Abstract
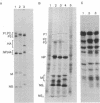
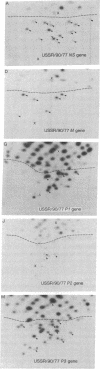
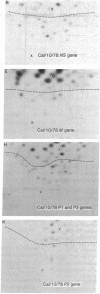
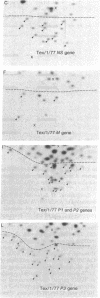
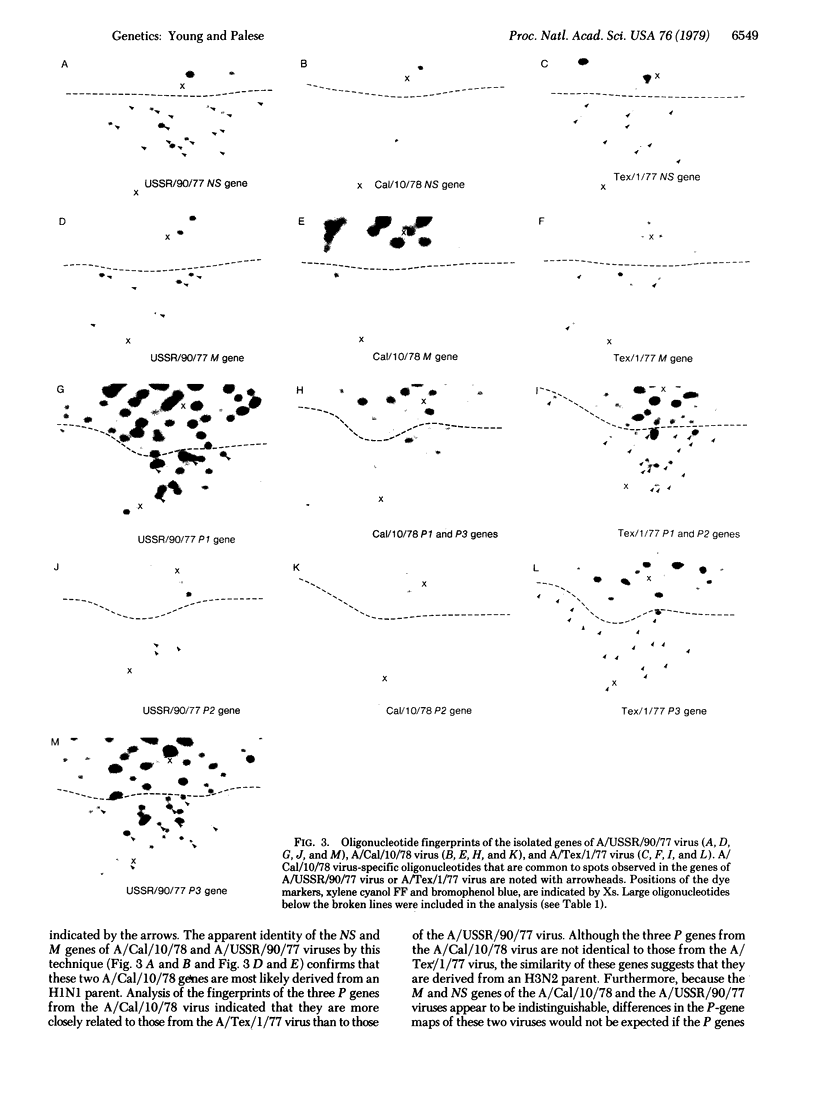
In June of 1977, a new influenza A pandemic was started by strains of the H1N1 serotype. Oligonucleotide fingerprint analysis of the RNA from viruses isolated during the early stage of this pandemic demonstrated that genetic variation among these 1977 strains could be attributed to sequential mutation [Young, J.F., Desselberger, U. & Palese, P. (1979) Cell, 18, 73-83]. Examination of more recent strains revealed that the H1N1 variants that were isolated in the winter of 1978-1979 differed considerably from the H1N1 viruses isolated the previous year. Oligonucleotide and peptide map analysis of the new prototype strain (A/Cal/10/78) suggested that it arose by recombination. It appears that only the HA, NA, M, and NS genes of this virus are derived from the earlier H1N1 viruses and that the P1, P2, P3, and NP genes most likely originate from an H3N2 parent. These data suggest that genetic variation in influenza virus strains of the same serotype is not restricted to mutation alone, but can also involve recombination (reassortment).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Desselberger U., Nakajima K., Alfino P., Pedersen F. S., Haseltine W. A., Hannoun C., Palese P. Biochemical evidence that "new" influenza virus strains in nature may arise by recombination (reassortment). Proc Natl Acad Sci U S A. 1978 Jul;75(7):3341–3345. doi: 10.1073/pnas.75.7.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaush C. R., Smith T. F. Replication and plaque assay of influenza virus in an established line of canine kidney cells. Appl Microbiol. 1968 Apr;16(4):588–594. doi: 10.1128/am.16.4.588-594.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G. Crystallization and peptide maps of neuraminidase "heads" from H2N2 and H3N2 influenza virus strains. Virology. 1978 May 1;86(1):78–87. doi: 10.1016/0042-6822(78)90009-0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Ecology of influenza viruses in lower mammals and birds. Br Med Bull. 1979 Jan;35(1):29–33. doi: 10.1093/oxfordjournals.bmb.a071537. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Studies on the origin of pandemic influenza. II. Peptide maps of the light and heavy polypeptide chains from the hemagglutinin subunits of A 2 influenza viruses isolated before and after the appearance of Hong Kong influenza. Virology. 1972 May;48(2):445–455. doi: 10.1016/0042-6822(72)90055-4. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Desselberger U., Palese P. Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature. 1978 Jul 27;274(5669):334–339. doi: 10.1038/274334a0. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. P1 and P3 proteins of influenza virus are required for complementary RNA synthesis. J Virol. 1977 Mar;21(3):1187–1195. doi: 10.1128/jvi.21.3.1187-1195.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2142–2146. doi: 10.1073/pnas.73.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. S., Haseltine W. A. A micromethod for detailed characterization of high molecular weight RNA. Methods Enzymol. 1980;65(1):680–687. doi: 10.1016/s0076-6879(80)65066-6. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Palese P. Influenza B virus genome: assignment of viral polypeptides to RNA segments. J Virol. 1979 Jan;29(1):361–373. doi: 10.1128/jvi.29.1.361-373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Kilbourne E. D. RNAs of influenza A, B, and C viruses. J Virol. 1976 May;18(2):738–744. doi: 10.1128/jvi.18.2.738-744.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Difference in protein patterns of influenza A viruses. Virology. 1977 Jan;76(1):122–128. doi: 10.1016/0042-6822(77)90289-6. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Rohde W., Von Hoyningen V., Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978 Jun 1;87(1):13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., von Hoyningen V., Rott R. Genetic relatedness between the new 1977 epidemic strains (H1N1) of influenza and human influenza strains isolated between 1947 and 1957 (H1N1). Virology. 1978 Sep;89(2):613–617. doi: 10.1016/0042-6822(78)90203-9. [DOI] [PubMed] [Google Scholar]
- Schulman J. L., Palese P. Selection and identification of influenza virus recombinants of defined genetic composition. J Virol. 1976 Oct;20(1):248–254. doi: 10.1128/jvi.20.1.248-254.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Isachenko V. A., Carter M. A new avian influenza virus from feral birds in the USSR: recombination in nature? Bull World Health Organ. 1974;51(4):325–332. [PMC free article] [PubMed] [Google Scholar]
- Yamane N., Arikawa J., Odagiri T., Sukeno N., Ishida N. Isolation of three different influenza A viruses from an individual after probable double infection with H3N2 and H1N1 viruses. Jpn J Med Sci Biol. 1978 Oct-Dec;31(5-6):431–434. doi: 10.7883/yoken1952.31.431. [DOI] [PubMed] [Google Scholar]
- Young J. F., Desselberger U., Palese P. Evolution of human influenza A viruses in nature: sequential mutations in the genomes of new H1N1. Cell. 1979 Sep;18(1):73–83. doi: 10.1016/0092-8674(79)90355-6. [DOI] [PubMed] [Google Scholar]