Abstract
Proximal tubule bicarbonate reabsorption is primarily mediated via the Na+/H+ exchanger, identified as NHE3 in adults. Previous studies have demonstrated a maturational increase in rat proximal tubule NHE3 expression, with a paucity of NHE3 expression in neonates, despite significant Na+-dependent proton secretion. Recently, a novel Na+/H+ antiporter (NHE8) was identified and found to be expressed on the apical membrane of the proximal tubule. To determine whether NHE8 may be the antiporter responsible for proton secretion in neonates, the present study characterized the developmental expression of NHE8 in rat proximal tubules. RNA blots and real-time RT-PCR demonstrated no developmental difference in the mRNA of renal NHE8. Immunoblots, however, demonstrated peak protein abundance of NHE8 in brush border membrane vesicles of 7- and 14-day-old compared with adult rats. In contrast, the level of NHE8 expression in total cortical membrane protein was higher in adults than in neonates. Immunohistochemistry confirmed the presence of NHE8 on the apical membrane of the proximal tubules of neonatal and adult rats. These data demonstrate that NHE8 does undergo maturational changes on the apical membrane of the rat proximal tubule and may account for the Na+-dependent proton flux in neonatal proximal tubules.
Keywords: sodium/hydrogen exchanger, development
Tubular immaturity of the neonatal kidney results in a lower threshold for bicarbonate reabsorption (7, 8, 26) and subsequent lower serum bicarbonate concentrations in neonates than in adults (15). Bicarbonate reabsorption is predominantly accomplished via luminal proton secretion mediated by a luminal Na+/H+ antiporter (25). The bicarbonate transport rate in the proximal tubule of the neonate is one-third that of the adult (3, 26). The maturational increase in proximal tubule acidification is largely due to the maturational increase in the apical membrane Na+/H+ antiporter (2, 5, 8).
At least 10 isoforms of the Na+/H+ exchanger (NHE) have been cloned (16). NHE3 is responsible for most of the luminal proton secretion in the adult proximal tubule (5, 13). Previous developmental studies have shown a maturational increase in the abundance of NHE3 on the apical membrane of proximal tubule cells (5, 27). There is a paucity of brush border membrane NHE3 expression in 7- to 14-day-old neonatal rats; however, in vitro perfusion studies demonstrated a significant rate of luminal Na+-dependent proton secretion in neonatal proximal tubules that was inhibited by ethylisopropylamiloride (EIPA) (27). Studies in NHE3-knockout mice showed only a 50% reduction in Na+-dependent proton secretion compared with wild-type mice. The majority of this residual Na+-dependent proton secretion was inhibited by luminal EIPA (13). These studies support the existence of another proximal tubule Na+/H+ antiporter.
NHE8 is a recently identified renal Na+/H+ antiporter isoform (17) that has been localized to the apical membrane of the rat proximal tubule (16). Given its location of expression, NHE8 has the potential to be the Na+/H+ antiporter responsible for the proton secretion in neonates as well as the isoform that mediates the residual proton secretion demonstrated in NHE3-knockout mice. The purpose of the present study was to determine whether NHE8 is developmentally expressed in rat proximal tubules.
Methods
Animals
Pregnant Sprague-Dawley rats were received on the 15th day of gestation. Neonatal rats were kept with their mother until they were studied at 1, 7, 14, and 26 days of age. Adult male Sprague-Dawley rats were ≥6 wk of age. The study conformed to the American Physiological Society “Guiding Principles in the Care and Use of Animals,” and all protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
RNA Isolation and Analysis
Kidneys were harvested from the rats, and the renal cortex was homogenized in RNazol [1:1, phenol-RNazol stock (4 M guanidine thiocyanate and 25 mM disodium citrate, pH 7.0) and 0.5% sarcosyl] containing β-mercaptoethanol (3.6 μl/ml). RNA was extracted with 3 M sodium acetate (pH 4.0) and chloroform, purified using isopropanol precipitation, and washed twice with 80% ethanol (14). Poly(A)+ RNA was purified from total RNA using oligo(dT) column chromatography. Poly(A)+ RNA (5 μg) was fractionated using agaroseformaldehyde gel electrophoresis and transferred to a nylon filter (GeneScreen Plus, New England Nuclear, Boston, MA). The filter was prehybridized at 42°C for 4 h with ULTRAhyb buffer (Ambion, Austin, TX) and subsequently hybridized to double-stranded uniformly 32P-labeled cDNA probes (>106 cpm/ml) in the ULTRAhyb buffer at 42°C for ∼16 h. The probes were synthesized by the random hexamer method using 50–100 ng of cDNA. The NHE8 probe was a 2.5-kb EcoR I fragment, and the GAPDH probe was a 1.2-kb Hind III/BamH I fragment. The filter was subjected to a series of washes: two washes with 2× saline-sodium citrate and 0.1% SDS for 5 min at room temperature followed by two washes with 0.1× saline-sodium citrate and 1% SDS for 40 min at 55°C. The mRNA abundance was quantitated by autoradiography and densitometry.
cDNA Synthesis and Real-Time PCR
Total RNA was isolated from rat renal cortex with use of the GenElute Mammalian Total RNA purification kit (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions. RNA estimation and quality were assessed using an LKB Ultra-spec III spectrophotometer at 260- and 280-nm wavelengths. Total RNA (1 μg) was pretreated with DNase I (Invitrogen, Carlsbad, CA) and then used for cDNA synthesis with random hexamer primers and StrataScript reverse transcriptase (Stratagene, La Jolla, CA) at an annealing temperature of 25°C for 10 min, extension at 42°C for 50 min, and termination at 70°C for 15 min. Real-time PCR was performed using an iCycler PCR thermal cycler (Bio-Rad, Hercules, CA) to quantify relative mRNA abundance. Primers for NHE8 [5′-AAGCCTATTCTTCCGGTGCAGACA-3′ (forward) and 5′-AGAGAAACAACAGCCACGCTCTCA-3′ (reverse)], NHE3 [5′-ACTGCTTAATGACGCGGTGACTGT-3′ (forward) and 5′-AAAGACGAAGCCAGGCTCGATGAT-3′ (reverse)], and 28S rRNA [5′-TTGAAAATCCGGGGGAGAG-3′ (forward) and 5′-ACATTGTTCCAACATGCCAG-3′ (reverse)] were mixed with cDNA and SYBR Green Master Mix (Bio-Rad) according to the manufacturer's instructions. The relative transcript levels of NHE8 and NHE3, with 28S rRNA used as the denominator, were determined using the method described by Vandesompele et al. (28).
Total Protein, Total Membrane Protein, and Brush Border Membrane Vesicle Isolation
Kidneys were removed and placed in an ice-cold isolation buffer containing 300 mM mannitol, 16 mM HEPES, and 5 mM EGTA titrated to pH 7.4 with Tris. The isolation buffer contained aprotinin (2 μg/ml), leupeptin (2 μg/ml), and phenylmethylsulfonyl fluoride (100 μg/ml) to inhibit peptidases. The cortex was homogenized with 15 strokes of a glass-Teflon homogenizer at 4°C (6, 27). Total protein samples were taken from the homogenate and stored separately. For preparation of total membranes, the homogenate was centrifuged at 400 g at 4°C for 4 min, and the supernatant was saved. The supernatant was centrifuged again at 400 g at 4°C for 4 min, and the resulting supernatant was centrifuged at 35,000 g at 4°C for 36 min. The fluffy white layer surrounding the pellet (containing the membranes) was resuspended with isolation buffer (5). Brush border membrane vesicles (BBMV) were isolated from the total protein homogenate by differential centrifugation and Mg2+ precipitation, as previously described (6, 19). The final BBMV fraction was resuspended in isolation buffer. Enrichment for leucine aminopeptidase activity in BBMV was comparable in 7-day-old (9.8 ± 1.3), 14-day-old (9.5 ± 0.6), and adult (9.2 ± 0.7) rats. All protein fractions were assayed using the Bradford method, with crystalline BSA as the standard (10).
SDS Gel Electrophoresis and Immunoblotting
Total protein (75 μg/lane), BBMV (25 μg/lane), and total membrane protein (25 μg/lane) were denatured and then separated on a 7.5% polyacrylamide gel using SDS-PAGE, as previously described (6, 27). The proteins were transferred to a polyvinylidene difluoride membrane at 120–140 mA at 4°C. The blots were blocked with fresh Blotto (5% nonfat milk and 0.1% Tween 20 in PBS, pH 7.4) for 1 h and then incubated with primary antibody to NHE8 or NHE3. The NHE8 antibody was the 9C9 monoclonal antibody raised in mice (16). NHE8 antibody was added at 1:500 dilution overnight at 4°C. The NHE3 antibody was a rabbit polyclonal antibody directed against a fusion protein of maltose-binding protein and rat NHE3 amino acids 405–831 (1). NHE3 antibody was added at 1:750 dilution overnight at 4°C. The blots were then washed extensively with Blotto. The secondary antibodies, horseradish peroxidase-conjugated donkey anti-mouse immunoglobulin for NHE8 and anti-rabbit immunoglobulin for NHE3, were added at 1:10,000 dilution for 1 h at room temperature. The blots were washed again with Blotto, and enhanced chemiluminescence (Amersham Life Science) was used to detect bound antibody. Relative NHE8 and NHE3 protein abundances were quantitated using densitometry. Equal loading of the samples was confirmed using an antibody to β-actin (1:10,000 dilution; Sigma Biochemicals and Reagents, St. Louis, MO).
NHE8 Immunohistochemistry
Tissue preparation for immunohistochemistry
Adult Sprague-Dawley rats were anesthetized with pentobarbital sodium. The kidneys were briefly perfused with PBS and then perfusion fixed with modified high-osmolar PLP fixative (2% paraformaldehyde, 75 mM lysine, 10 mM sodium periodate, and 750 mM sucrose in phosphate buffer, pH 7.4) via aortic puncture. Neonatal rat kidneys were perfusion fixed with modified isosmolar PLP fixative (2% paraformaldehyde, 75 mM lysine, 10 mM sodium periodate, and 285 mM sucrose in phosphate buffer, pH 7.4) via cardiac puncture. Kidneys were removed and postfixed by immersion in the same fixative for 4 h.
Indirect immunofluorescence microscopy
Frozen sections (4 μm) were washed with TBS (pH 7.2, 50 mM Tris, and 150 mM NaCl) followed sequentially by 500 mM ammonium chloride, 10 mM glycine, and then 0.1% Triton X-100. Sections were incubated with a blocking solution (TBS, 1.5% BSA, and 10% goat serum) for 30 min and then incubated with the primary antibody (anti-NHE8 monoclonal antibody, 1:10 dilution in blocking solution) overnight. After they were washed with a high-salt wash solution (TBS with 2.5% NaCl and 0.1% BSA), the sections were incubated with the appropriate FITC-labeled secondary antibody for 1 h. After additional high-salt washes, the sections were incubated with rabbit anti-villin antibody (1:100 dilution; Santa Cruz Biotechnology) or anti-Na+/Ca2+ exchanger (NCX) antibody (1:200 dilution; Santa Cruz Biotechnology) for 1 h followed by appropriate secondary antibody for 1 h and then washed with TBS. For Lotus tetragonolobus agglutinin (LTA) staining, sections were incubated with biotinylated LTA (1:800 dilution; Vector Laboratories) for 1 h, washed with TBS, incubated with rhodamine-labeled avidin (1:400 dilution; Vector Laboratories) for 40 min, mounted, and then visualized with a Zeiss LSM510 microscope.
Statistical Analysis
Values are means ± SE; n is the number of experiments performed using different animals. Statistical significance was determined by analysis of variance with the post hoc Student-Newman-Keuls method.
Results
NHE8 and NHE3 mRNA Expression
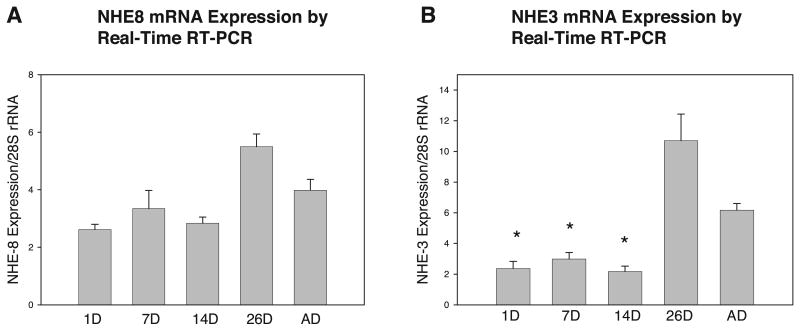
Relative expression of NHE8 and NHE3 mRNA was assessed using RNA blots of poly(A)+-selected RNA (Fig. 1), as well as real-time RT-PCR analysis of total RNA (Fig. 2). Both methods of evaluating gene expression produced the same results. There was no significant difference in the mRNA expression of NHE8 in any of the age groups. NHE3 mRNA expression was consistent with that reported previously (5, 8, 27): it began to increase at 14 days, rose dramatically at 26 days, and remained elevated in the adult.
Fig. 1.
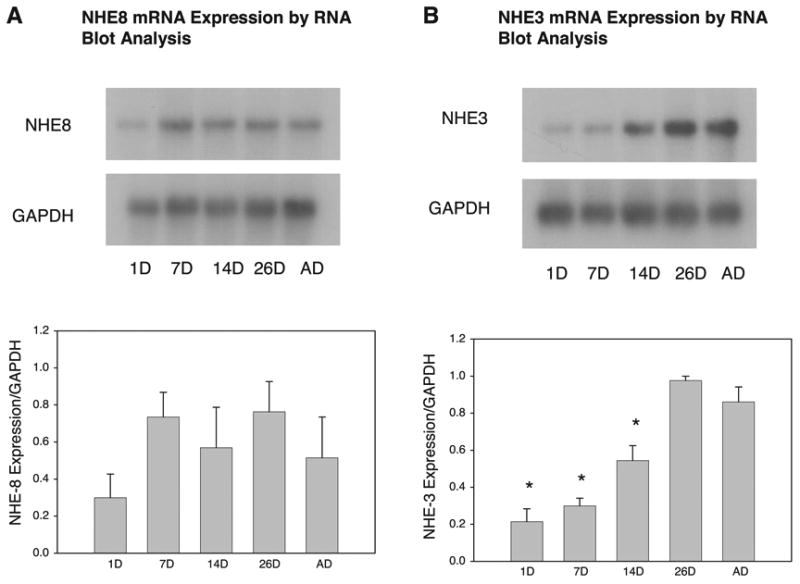
Maturational changes in Na+/H+ exchanger [NHE8 (A) and NHE3 (B)] mRNA abundance. RNA blots with 5 μg of poly(A)+ RNA from rat renal cortex are shown for 1-, 7-, 14-, and 28-day-old (1D, 7D, 14D, and 28D) and adult (AD) rats. GAPDH mRNA was used to ensure equal loading of lanes. There was no significant maturational change in NHE8 mRNA expression. There was a significant maturational increase in NHE3 expression. Values are means ± SE of 3 measurements in each age group. *P < 0.05 vs. 26D and AD.
Fig. 2.
NHE8 (A) and NHE3 (B) mRNA abundance. cDNA synthesized from rat renal cortex total RNA was analyzed by real-time RT-PCR, with 28S rRNA as a control. Results are consistent with RNA blot analysis. There was no significant maturational change in NHE8 expression. There was a significant maturational increase in NHE3 expression. Values are means ± SE of ≥7 measurements in each age group. *P < 0.05 vs. 26D and AD.
Maturational Changes in BBMV Expression of NHE8 and NHE3
Protein abundance of NHE8 relative to (3-actin in rat BBMV was estimated using immunoblots (Fig. 3). Abundance of NHE8 relative to (3-actin in BBMV was significantly higher in the 7-day-old group (0.96 ± 0.04) than in the 1-day-old (0.29 ± 0.07), 26-day-old (0.52 ± 0.13), and adult (0.22 ± 0.09) groups (P < 0.05). Abundance of NHE8 relative to β-actin in BBMV was also higher in the 14-day-old group (0.64 ± 0.12) than the 1-day-old and adult groups (P < 0.05). Abundance of NHE3 relative to β-actin in BBMV was similar to that reported previously (5, 8, 27); however, this experiment demonstrated significant expression in the 1-day-old group that was not previously reported. Expression was lower in the 7-day-old (0.10 ± 0.02) and 14-day-old (0.11 ± 0.03) groups than in the 26-day-old (0.71 ± 0.10) and adult (0.88 ± 0.06) groups (P < 0.05).
Fig. 3.
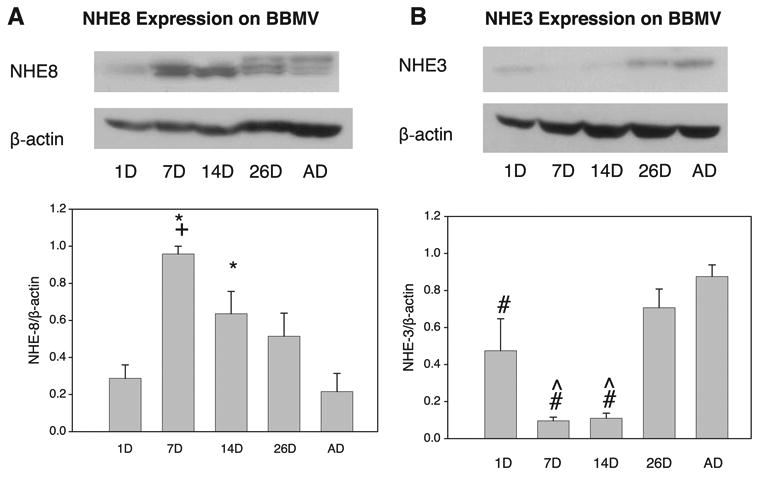
Maturational changes in NHE8 (A) and NHE3 (B) protein abundance in renal brush border membrane vesicles (BBMV). NHE8 and NHE3 protein abundance relative to β-actin was determined by immunoblots of 50 μg of rat renal BBMV (NHE8 and NHE3 were ∼80 kDa). Developmental pattern of NHE8 is the reciprocal of developmental pattern of NHE3. Equal loading of samples was verified by β-actin. Values are means ± SE of ≥5 measurements in each age group. *P < 0.05 vs. 1D and AD. +P < 0.05 vs. 26D. #P < 0.05 vs. 26D and AD. ˆP < 0.05 vs. 1D.
Total Protein and Total Membrane Protein NHE8 Abundance
Immunoblots of total protein and total membrane protein abundance of NHE8 relative to β-actin are shown in Figs. 4 and 5, respectively. The significantly higher total protein abundance of NHE8 relative to β-actin in the adult group than in the 7- and 14-day-old groups (P < 0.05) demonstrates a sharp contrast to the results of BBMV expression analysis. Total membrane protein and total protein abundance of NHE8 relative to β-actin were similar, with the highest expression in the adult group (P < 0.05). These data suggest a greater fraction of NHE8 on cellular membranes than in BBMV in adults than in neonates.
Fig. 4.
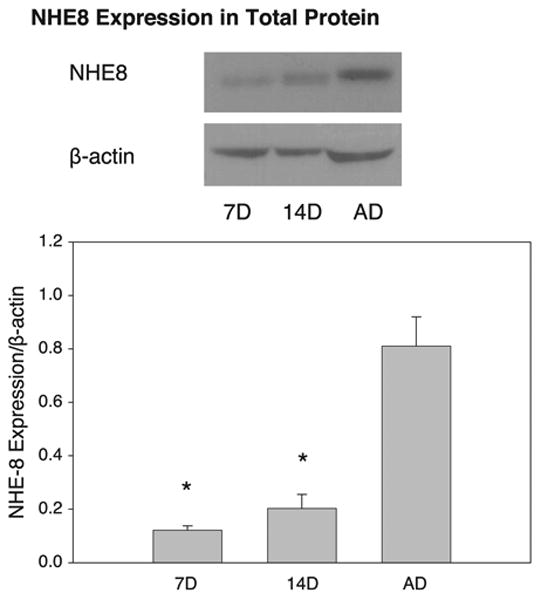
NHE8 protein abundance in rat cortical total protein. Immunoblots of cortical total protein demonstrate highest relative abundance of NHE8 in adult. Equal loading of samples was confirmed using β-actin. Values are means ± SE of 4 measurements in each age group. *P < 0.05 vs. AD.
Fig. 5.
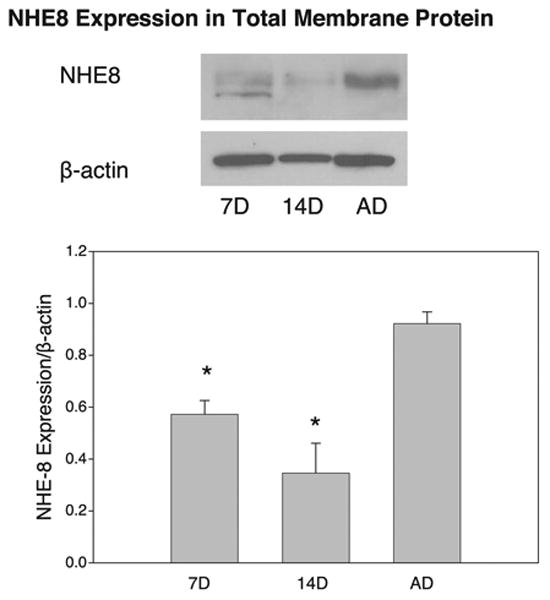
NHE8 abundance in rat cortical total membrane protein relative to β-actin. Immunoblots of rat cortical total membrane protein (including apical, basolateral, and intracellular membranes) show highest relative abundance of NHE8 in adult. Values are means ± SE of 4 measurements in each age group. *P < 0.05 vs. AD.
Rat Proximal Tubule Immunohistochemistry
Immunohistochemistry of rat proximal tubules demonstrates identifiable expression of NHE8 on the apical membrane in the neonate and adult rat (Fig. 6). NHE8 expression colocalized with villin, LTA, and NCX-1 as proximal brush border, proximal tubule, and distal tubule markers, respectively, in 1-day-old, 10-day-old, and adult rats is shown in Fig. 7. NHE8 is a proximal tubule protein throughout this developmental period, with absolutely no colocalization with NCX-1 (Fig. 7). In the 1-day-old rats, NHE8 is expressed but is primarily an intercellular protein underneath the brush border (Fig. 7A). In contrast, the 10-day-old rats show clear brush border expression (Fig. 7B). NHE8 is also expressed in the brush border in adult rats, but expression is less intense than in the 10-day-old rats (Fig. 7C). The immunohistochemical data are compatible with the data from immunoblots showing low brush border NHE8 expression at 1 day of age and peak expression at 10 days of age (Fig. 3).
Fig. 6.
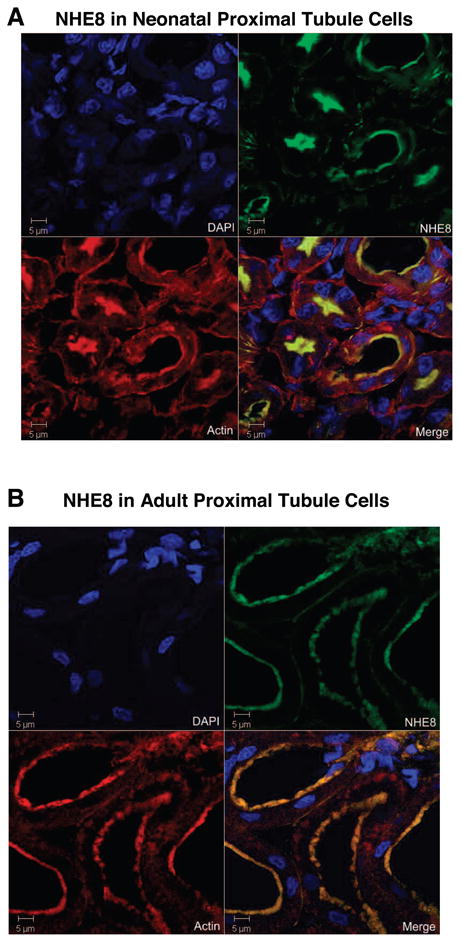
Immunohistochemistry of NHE8 in neonatal and adult rat proximal tubule. Neonatal (10 days) and adult rat kidneys were fixed by perfusion, and sections were probed with anti-NHE8 with fluorescein secondary antibody for NHE8 protein (green), rhodamine-phalloidin for actin (red), and 4,6-diamidino-2-phenylindole (DAPI) for nuclei (blue). Images are representative of 4 independent sets of animals that showed similar results.
Fig. 7.
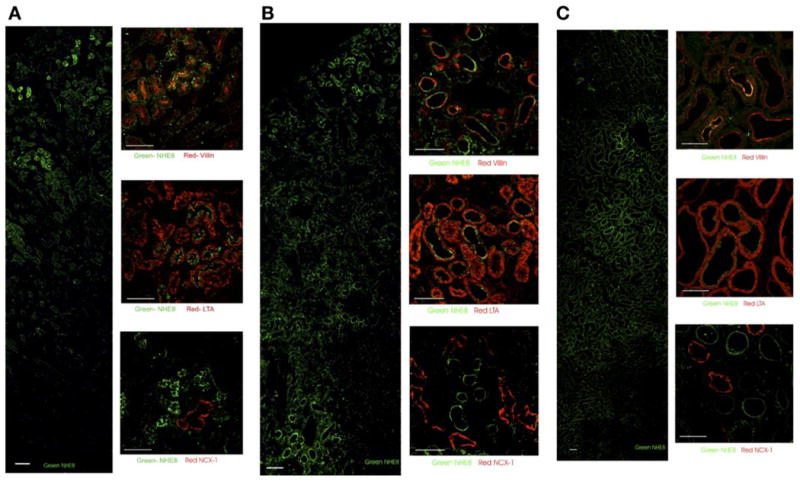
Immunohistochemistry of NHE8 in 1-day-old (A), 10-day-old (B), and adult (C) rat kidney. NHE8 is costained with villin (brush border), Lotus tetragonolobus agglutinin (LTA, proximal tubule), and Na+/Ca2+ exchanger (NCX-1, distal tubule). Superficial cortex is shown at top of the low-magnification figures stained for NHE8 alone. Nephrogenic zone is still present in 1-day-old neonates (A, left). In low magnification of developing kidney, NHE8 staining is most intense, but not limited to proximal tubule. Although it is likely that this staining represents background, we cannot rule out expression in other nephron segments. NHE8 staining appears to be heterogeneous on apical membrane of proximal tubules, and NHE8 labeling may be limited to certain segments of proximal tubule in neonatal and adult kidney. Scale bars, 50 μm.
Discussion
Previous studies from our laboratory showed a disparity between the rate of Na+-dependent proton secretion in neonatal and adult rat proximal tubule and relative NHE3 BBMV expression (18, 19, 27). The rate of Na+-dependent proton secretion was higher than could be accounted for by NHE3 (18, 19, 27). This discrepancy suggests that another Na+/H+ anti-porter in neonatal rats accounts for much of the Na+-dependent proton secretion. The functional existence of another Na+/H+ antiporter in the proximal tubule apical membrane was demonstrated by Na+-dependent Na+/H+ activity in NHE3-deficient mice (13). The present study demonstrates a maturational change in the relative abundance of NHE8 in rat BBMV. NHE8 abundance was greatest in rats at 7 and 14 days of age, which corresponds to the lowest expression of NHE3. Thus the Na+/H+ antiporter activity measured in neonates may be due to NHE8.
In the present study, we found a reciprocal developmental expression of BBMV NHE3 and NHE8 protein abundance. The factors that affect the developmental increase in NHE3 have been studied. There is a threefold increase in thyroid hormone level (6, 29, 29) and a 30-fold increase in corticosterone level in the rat during postnatal maturation (6, 20), which correspond to maturational changes in NHE3 expression. Previous studies have provided substantive evidence that postnatal increases in glucocorticoids and thyroid hormone result in the maturational increase in Na+/H+ exchanger activity, NHE3 mRNA, and NHE3 protein abundance (4, 6, 12, 18, 19). The factors that affect the maturational changes in brush border membrane NHE8 abundance are unknown but may be the same factors that increase NHE3 expression. Further studies are needed to elucidate any regulatory role of these hormones.
In the present study, the level of relative NHE3 expression in the 1-day-old rats was higher than that previously described by our laboratory (27). Immediately after birth, there is a transient increase in serum glucocorticoid levels (21) that is equal to that seen after weaning (20). Glucocorticoids have been shown to increase NHE3 protein trafficking to the apical membrane (9). The high levels of glucocorticoids in the early postnatal period may stimulate trafficking of NHE3 to the apical membrane, resulting in a higher level of expression immediately after birth that disappears until the second increase in thyroid hormone and glucocorticoids at the time of weaning. Thus the 1-day-old animals in this study may have been studied when they were closer to parturition and had higher glucocorticoid levels than in our previous study.
RNA blot and real-time quantitative RT-PCR confirm a uniform expression of NHE8 mRNA in all age groups. Our data show higher expression of NHE8 on BBMV of neonates than adults, when there is a paucity of BBMV NHE3. However, abundance of NHE8 in the total membrane fraction is greater in adults than in neonates. Using a phylogenetic analysis of the NHE family, Brett et al. (11) classified NHE8 as an endosomal/trans-Golgi protein. The orthologous isoform of NHE8 in Caenorhabditis elegans has a perinuclear distribution in transgenic nematodes (23). Expression of green fluorescent protein-tagged NHE8 in yeast results in a distribution to the mid- to trans-Golgi (22). A similar distribution has been found in COS7 cells (22). In unpublished data, expression of epitope-tagged human NHE8 in HeLa cells had a diffuse intracellular distribution (24). The cellular distribution of NHE8 in adults and neonates is unknown.
Our present work confirms that NHE8 is also on the apical membrane of the proximal tubule (16), which suggests that it may mediate Na+/H+ exchange in that segment. Recent work demonstrates that NHE8 is a functional Na+/H+ antiporter, which is compatible with the data derived from heterologously expressed, purified, and reconstituted NHE8 (22). We recently demonstrated that normal rat kidney cells express NHE8 on the apical membrane (30). Transcripts for NHE2, NHE3, and NHE4 were not present in this cell line. Normal rat kidney cells have apical EIPA-inhibitable Na+-dependent proton secretion, and this Na+/H+ exchange activity was reduced with an NHE8 small interfering RNA. These data are consistent with a functional role for NHE8 in mediation of apical Na+/H+ exchange in a mammalian kidney cell line. The reason for the developmental isoform change in postnatal proximal tubule maturation remains unclear.
Acknowledgments
Grants: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-41612 (to M. Baum) and R01-DK-48482 and PO1-DK-20543 (to O. W. Moe).
References
- 1.Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int. 1995;48:1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 2.Baum M. Neonatal rabbit juxtamedullary proximal convoluted tubule acidification. J Clin Invest. 1990;85:499–506. doi: 10.1172/JCI114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum M. Developmental changes in rabbit juxtamedullary proximal convoluted tubule acidification. Pediatr Res. 1992;31:411–414. doi: 10.1203/00006450-199204000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Baum M, Amemiya M, Dwarakanath V, Alpern RJ, Moe OW. Glucocorticoids regulate NHE-3 transcription in OKP cells. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;270:F164–F169. doi: 10.1152/ajprenal.1996.270.1.F164. [DOI] [PubMed] [Google Scholar]
- 5.Baum M, Biemesderfer D, Gentry D, Aronson PS. Ontogeny of rabbit renal cortical NHE3 and NHE1: effect of glucocorticoids. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;268:F815–F820. doi: 10.1152/ajprenal.1995.268.5.F815. [DOI] [PubMed] [Google Scholar]
- 6.Baum M, Dwarakanath V, Alpern RJ, Moe OW. Effects of thyroid hormone on the neonatal renal cortical Na+/H+ antiporter. Kidney Int. 1998;53:1254–1258. doi: 10.1046/j.1523-1755.1998.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum M, Quigley R. Prenatal glucocorticoids stimulate neonatal juxtamedullary proximal convoluted tubule acidification. Am J Physiol Renal Fluid Electrolyte Physiol. 1991;261:F746–F752. doi: 10.1152/ajprenal.1991.261.5.F746. [DOI] [PubMed] [Google Scholar]
- 8.Baum M, Quigley R. Ontogeny of proximal tubule acidification. Kidney Int. 1995;48:1697–1704. doi: 10.1038/ki.1995.467. [DOI] [PubMed] [Google Scholar]
- 9.Bobulescu IA, Dwarakanath V, Zou L, Zhang J, Baum M, Moe OW. Glucocorticoids acutely increase cell surface Na+/H+ exchanger-3 (NHE3) by activation of NHE3 exocytosis. Am J Physiol Renal Physiol. 2005;289:F685–F691. doi: 10.1152/ajprenal.00447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223–C239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- 12.Cano A, Baum M, Moe OW. Thyroid hormone stimulates the renal Na/H exchanger NHE3 by transcriptional activation. Am J Physiol Cell Physiol. 1999;276:C102–C108. doi: 10.1152/ajpcell.1999.276.1.C102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JY, Shah M, Lee MG, Schultheis PJ, Shull GE, Muallem S, Baum M. Novel amiloride-sensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J Clin Invest. 2000;105:1141–1146. doi: 10.1172/JCI9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Edelmann CMJ, Soriano JR, Boichis H, Gruskin AB, Acosta MI. Renal bicarbonate reabsorption and hydrogen ion excretion in normal infants. J Clin Invest. 1967;46:1309–1317. doi: 10.1172/JCI105623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol. 2005;288:F530–F538. doi: 10.1152/ajprenal.00229.2004. [DOI] [PubMed] [Google Scholar]
- 17.Goyal S, Vanden HG, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol. 2003;284:F467–F473. doi: 10.1152/ajprenal.00352.2002. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Dwarakanath V, Baum M. Maturation of the Na+/H+ antiporter (NHE3) in the proximal tubule of the hypothyroid adrenalectomized rat. Am J Physiol Renal Physiol. 2004;287:F521–F527. doi: 10.1152/ajprenal.00005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta N, Tarif SR, Seikaly M, Baum M. Role of glucocorticoids in the maturation of the rat renal Na+/H+ antiporter (NHE3) Kidney Int. 2001;60:173–181. doi: 10.1046/j.1523-1755.2001.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henning SJ. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol Endocrinol Metab Gastrointest Physiol. 1978;235:E451–E456. doi: 10.1152/ajpendo.1978.235.5.E451. [DOI] [PubMed] [Google Scholar]
- 21.Malinowska KW, Hardy RN, Nathanielsz PW. Plasma adrenocorticosteroid concentrations immediately after birth in the rat, rabbit and guinea-pig. Experientia. 1972;28:1366–1367. doi: 10.1007/BF01965349. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem. 2005;280:1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- 23.Nehrke K, Melvin JE. The NHX family of Na+-H+ exchangers in Caenorhabditis elegans. J Biol Chem. 2002;277:29036–29044. doi: 10.1074/jbc.M203200200. [DOI] [PubMed] [Google Scholar]
- 24.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflügers Arch. 2004;447:549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 25.Preisig PA, Ives HE, Cragoe EJ, Jr, Alpern RJ, Rector FC., Jr Role of the Na+/H+ antiporter in rat proximal tubule bicarbonate absorption. J Clin Invest. 1987;80:970–978. doi: 10.1172/JCI113190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Evan AP. Development of solute transport in rabbit proximal tubule. I. and glucose absorption. Am J Physiol Renal Fluid Electrolyte Physiol. 1983;245:F382–F390. doi: 10.1152/ajprenal.1983.245.3.F382. [DOI] [PubMed] [Google Scholar]
- 27.Shah M, Gupta N, Dwarakanath V, Moe OW, Baum M. Ontogeny of Na+/H+ antiporter activity in rat proximal convoluted tubules. Pediatr Res. 2000;48:206–210. doi: 10.1203/00006450-200008000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandesompele J, De PK, Pattyn F, Poppe B, Van RN, De PA, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(RESEARCH0034) doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker P, Dubois JD, Dussault JH. Free thyroid hormone concentrations during postnatal development in the rat. Pediatr Res. 1980;14:247–249. doi: 10.1203/00006450-198003000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Bobulescu IA, Goyal S, Aronson PS, Baum M, Moe OW. Characterization of Na+/H+ exchanger-8 (NHE8) in rat kidney (NRK) cells (Abstract) J Am Soc Nephrol. 2006;17:578A. [Google Scholar]