed] [Google Scholar]
- 57.Stone LS, Vulchanova L. The pain of antisense: in vivo application of antisense oligonucleotides for functional genomics in pain and analgesia. Advanced drug delivery reviews. 2003;55:1081–1112. doi: 10.1016/s0169-409x(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 58.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Human reproduction update. 2011;17:327–346. doi: 10.1093/humupd/dmq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 60.Svensson P, Cairns BE, Wang K, Arendt-Nielsen L. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003;104:241–247. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 61.Umezawa M, Sakata C, Tanaka N, Kudo S, Tabata M, Takeda K, Ihara T, Sugamata M. Cytokine and chemokine expression in a rat endometriosis is similar to that in human endometriosis. Cytokine. 2008;43:105–109. doi: 10.1016/j.cyto.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 62.Wang G, Tokushige N, Russell P, Dubinovsky S, Markham R, Fraser IS. Hyperinnervation in intestinal deep infiltrating endometriosis. Journal of minimally invasive gynecology. 2009;16:713–719. doi: 10.1016/j.jmig.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Nesbitt JE, Fuentes NL, Fuller GM. Molecular cloning and characterization of the rat liver IL-6 signal transducing molecule, gp130. Genomics. 1992;14:666–672. doi: 10.1016/s0888-7543(05)80166-1. [DOI] [PubMed] [Google Scholar]
- 64.Wickiewicz D, Chrobak A, Gmyrek GB, Halbersztadt A, Gabryś MS, Goluda M, Chełmońska-Soyta A. Diagnostic accuracy of interleukin-6 levels in peritoneal fluid for detection of endometriosis. Archives of gynecology and obstetrics. 2013 doi: 10.1007/s00404-013-2828-6. [DOI] [PubMed] [Google Scholar]
- 65.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. British journal of pharmacology. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wykes CB, Clark TJ, Chakravati S, Mann CH, Gupta JK. Efficacy of laparoscopic excision of visually diagnosed peritoneal endometriosis in the treatment of chronic pelvic pain. European journal of obstetrics, gynecology, and reproductive biology. 2006;125:129–133. doi: 10.1016/j.ejogrb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Yan J, Melemedjian OK, Price TJ, Dussor G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6) Molecular pain. 2012;8:6. doi: 10.1186/1744-8069-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang G, Dmitrieva N, Liu Y, McGinty KA, Berkley KJ. Endometriosis as a neurovascular condition: estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat. American journal of physiology Regulatory, integrative and comparative physiology. 2008;294:R162–171. doi: 10.1152/ajpregu.00649.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


