Glucose polymers with both the right branching pattern and the right chain length distribution are required to produce semicrystalline starch granules.
Abstract
The major component of starch is the branched glucan amylopectin. Structural features of amylopectin, such as the branching pattern and the chain length distribution, are thought to be key factors that enable it to form semicrystalline starch granules. We varied both structural parameters by creating Arabidopsis (Arabidopsis thaliana) mutants lacking combinations of starch synthases (SSs) SS1, SS2, and SS3 (to vary chain lengths) and the debranching enzyme ISOAMYLASE1-ISOAMYLASE2 (ISA; to alter branching pattern). The isa mutant accumulates primarily phytoglycogen in leaf mesophyll cells, with only small amounts of starch in other cell types (epidermis and bundle sheath cells). This balance can be significantly shifted by mutating different SSs. Mutation of SS1 promoted starch synthesis, restoring granules in mesophyll cell plastids. Mutation of SS2 decreased starch synthesis, abolishing granules in epidermal and bundle sheath cells. Thus, the types of SSs present affect the crystallinity and thus the solubility of the glucans made, compensating for or compounding the effects of an aberrant branching pattern. Interestingly, ss2 mutant plants contained small amounts of phytoglycogen in addition to aberrant starch. Likewise, ss2ss3 plants contained phytoglycogen, but were almost devoid of glucan despite retaining other SS isoforms. Surprisingly, glucan production was restored in the ss2ss3isa triple mutants, indicating that SS activity in ss2ss3 per se is not limiting but that the isoamylase suppresses glucan accumulation. We conclude that loss of only SSs can cause phytoglycogen production. This is readily degraded by isoamylase and other enzymes so it does not accumulate and was previously unnoticed.
Starch, the major storage carbohydrate in plants, is composed of two α-1,4- and α-1,6-linked glucan polymers: moderately branched amylopectin and predominantly linear amylose. Amylopectin, which constitutes approximately 80% of most starches, is synthesized by three enzyme activities. Starch synthases (SSs) transfer the glucosyl moiety of ADP-Glc to a glucan chain, forming a new α-1,4 glucosidic linkage, extending the linear chains. Branching enzymes (BEs) cleave some α-1,4 linkages and reattach chains of six Glc units or more via α-1,6 linkages, creating branch points. Debranching enzymes (DBEs) hydrolyze some of these branches, tailoring the structure of the polymer. However, the way in which the individual enzymes work together to create crystallization-competent amylopectin remains unclear.
The coordinated actions of SSs, BEs, and DBEs are thought to produce a glucan with a tree-like architecture in which the branch points are nonrandomly positioned. According to models of amylopectin, clusters of unbranched chain segments are formed. Within these clusters, adjacent chains form double helices, which align in parallel giving rise to crystalline lamellae. These alternate with amorphous lamellae containing the branch points and chain segments that span the clusters (Zeeman et al., 2010). In the context of this amylopectin model, glucan chains can be categorized according to their length and connection to other chains. The A chains are external chains that do not carry other branches. The B chains carry one or more branches (either an A chain or another B chain) and have both external and internal segments. The B chains can span one or more clusters (e.g. a B1 chain spans one cluster). The C chain is the single chain that has a reducing end (Manners, 1989). The A chains tend to be the shortest, having an average chain length (ACL) of 12 to 16, depending on the species (Hizukuri, 1986). Together with the B1 chains, the A chains are thought to make up the crystalline clusters. Longer chains such as B2 chains (ACL 20–24) or B3 chains (ACL 42–48) are presumed to connect clusters (Hizukuri, 1986). Amylose is a distinct polymer synthesized within the amylopectin matrix by granule-bound SS (Tatge et al., 1999). Mutants lacking granule-bound SS also lack amylose but still make starch granules, showing that amylose synthesis is not required for this (Zeeman et al., 2010).
The structural properties of amylopectin contrast with those of glycogen, the Glc polymer synthesized in organisms such as fungi, animals, and most bacteria. Glycogen also consists of α-1,4-linked Glc chains with α-1,6-linked branches, but differs in three major ways from amylopectin. First, its external branches are considerably shorter (6–8 Glc units compared with 12–16 in amylopectin). Second, the branch frequency (10%) is twice as high as in amylopectin. Third, its branch points are assumed to be distributed homogeneously, whereas branching in amylopectin is thought to be nonhomogeneous. These differences prevent the formation and parallel alignment of double helices in glycogen, rendering it soluble. Glycogen synthesis requires only a single glycogen synthase enzyme and a single glycogen BE, whereas several SS and BE isoforms are involved in amylopectin synthesis. In Arabidopsis (Arabidopsis thaliana), there are four SSs (SS1–SS4) and two BEs (BE2 and BE3; Li et al., 2003; Streb and Zeeman, 2012). In addition, Arabidopsis has three DBEs. ISOAMYLASE1-ISOAMYLASE2 (hereafter referred to simply as ISA), a heteromultimeric enzyme composed of the two subunits ISA1 and ISA2, is implicated in amylopectin synthesis (Delatte et al., 2005). The other two DBEs, ISA3 and LIMIT DEXTRINASE (LDA), are implicated in starch degradation (Delatte et al., 2006).
Loss of specific SS isoforms has different effects on the starch amount, amylopectin chain length distribution (CLD), and starch granule morphology, suggesting distinct functions for each isoform. For example, amylopectin from SS1-deficient mutants of Arabidopsis (Delvallé et al., 2005; Szydlowski et al., 2011) and rice (Oryza sativa; Fujita et al., 2006) has fewer chains with a degree of polymerization (DP; i.e. chain length) between 8 and 12 and more chains with a DP between 17 and 20 compared with the wild-type starches. This is consistent with in vitro data for the maize (Zea mays; Commuri and Keeling, 2001) and rice SSI enzymes (Fujita et al., 2006), which preferentially elongate short chains of DP 6 or 7 up to a length of DP 10. This indicates that SSI functions to elongate the short chains created by BEs by a few Glc units (Commuri and Keeling, 2001; Delvallé et al., 2005). Comparable studies in SS2-deficient mutants reveal amylopectin with more chains with DP 6 to 11, but depletion in chains with DP 13 to 20 compared with the corresponding wild-type amylopectins. Thus, SS2 is suggested to elongate shorter chains (e.g. those made by SS1) to a length of between DP 13 and 20 (Edwards et al., 1999; Yamamori et al., 2000; Umemoto et al., 2002; Morell et al., 2003; Zhang et al., 2004, 2008). SS3 was proposed to be important for the generation of long, cluster-spanning chains (Jeon et al., 2010; Tetlow and Emes, 2011), as well as contributing to A chain and B1 chain elongation (Edwards et al., 1999; Zhang et al., 2005, 2008). By contrast, SS4 appears to have a specialized role in initiating or coordinating granule formation (Roldán et al., 2007; Crumpton-Taylor et al., 2012, 2013). Arabidopsis ss4 mutants have just one round starch granule per chloroplast rather than five or more lenticular granules observed in the wild type.
Partial loss of BE activity in maize (Stinard et al., 1993), rice (Mizuno et al., 1993), and potato (Solanum tuberosum; Schwall et al., 2000) leads to starches with high apparent amylose, most likely caused by the accumulation of less frequently branched amylopectin. A total lack of branching activity in Arabidopsis be2be3 mutants, however, abolishes starch production. Instead, maltose accumulates, suggesting that linear glucans are produced, but degraded by α- and β-amylases (Dumez et al., 2006).
Loss of DBE of the ISA1 class causes a dramatic phenotype, with production of a soluble glucan (phytoglycogen) in place of starch. This has been observed in starch-synthesizing tissues of several species, including Chlamydomonas reinhardtii cells (Mouille et al., 1996), Arabidopsis leaves (Delatte et al., 2005; Wattebled et al., 2005), and the endosperms of maize (Zea Mays; James et al., 1995), rice (Oryza sativa; Nakamura et al., 1997), and barley (Hordeum vulgare) seeds (Burton et al., 2002). Phytoglycogen has structural similarities to glycogen in that both are water soluble and have a higher branch frequency than amylopectin. Accordingly, it was proposed that the trimming of glucans produced by SS and BE isoforms by ISA1 removes branches that interfere with the formation of secondary and tertiary structures (i.e. organized arrays of double helices), thereby facilitating amylopectin biosynthesis and crystallization (Ball et al., 1996). Compared with ISA1, the other two DBEs (LDA and ISA3) have different substrate specificities, both preferring substrates with short outer chains, such as β-limit dextrins, suggesting that their role is primarily in starch degradation. Consistently, mutating these genes in Arabidopsis causes a starch-excess phenotype rather than phytoglycogen accumulation (Delatte et al., 2006).
Although it is now widely accepted that a degree of debranching occurs to control branch number and positioning in amylopectin, the importance of this for crystalline starch production is still uncertain. Several studies have shown that some cell types in isa1-deficient mutants still produce some starch (e.g. epidermal and bundle sheath cells in Arabidopsis mutants; Delatte et al., 2005), indicating that other factors can also affect the partitioning between phytoglycogen and starch.
No starch granules are made in the Arabidopsis isa1isa2isa3lda quadruple mutant, which lacks all three DBEs (Streb et al., 2008). Although suggestive of redundancy between the DBEs, the loss of each enzyme has distinct effects on amylopectin or phytoglycogen structure, consistent with their different substrate specificities. Furthermore, the loss of starch granules in isa1isa2isa3lda was shown to be at least partly due to the actions of α-amylase; typical α-amylolytic products (short malto-oligosaccharides) accumulated alongside phytoglycogen. Mutation of the gene encoding the chloroplastic α-AMYLASE3 (AMY3) eliminated these short malto-oligosaccharides and restored starch granule biosynthesis in all cell types examined. This unexpected result showed that crystalline glucans can be produced in the absence of DBE activity, despite an altered branching pattern. Streb et al. (2008) proposed that AMY3 shortens external chains of the glucans made by SSs and BEs so that they cannot form double helices with their neighbors. This idea is consistent with models for amylopectin, in which a suitable CLD is a critical factor in the formation of the secondary and higher-order crystalline structures (Gidley and Bulpin, 1987; Pfannemüller, 1987). Thus, factors that affect the CLD, such as a failure to sufficiently elongate new branches or concomitant chain degradation by amylases, should also affect crystallinity. Indeed, early studies of maize mutants (that were subsequently shown to be affected in DBE and SS activities) reported that loss of SS in a DBE mutant background altered the ratio of starch to phytoglycogen compared with the DBE mutants alone (Cameron and Cole, 1954; Creech, 1965).
The aim of this work was to use genetics to systematically vary both branch point position and chain lengths and determine the impact on glucan amount, structure, and starch granule formation in Arabidopsis. We analyzed mutants lacking combinations of SSs (to vary chain lengths) in the absence of the debranching enzyme ISA1-ISA2 (to change branch point distribution/frequency). This revealed that the length of external chains is a key factor in the production of a crystallization-competent glucan. Remarkably, our results also provide evidence for phytoglycogen production due to mutations just in SSs. Our results indicate that this phenomenon is largely masked by the presence of ISA1-ISA2, which degrades the aberrant glucan instead of trimming it to amylopectin.
RESULTS
Isolation of Homozygous Mutants
We crossed the mutant lacking SS1, SS2, and SS3 (ss1ss2ss3; Szydlowski et al., 2011) with the mutant lacking ISA1 and ISA2 (isa1isa2; Delatte et al., 2005). Mutants of SS4 were not included in this study because SS4 has a special role in granule initiation and has not been implicated in the control of chain lengths per se (Roldán et al., 2007; Szydlowski et al., 2009). All multiple mutants were selected using PCR-based genotyping (see the “Materials and Methods”; Supplemental Table S1). Because isa1, isa2, and isa1isa2 mutants were previously shown to be phenotypically identical due to the lack of the ISA1-ISA2 enzyme activity (Delatte et al., 2005), we selected mutants lacking either ISA1 or both ISA1 and ISA2 and assigned them hereafter as isa mutants for simplicity (Supplemental Table S2). The absence of the corresponding SS and ISA enzyme activities was confirmed using native PAGE and in-gel activity assays (zymograms; Supplemental Fig. S1; Supplemental Methods S1), with the exception of SS2, which was not detectable on our gels. We observed small changes concerning the activity of SS1 and the migration of BE2 and PHOSPHORYLASE1 through our set of mutant lines. This variability, however, was also visible in the two wild types (ecotype Wassilewskija of Arabidopsis [WS] and ecotype Columbia-0 of Arabidopsis [Col-0]) and is likely due to an ecotype effect. In addition, there was variation in the activity of a β-AMYLASE5 apparent on our gels. The expression of this enzyme is known to be variable and responds to fluctuation in sugar levels (Caspar et al., 1989; Fulton et al., 2008). However, the enzyme is nonchloroplastic and does not appear to be involved in starch metabolism (Wang et al., 1995; Laby et al., 2001).
Phenotypes of ssisa Mutants
Previous studies have shown that isa mutants have lower starch levels than wild-type plants, but accumulate soluble phytoglycogen instead. To investigate whether the additional loss of SSs affects this phenotype, we analyzed our newly isolated set of ssisa mutants for glucan content and structure, as well as their growth phenotypes. For comparison, it was necessary to grow and reanalyze the parental ss and isa mutants in parallel.
Among the ss mutants, ss1ss3, ss2ss3, and ss1ss2ss3 were smaller than the respective WS or Col-0 wild type (Fig. 1). In the case of ss1ss3, this contrasts with a previous report in which no growth phenotype was observed, although no quantitative data were presented (Szydlowski et al., 2011). The ss1ss2ss3 triple mutant had the strongest retardation in growth, together with pale and hyponastic leaves. The isa1, isa2, and isa1isa2 mutants grew slightly slower than the wild type (Col-0). The ssisa mutants also grew slowly, displaying a lower average fresh weight (FW) than the wild types. Among the multiple mutants, ss1ss2ss3isa was the smallest and was similar in appearance to ss1ss2ss3.
Figure 1.
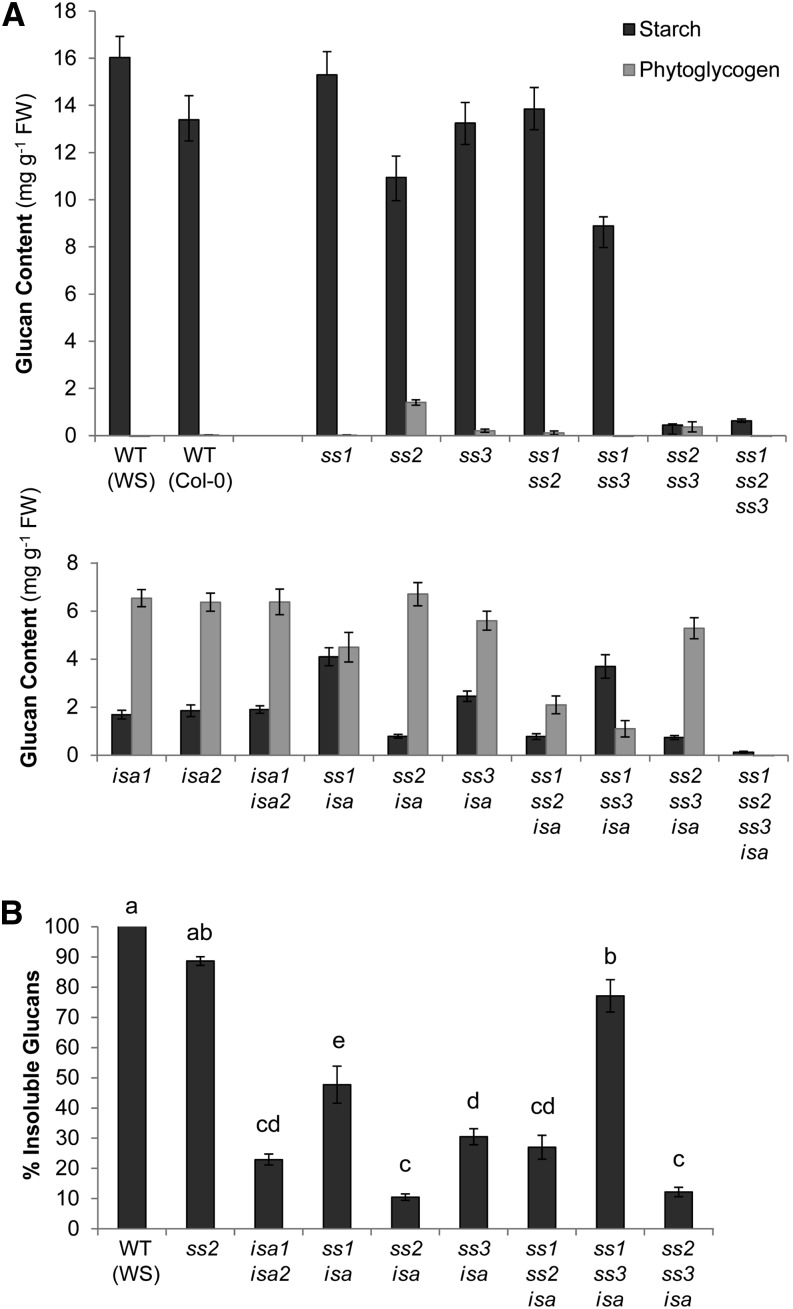
Iodine staining of ssisa mutants. Plants were harvested at the end of the day, decolorized in hot ethanol, stained for starch with iodine solution, rinsed in water, and photographed. FW (in grams) of whole rosettes at the time of harvest is given as the mean ± se (n = 6, except n = 10 for ss1ss2ss3 and ss1ss2ss3isa). Starch stains dark and bluish, whereas phytoglycogen stains weakly with an orange-brown color. WT, Wild type.
Iodine staining at the end of a normal 12-h day revealed that all single ss mutants (ss1, ss2, and ss3) and the ss1ss2 and ss1ss3 double mutants stained similarly to the wild type (Fig. 1). The ss1ss2ss3 plants stained only slightly and ss2ss3 plants hardly stained at all, indicating a very low starch content. The isa mutants had an altered, more reddish-brown staining, reflecting their phytoglycogen accumulation and low starch content. These observations are in agreement with previous reports (Delatte et al., 2005; Zhang et al., 2008). Iodine staining of ss1isa and ss1ss3isa resulted in a darker, more bluish color than the isa parental line, suggesting the presence of considerably higher levels of starch. No major alteration in staining was observed in the other ssisa mutant combinations compared with isa.
Starch and Soluble Glucan Content
Iodine straining gives a rapid indication of starch content and/or structure, but is largely qualitative. Therefore, we harvested plants at the end of the day and measured starch and phytoglycogen. Extraction was done using perchloric acid to inactivate enzymes and prevent changes in starch amount/structure (Delatte et al., 2005). Previous work showed that isa mutants contain a spectrum of glucans ranging from insoluble starch granules to soluble phytoglycogen. Here, we define insoluble glucans (including starch) as that pelleted by centrifugation at 3,000g for 10 min. Phytoglycogen in the supernatant was precipitated using 75% (v/v) methanol. Both insoluble glucans and phytoglycogen were quantified after digestion to Glc (see the “Materials and Methods”).
As expected from the iodine staining and previous studies, all ss single and double mutants except for ss2ss3 had considerable amounts of starch (Fig. 2A). However, reduced levels were found in ss1ss3 and, to a smaller extent, in ss2 mutants compared with the wild type. Interestingly, in ss2, small amounts of phytoglycogen were found. The ss2ss3 mutant had a much lower glucan content, part of which was also in the phytoglycogen fraction. This was also observed in two additional ss2ss3 lines of different ecotype backgrounds (Supplemental Fig. S2B). The ss1ss2ss3 mutants had a very low glucan content, all of which was insoluble.
Figure 2.
Starch and soluble glucan content from ssisa mutants at the end of the day. Whole rosettes from plants were harvested at the end of the day and immediately frozen in liquid nitrogen. Starch and soluble glucans (phytoglycogen) were extracted using perchloric acid, and glucans were quantified after enzymatic digestion to Glc. Each value is the mean ± se (n = 6, except n = 10 for ss1ss2ss3 and ss1ss2ss3isa). A, Starch (black bars) and phytoglycogen (gray bars) content from the wild types and ss, isa, and ssisa mutant lines. B, Insoluble glucans given as a percentage of the total glucan measured in selected lines. Different letters correspond to values that are statistically different (tested with one-way ANOVA [Tukey test], P ≤ 0.01). WT, Wild type.
The isa1, isa2, and isa1isa2 mutants were indistinguishable from each other, displaying a strong shift toward phytoglycogen accumulation (Fig. 2A; Delatte et al., 2005; Wattebled et al., 2005). All combined ssisa mutants also contained phytoglycogen, except for ss1ss2ss3isa, in which essentially no glucan was measurable. However, the amount of insoluble, soluble, and total glucans varied strongly from line to line. Calculation of the percentage of insoluble glucans from total glucans showed that there was a specific impact for the loss of each individual SS (Fig. 2B). The loss of SS1 increased the amount of insoluble glucan in the absence of ISA activity, both in absolute terms and when expressed as percentage of the total glucan (ss1isa and ss1ss3isa). Remarkably, the loss of SS2 had the opposite effect, decreasing the insoluble glucan contents in the absence of ISA activity (ss2isa and ss2ss3isa). The effect of losing SS3 was smaller, tending to increase the percentage of insoluble glucans (compare ss3isa with isa mutants and ss1ss3isa with ss1isa). In the ss1ss2isa mutant, which produced low amounts of total glucan, the percentage of insoluble glucans was similar to the isa mutants. This suggests that the opposite effects observed when losing SS1 or SS2 are balanced when both are lost.
We also analyzed our mutant set for starch and phytoglycogen levels at the end of a normal night. At this point, all lines were essentially free of phytoglycogen and contained only low levels of insoluble glucans (Supplemental Table S3).
Chain Length Profile Differences in ssisa Mutants Correlate with the Synthesis of Insoluble Glucans
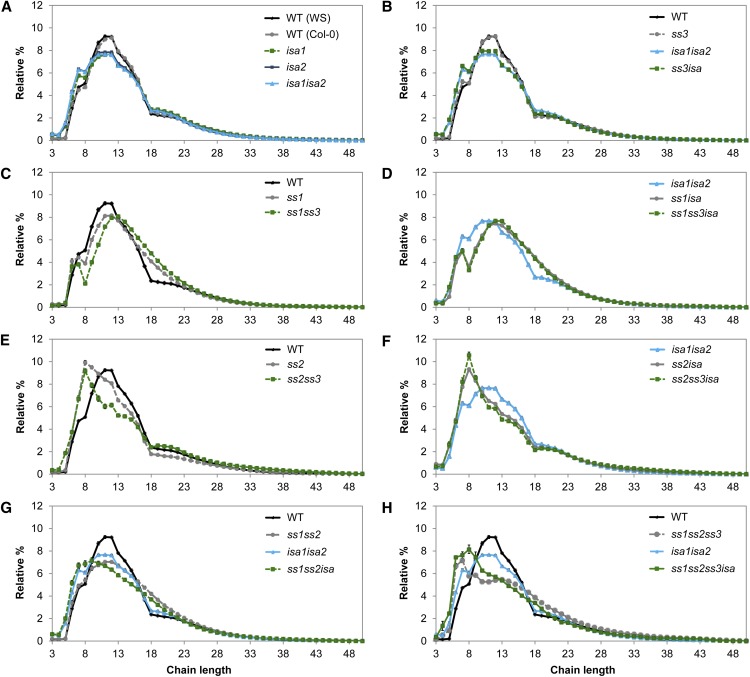
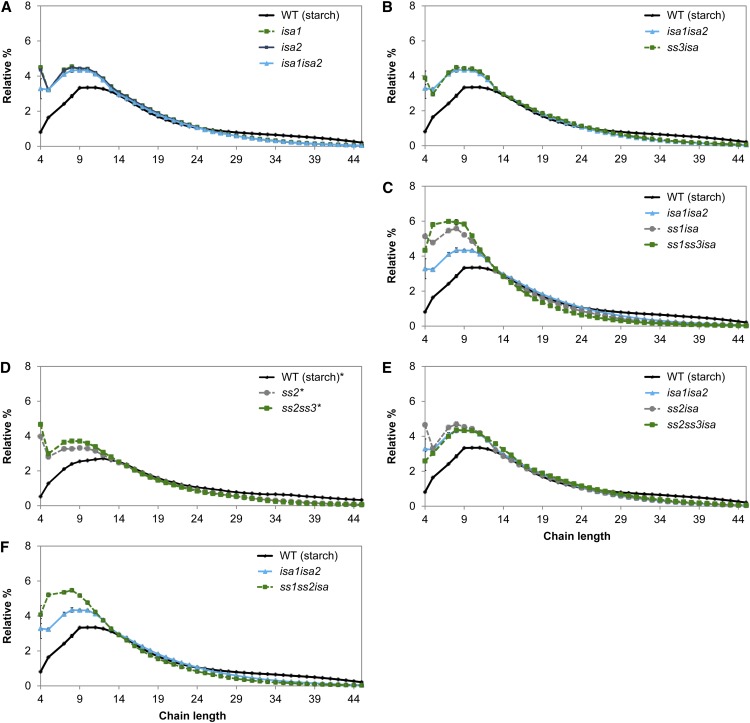
Loss of SSs often results in changes in starch structure, particularly the lengths of the glucan chains. We obtained the CLD profiles from insoluble glucans and, where appropriate, phytoglycogen from the ssisa mutants. The amylopectin CLD profiles from the WS and Col-0 wild types were indistinguishable, indicating that there was no ecotype background effect (Fig. 3A). The loss of ISA activity (in isa1, isa2, and isa1isa2) resulted in an increased number of chains from DP 3 to 8 and a decreased number of chains from DP 10 to 16 in the insoluble glucans (Fig. 3A), in line with earlier results (Delatte et al., 2005).
Figure 3.
Influence of ssisa mutations on CLD of insoluble glucans. A to H, Insoluble glucans from the given mutants were debranched and the resultant linear chains analyzed using high-performance anion-exchange chromatography with pulsed-amperometric detection (HPAEC-PAD). Peak areas from DP 3 to 50 were summed and the relative peak area for each chain length was calculated. Values are the means ± se from three or four biological replicates. B to H, The CLD of the wild type (WS, in black) or isa1isa2 (in cyan), or both are shown for ease of comparison. Further comparisons (difference plots) are presented in Supplemental Figure S3. WT, Wild type.
We sorted the CLDs of insoluble glucans from the ssisa mutants, examining the impact of the loss of individual SS isoforms in the isa background. For the ss mutants, our data generally confirmed earlier reports (Zhang et al., 2008; Szydlowski et al., 2011). The CLD from the ss3 mutant was comparable to that of the wild type (Fig. 3B). Similarly, the CLD of ss3isa was almost identical to the CLD of isa, suggesting that loss of SS3 alone has only little measurable effect on CLD.
The loss of SS1 resulted in significant changes in the amylopectin CLD, showing a shift toward longer chains. There were fewer chains from DP 7 to 12 and more chains of DP 14 to 23 compared with the wild type (Fig. 3C). A similar trend was observed for the loss of SS1 in the isa background (Fig. 3D), in which ss1isa displayed a comparable shift toward longer chains compared with isa1isa2. Notably, ss1isa had significantly more insoluble glucans compared with isa (Fig. 2). The opposite was observed in mutants lacking SS2: Insoluble glucans from ss2 were enriched in short chains (particularly around DP 8) and had fewer chains in the region of DP 10 to 18 (Fig. 3E). The same trend toward shorter chains was also visible in the ss2isa mutant (Fig. 3F), in which a decrease of insoluble glucans was measured compared with isa (Fig. 2).
Next, we examined the impact of the loss of multiple SS isoforms. The CLD of ss1ss3 was quite similar to that of ss1 in having a shift toward longer chains compared with the wild type (Fig. 3C). The ss1ss3isa triple mutant had CLD changes that were comparable to ss1isa compared with isa (Fig. 3D). We also observed more insoluble glucans than in isa (Fig. 2). The CLD of ss2ss3 had a shift toward shorter chains compared with the wild type, although the profile was not quite identical to that of the ss2 single mutant (Fig. 3E). The double mutant had fewer chains between DP 8 and 17 than ss2. Zhang et al. (2008) also reported a difference between these mutants, although the previously reported CLDs and those presented here are not directly comparable. Despite the differences between ss2 and ss2ss3, the ss2ss3isa triple mutant had a comparable CLD to the ss2isa (Fig. 3F). As with ss2isa, ss2ss3isa had fewer insoluble glucans than isa (Fig. 2).
The CLDs from ss1ss2 and ss1ss2isa mutants had features resulting both from the loss of SS1 (i.e. more chains from approximately DP 17–23 relative to ss2 and ss2isa, respectively) and from the loss of SS2 (i.e. more chains around DP 8 relative to ss1 and ss1isa, respectively; Fig. 3G). Similarly, the CLDs from ss1ss2ss3 and ss1ss2ss3isa display characteristics from the loss of both SS1 and SS2. These profiles also reveal an impact of the additional lack of SS3 (Fig. 3H). For further comparisons of the changes in CLD profiles (difference plots) upon the loss of individual SSs, see Supplemental Figure S3. Overall, these data reveal an important relationship between the lengths of glucan chains and the production of insoluble and soluble glucans. Within the DP range associated with cluster-forming A chains and B1 chains, longer chain lengths correlate with an increased production of insoluble glucans and shorter chains correlate with an increased production of phytoglycogen.
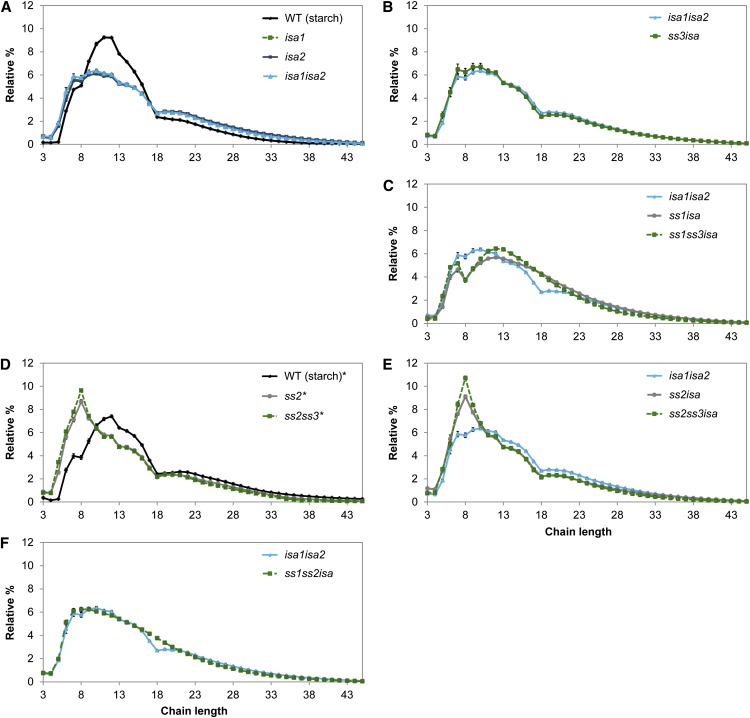
We also obtained the CLDs from phytoglycogen, where present. It is worth noting that phytoglycogen is susceptible to modifications during its synthesis by glucan-degrading enzymes (e.g. amylases; Delatte et al., 2005; Streb et al., 2008). Consequently, the structure obtained does not only result from the action of synthesizing enzymes, but also from degrading enzymes, and should thus be interpreted with caution. The specific changes in chain lengths observed upon the loss of SS1 and SS3 in insoluble glucans were also visible in the CLDs of phytoglycogen (Fig. 4, B and C; Supplemental Fig. S4, A–C and G–I). Loss of SS2 again resulted in a prominent increase of chains around DP 8, but the decrease of DP 10 to 18 was less pronounced than in the insoluble glucans (Fig. 4, D and E; Supplemental Fig. S4, D–F). Thus, the impact of losing SS1, SS2, or SS3 on the structure of both insoluble and soluble glucans is similar. Interestingly, the phytoglycogen CLDs exhibited a decrease in the number of chains between DP 8 and 17 compared with the insoluble glucans from the same line. The lines ss2isa and ss2ss3isa were exceptions, because the CLDs from the soluble and insoluble glucans were almost identical (compare Figs. 3F and 4E).
Figure 4.
Influence of ssisa mutations on CLD of phytoglycogen. A to F, Phytoglycogen from the given mutants was debranched and the resultant linear chains were analyzed using HPAEC-PAD. Peak areas from DP 3 to 45 were summed and the relative peak area for each chain length was calculated. Values are the means ± se from four replicates (except n = 2 for ss1ss3isa, n = 3 for ss1isa and ss2isa, and n = 5 for ss2 and ss2ss3,). For ease of comparison, the CLD from the wild-type (WS) starch is shown in A and D, whereas the CLD for isa1isa2 phytoglycogen is shown in B, C, E, and F. Further comparisons (difference plots) are presented in Supplemental Figure S4. The data for ss2 and ss2ss3 (marked by asterisks) were obtained from a separately grown batch of plants and the corresponding CLD from wild-type starch is included (Supplemental Fig. S2 contains further analyses of ss2ss3). WT, Wild type.
Branch Point Distribution Is Altered in ssisa Mutants
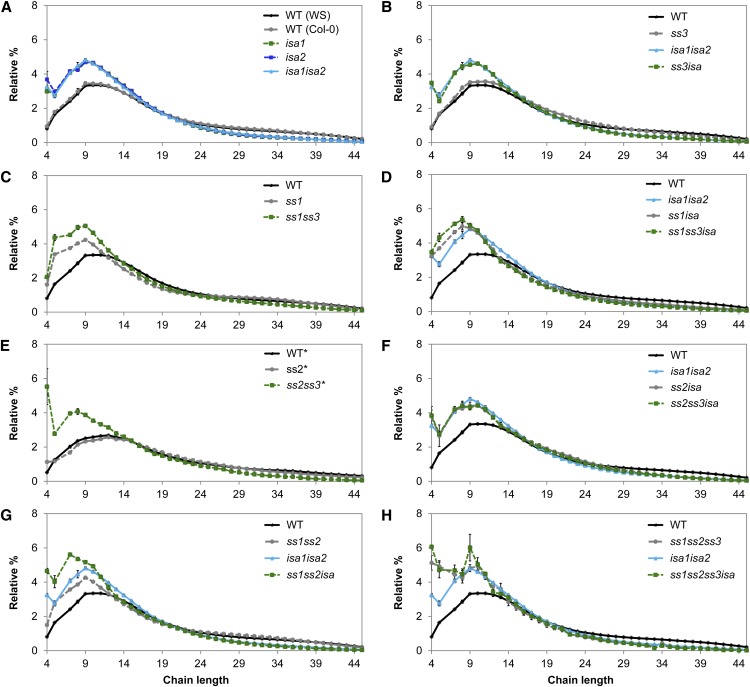
Normal CLDs do not give information about how chains are connected to each other. Therefore, we generated CLDs of β-limit dextrins in which the glucans are first digested with β-amylase prior to the enzymatic debranching and separation by HPLC. β-Amylase removes maltose units from the nonreducing ends of all chains until reaching a branch point. It leaves A-chain stubs of 2 or 3 Glc units and, when acting on B chains, stops 1 or 2 Glc units from the branch point. Accordingly, the length of the remaining B chains reflects the distance between their attachment to another chain and the outermost branch they carry (plus 1 or 2 Glc units). When B chains carry two branches, this method gives only the distance to the outermost branch.
The β-limit dextrins of amylopectin from isa1, isa2, and isa1isa2 have an increase in chains from DP 4 to 17, with slightly fewer chains above this length (Fig. 5A; Delatte et al., 2005), showing that the branch point distributions are altered. In particular, the increase in short residual B chains (which unlikely carry additional branches themselves) suggests that the branch points are closer together than in the wild type (Delatte et al., 2005). Similar tendencies were found in all ss mutant combinations lacking SS1 (Fig. 5, C, G, and H), in which chains of DP 4 to 12 were increased relative to the wild type. In the ss2 and ss3 single mutants, the β-limit CLD was almost like that of the wild type, indicating that the branch point distribution is essentially unaltered in these mutants. These results from the ss mutants are mostly in accordance with data published in Szydlowski et al. (2011). Interestingly, the β-limit CLD of the ss2ss3 double mutant differed considerably from the wild type and had more short chains, particularly DP 4. These alterations were even more pronounced in ss1ss2ss3.
Figure 5.
β-Limit CLD of insoluble glucans. A to H, Insoluble glucans of the given lines were first treated with β-amylase to digest the exterior chains until a branch point was reached. The resultant β-limit glucan was then debranched to obtain linear chains that were analyzed by HPAEC-PAD. Peak areas for chains of DP 2 to 45 were summed. DP 6 was excluded because it showed variable inconsistent abundances (Delatte et al., 2005). Relative peak areas were calculated for each chain length. Values are the mean ± se (n = 4, except n = 3 for ss1isa, ss3isa, and ss1ss2ss3isa). For ease of comparison, the β-CLD from wild-type (WS) and/or isa1isa2 starch is shown in each graph. The data for ss2 and ss2ss3 (marked by asterisks) were obtained from a separately grown batch of plants and the β-CLD from the corresponding wild-type starch is included. WT, Wild type.
The β-limit dextrins of insoluble glucans from the ssisa mutants all had an increased number of chains of DP 4 to around 12 (Fig. 5). In ss2isa and ss3isa, in which no change was observed in the corresponding ss line, the CLD was almost identical to the isa CLD (Fig. 5, B and F), again suggesting little influence of SS2 or SS3 on the branching pattern in these backgrounds. Surprisingly, the β-limit CLD of ss2ss3isa was also very similar to the isa mutant, indicating that the individual effects from loss of SS and ISA do not sum up. Similarly, the β-limit CLDs from ss1isa, ss1ss3isa, and ss1ss2ss3isa were only slightly different from those of ss1, s1ss3, and ss1ss2ss3, respectively. Only in the case of ss1ss2isa did we obtain a clear additive effect from the loss of SS1 and SS2 and ISA. Together, these data show that branching patterns of insoluble glucans from all ssisa mutants are altered. It should be noted that these distributions only provide an average picture, so similar distributions may arise from different structures. This might explain why no additive effects were observed upon loss of SS and ISA in some cases.
We obtained the β-limit CLDs from phytoglycogen where appropriate. Most of these distributions resembled the distributions from the insoluble glucans (Fig. 6). The only exception was SS2. Whereas the β-limit CLD of starch is similar to the distribution of the wild type, the β-limit distribution of phytoglycogen more resembled the distribution of isa mutants, with more short chains of DP 4 to 13 (Fig. 6, A and D). This suggests that for some reason the branching is distorted in phytoglycogen from ss2, but not in its insoluble glucans.
Figure 6.
β-Limit CLD of phytoglycogen. A to F, The β-CLDs of soluble glucans were obtained as described in Figure 5. Values are means ± se (n = 4, except n = 3 for ss1isa and ss1ss3isa and n = 5 for ss2 and ss2ss3). For ease of comparison, the β-CLDs from isa1isa2 phytoglycogen and/or wild-type starch (WS) are shown in each graph. The data for ss2 and ss2ss3 (marked by asterisks) were obtained from a separately grown batch of plants and the β-CLD from the corresponding wild-type starch is included. WT, Wild type.
Formation of Starch Granules Is Restored in the Mesophyll in ss1isa Mutants, But Is Abolished in All Cell Types in ss2isa Mutants
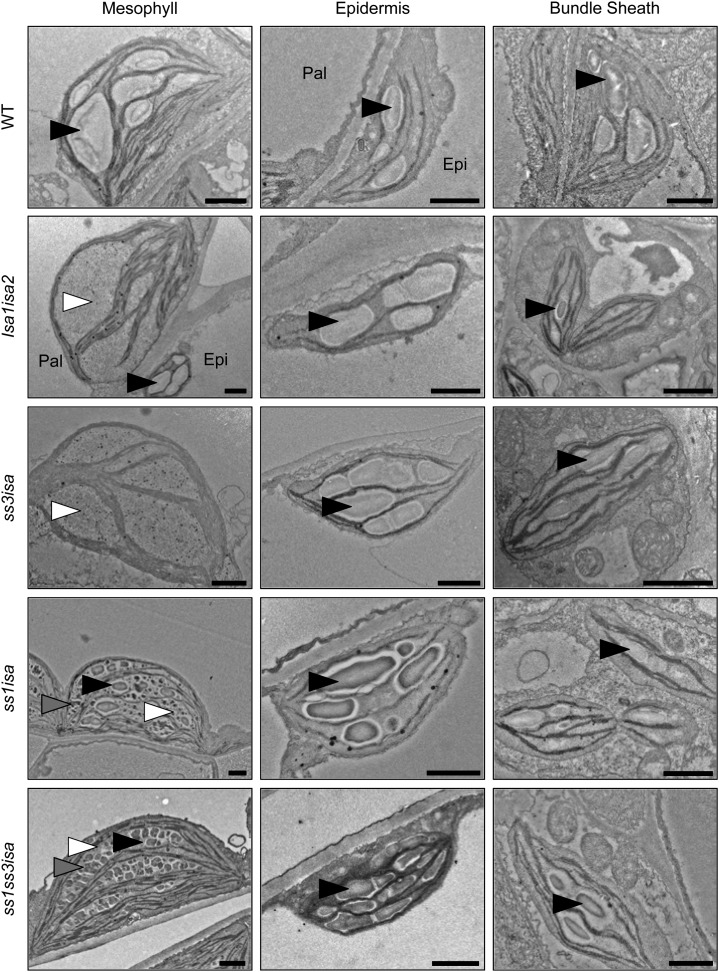
We analyzed mesophyll, epidermal, and bundle sheath cells from leaves by transmission electron microscopy (TEM) to observe the appearance of the soluble and insoluble glucans in the ssisa mutants. The wild type contains starch granules in all of these cell types (Fig. 7). In isa mutants, only phytoglycogen-like particles were detected in most mesophyll chloroplasts, but starch granules were present in all epidermal and bundle sheath cell plastids (Fig. 7), as previously observed (Delatte et al., 2005; Streb et al., 2008).
Figure 7.
Production of starch in the mesophyll is restored by loss of SS1 in the isa background. Leaves from the indicated lines were harvested close to the end of day, fixed with glutaraldehyde, and embedded in resin, and plastids in different cell types were visualized by TEM. Starch granules (black arrowheads), phytoglycogen (white arrowheads), and intermediate structures between starch and phytoglycogen (gray arrowheads) are indicated. Epi, Epidermal cell; Pal, palisade cell; WT, wild type. Bars = 1 µm.
The ss3isa mutant, in which we had measured similar glucan levels as in isa, was phenotypically very similar to isa with phytoglycogen in the mesophyll but starch granules in other leaf cell types (Fig. 7). By contrast, the chloroplasts from all mesophyll cells of the ss1isa mutant contained several starch granules, together with structures that appeared intermediate between starch and phytoglycogen (Fig. 7). The plastids from epidermal and bundle sheath cells all had starch granules and were free of phytoglycogen, as in the wild type and the isa mutants. Thus, the increase in insoluble glucans measured in this mutant is due to the appearance of starch granules in the mesophyll. In the mesophyll cell chloroplasts of ss1ss3isa, we observed structures that were clearly distinct from phytoglycogen, appearing as discrete, well-defined particles. However, they did not resemble normally shaped starch granules as in the ss1isa mutant; rather, they appeared as small cracked granules (Fig. 7). Epidermal and bundle sheath cell plastids contained starch granules, which were similar in appearance to the wild type and the isa mutants. Thus, the cracked particles in the mesophyll probably represent the increased insoluble glucan measured in this line relative to isa.
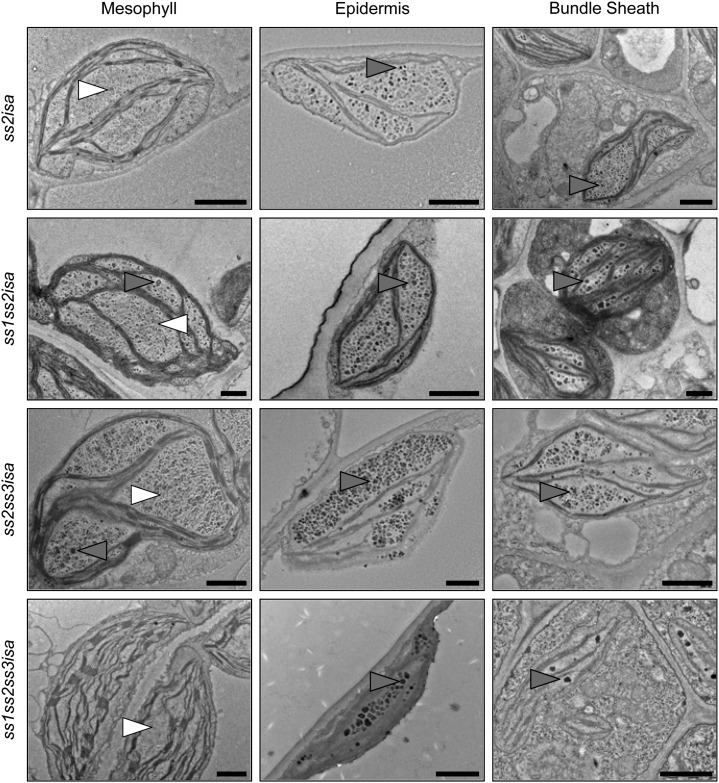
Remarkably, we did not find any starch granules in different sections of mutants lacking both SS2 and ISA activity (ss2isa, ss1ss2isa, ss2ss3isa, and ss1ss2ss3isa), neither in the mesophyll nor in the other leaf cell types examined (Fig. 8). Again, some glucans, particularly in the epidermal and bundle sheath cells, appeared as intermediate between starch and phytoglycogen. The ss1ss2ss3isa mutant generally contained only few glucans (Fig. 8), which is consistent with our measurements (Fig. 2). These micrographs strengthen our initial finding that SS activities present influence whether starch or phytoglycogen is produced in the isa background. Whereas loss of SS1 promotes the formation of starch granules or other insoluble glucans, loss of SS2 seems to prevent it, suggesting that it has a particularly important role in establishing the structures required for starch granule formation in the isa background.
Figure 8.
Production of starch is abolished in plants lacking SS2 and ISA. Electron micrographs of leaf cell plastids of the indicated plant lines were obtained as described in Figure 7. No starch granules were observed, but phytoglycogen (white arrowheads) and intermediate (gray arrowheads) structures are indicated. Bars = 1 µm.
Evidence for Phytoglycogen Synthesis and Turnover in Mutants Lacking SS2
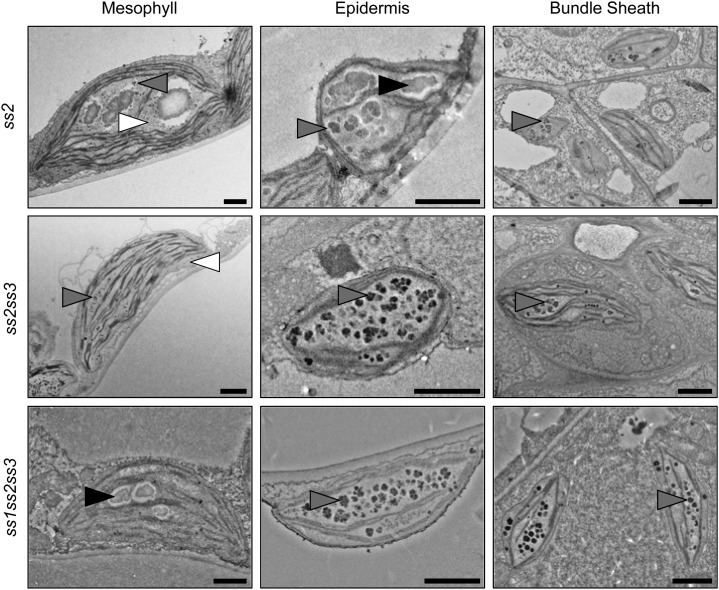
We analyzed the ultrastructure of leaf cell plastids of the ss2, ss2ss3, and ss1ss2ss3 mutants in which we had detected soluble glucans despite the presence of the ISA1-ISA2 activity (Figs. 2 and 9). Remarkably, mesophyll cells from ss2 contained irregularly shaped and unusually big granules together with particles resembling phytoglycogen and intermediate structures (Fig. 9). In epidermal cell plastids, the glucans appeared as mostly insoluble, but cracked and uneven particles. In the bundle sheath cells, we observed small, intermediate particles. The ss2ss3 mutant displayed an even stronger phenotype: In all cell types examined, we detected only phytoglycogen and intermediate particles but could not find any starch granules. This was confirmed in the other two ss2ss3 lines with different ecotype backgrounds (Fig. 9; Supplemental Fig. S2). The phytoglycogen content in the mesophyll cell chloroplasts was generally low (Fig. 9; Supplemental Fig. S2). By contrast, ss1ss2ss3 contained a few small starch granules in the mesophyll and intermediate structures in the epidermal and bundle sheath cells (Fig. 9).
Figure 9.
Plants lacking SS2 produce altered granules and phytoglycogen. Electron micrographs of leaf cell plastids of the indicated plant lines were obtained as described in Figure 7. Starch granules (black arrowheads), phytoglycogen (white arrowheads), and intermediary structures (gray arrowheads) are indicated. Bars = 1 µm.
We measured very low levels of glucans in ss2ss3, ss1ss2ss3, and ss1ss2ss3isa (Fig. 2). It is possible that there is too little SS activity remaining in ss1ss2ss3 to catalyze normal rates of glucan synthesis (only SS4 remains). However, this is unlikely to explain the low glucan levels in ss2ss3, because both SS1 and SS4 remain. Furthermore, ss2ss3isa has a total glucan content (predominantly phytoglycogen) comparable to the isa parental line (Fig. 2), indicating that SS activity is not limiting. To obtain direct evidence for a possible limitation in SS activity, we measured ADP-Glc levels in selected mutant lines at the end of the day. The ADP-Glc levels were 35-fold higher in ss1ss2ss3 (151.3 ± 23.5 nmol g−1 FW) than in the wild type (4.1 ± 0.8 nmol g−1 FW), consistent with a limitation of SS activity to consume it. However, in ss2ss3 (14.0 ± 0.7 nmol g−1 FW), the levels were only slightly elevated compared with the wild type and were comparable to the levels found in ss2ss3isa (9.5 ± 3.7 nmol g−1 FW). Thus, in both ss2ss3 and ss2ss3isa, ADP-Glc is being consumed by SSs, but only in the latter line does the glucan product accumulate.
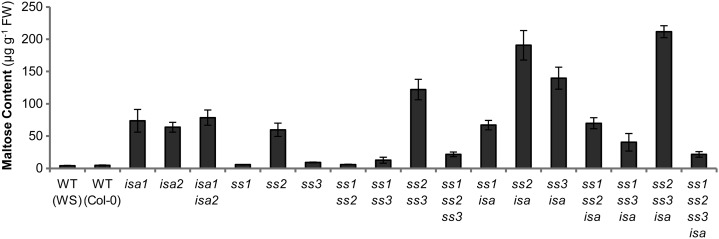
An explanation for the low level of glucans in ss2ss3 is that they are subject to turnover (i.e. degradation occurring concomitantly with synthesis), and that the ISA1-ISA2 enzyme is partially responsible for this. To obtain further information about turnover in these lines, we measured maltose levels at the end of the day. Maltose is a major intermediate of starch degradation (Niittylä et al., 2004; Zeeman et al., 2007) and has been used as an indicator for soluble glucan turnover in isa mutants, as maltose levels increase during the day, correlating with the accumulation of phytoglycogen (Delatte et al., 2005; Fig. 10). Interestingly, maltose levels were similarly elevated in the ss2 mutant and were even higher in ss2ss3, despite being normal or close to normal in the other ss mutants. These data suggest that in ss2 and ss2ss3, a proportion of the produced glucans is subject to degradation by enzymes including chloroplastic β-amylases. Among the ssisa mutants, most lines had maltose levels comparable to the isa parental line, or higher. Notably, maltose levels were highest in ss2isa and ss2ss3isa, in which we had measured the lowest proportion of insoluble glucans and observed only phytoglycogen particles but no starch granules with TEM (Figs. 2B and 8).
Figure 10.
Leaf maltose content at the end of the day. Whole rosettes were harvested at the end of the day and immediately frozen in liquid nitrogen. Maltose in the soluble fraction after perchloric-acid extraction was measured using HPAEC-PAD. Values are mean ± se (n = 4, except n = 5 for wild-type Col-0, ss1, and ss2ss3 and n = 3 for isa1isa2). WT, Wild type.
DISCUSSION
Isoamylase activity has long been considered as the most important determinant for producing semicrystalline starch granules, with isa mutants accumulating soluble phytoglycogen in what would normally be starch-storing tissues such as Arabidopsis leaves, potato tubers, cereal endosperms, and C. reinhardtii cells (James et al., 1995; Mouille et al., 1996; Nakamura et al., 1997; Burton et al., 2002; Bustos et al., 2004; Delatte et al., 2005; Wattebled et al., 2005). Mutations of the other starch biosynthetic enzymes have not been reported to cause phytoglycogen accumulation. Despite the importance of ISA, the phytoglycogen-accumulating phenotype was shown to be incomplete in many cases (e.g. starch granules in epidermal and bundle sheath cells of Arabidopsis isa mutants). Even when all debranching activity was lost from Arabidopsis, the formation of starch granules was still possible under some circumstances (Streb et al., 2008).
Here, we have demonstrated that the balance of specific SS activities is an equally important factor for the formation of starch granules. Our analysis of the set of mutants deficient in SS1, SS2, and SS3 and the ISA1-ISA2 isoamylase showed that by determining the lengths of glucan chains, SSs influence the extent to which a glucan with an aberrant branching pattern will crystallize. When SS activity is biased in favor of producing short external chains, it promotes phytoglycogen biosynthesis, whereas when SS activity is biased in favor of producing longer external chains, it promotes starch granule biosynthesis. Although this effect is most apparent in the isa background, it also appears to be the case when ISA is present, because mutation of SS2 alone results in small amounts of phytoglycogen production. Furthermore, our data suggest that ISA activity does not always promote granule synthesis. Instead, it can suppress glucan accumulation altogether, presumably by debranching a precursor glucan that lacks the chain-length characteristics needed for granule formation.
Modulation of the ISA-Deficient Phenotype by SSs
In the isa background, loss of each of the three SSs has a distinct effect. Loss of SS1 (ss1isa) partially suppresses the isa phenotype, allowing increased starch granule formation. This is shown by an increase of iodine staining intensity of whole rosettes (Fig. 1), higher quantities of insoluble glucans (Fig. 2), and the frequent occurrence of starch granules in ss1isa mesophyll cell chloroplasts, compared with isa (Fig. 7). By contrast, the loss of SS2 in the isa background (ss2isa) enhances the isa phenotype. Lower quantities of insoluble glucans were measured compared with isa (Fig. 2) and only phytoglycogen and small intermediate particles were observed using TEM, but no starch granules (Fig. 8). It is likely that some of the intermediate particles partition into the insoluble fraction during extraction, explaining why we still measure small amounts of insoluble glucan in this line (Fig. 2). In ss1ss2isa, the ratio of soluble to insoluble glucan was similar to the isa mutant. However, in some ways, the effect of losing SS2 appeared to dominate over the effect of losing SS1. For example, in the epidermal and bundle sheath cells, we did not find any starch granules in the plastids (Fig. 8).
Loss of SS3 in the isa background (ss3isa) did not cause measurable changes in the starch and phytoglycogen content compared with isa (Figs. 2 and 7). The effect of losing SS3 was more apparent when other SSs were also missing. For example, although ss1ss3isa had similar amounts of starch and phytoglycogen to ss1isa, the appearance of the insoluble glucans by TEM differed. The loss of SS3 in this background resulted in granules with a more fractured appearance, a feature also observed upon suppression of SS3 expression in potato (Marshall et al., 1996). Interestingly, the ss2ss3isa mutant was very similar to ss2isa in having no starch granules, despite the fact that the ss2 and ss2ss3 phenotypes are quite distinct (see below).
Some of the observed effects of ss mutations in the isa background are in line with other studies. Early reports from maize showed that deficiencies in SSIIa and ISA1 resulted in fewer insoluble glucans than isa1 single mutants (Cameron and Cole, 1954; Creech, 1965). More recently, expression of an active SS2 isoform in the endosperm of a japonica rice cultivar (which itself expresses an inactive SS2) increased the proportion of insoluble glucans (Fujita et al., 2012). However, other results appear to differ from our findings. For example, an enhanced accumulation of soluble glucans was reported for maize mutated in SSIII and ISA1 compared with isa1 single mutants (Creech, 1965). A recent analysis showed that, surprisingly, phytoglycogen accumulates in maize lacking SSIII and ISA2, even though both single mutants contain only small amounts of soluble glucans (Lin et al., 2012). This is intriguing because isoamylase activity is still present in this line in the form of the ISA1 homomultimeric complex (a form thus far found only in cereal endosperms). These differences between Arabidopsis and maize might reflect variation in the properties of a given isoform between the two species, variation in the relative activities of the isoforms of starch biosynthetic enzymes, or variation in other, as-yet unidentified factors.
External Chain Length as a Determinant for Glucan Insolubility
There is a strong correlation among ssisa mutant lines between the phenotypes in terms of insoluble versus soluble glucan accumulation and their CLDs. Glucans from both ss1isa and ss1ss3isa have fewer short chains (DP 7–10) but more longer chains (DP 13–21), whereas the glucans from both ss2isa and ss2ss3isa show contrary changes with more short chains (DP 5–9) and fewer longer chains (DP 11–18) compared with isa (Figs. 3 and 4). The lack of SS1 or SS2 function, respectively, is likely to directly cause these changes. The differences upon losing these SS isoforms were consistent in all backgrounds analyzed and were also similar in the insoluble and soluble glucans (Supplemental Figs. S3 and S4). Moreover, the observed changes are consistent with the reported functions of SS1 and SS2 orthologs in other organisms and from in vitro studies (see introduction).
The differences in chain length described above mostly concern chains from DP 5 to 20. Notably, these chains constitute the A chains and B1 chains, which are proposed to form the secondary and tertiary structures and make up the crystalline domains of amylopectin (Hizukuri, 1986). Analyses of crystallization properties of linear malto-oligosaccharides in aqueous solution have shown that chains of DP < 10 do not crystallize (i.e. do not form double helices) unless mixed with longer chains (Gidley and Bulpin, 1987). It has also been shown that longer chains (DP 14–20) crystallize more rapidly than shorter ones (DP 10–13; Pfannemüller, 1987). It therefore seems likely that the different crystallization capacities of the glucans from the ssisa mutants are caused by the observed differences in exterior chain length, with longer exterior chains made by SS2 and SS3 enhancing crystallization and shorter chains made by SS1 preventing it.
Branch point distribution has also been proposed as a critical factor in the formation of a glucan structure capable of crystallization and is altered in the isa mutants in addition to the CLDs (Delatte et al., 2005; this work). Altered branch point distributions were also detected in a number of the SS mutants (ss1, ss1ss3, ss2ss3, ss1ss2, and ss1ss2ss3; Szydlowski et al., 2011). An altered branching pattern can be explained by the fact that SSs generate the substrates for the BEs to act upon, so loss of SS isoforms could alter the available substrates. Interestingly, our data also show that an altered branch point distribution typical of isa mutants is still visible in lines in which starch biosynthesis is partially restored (e.g. ss1isa; Figs. 5D and 7), which suggests that longer chain lengths can compensate for an imperfect branching pattern.
The question remains as to why different mutants synthesize insoluble glucans with distinct structures, whereas many of these mutants simultaneously accumulate soluble glucans that appear structurally quite similar to their insoluble counterparts. Why do some glucans crystallize, whereas other, apparently similar glucans do not? In all cases, the CLDs from phytoglycogen and insoluble glucans from a given ssisa combination show comparable alterations toward shorter or longer chains. However, the phytoglycogen generally has fewer chains between DP 8 and 17. Because these are the chain lengths most likely to be involved in double helix formation, this could help to explain the difference in solubility (compare Figs. 3 and 4). Nevertheless, there were some genotypes in which no differences between the CLDs of the soluble and insoluble glucans could be detected (e.g. ss2isa and ss2ss3isa). In most cases, the β-limit CLDs were almost identical (with the exemption of ss2, in which the β-limit CLDs from the soluble glucans are more altered than from the insoluble ones; compare Figs. 5 and 6).
In general, it is possible that crystallization is a stochastic event, such that alterations in chain length or branching would not necessarily block crystallization but rather determine its frequency, explaining why soluble and insoluble glucans could appear structurally similar. Alternatively, there may be structural features (e.g. arrangement of chains of different lengths relative to each other) that vary between the soluble and insoluble glucans but are not captured by our analytical methods. A further possible explanation is that a defined centrifugation step might not fully sediment glucans that are partially crystalline, especially if they form a range of particle sizes (sedimentation rate is influenced by particle density, shape, and size). Thus, centrifugation for 10 min at 3,000g might leave small particles of a partially crystalline glucan in the supernatant while pelleting larger particles that have the same basic structure.
SS2 as a Determinant for Starch Granule Formation
Our data reveal that the effect of having longer external chains on crystallization is already visible in the presence of ISA1-ISA2, albeit less obvious. Glucans from ss2 mutants are enriched in short chains (DP 5–9) and have fewer chains of DP 11 to 25. Notably, we measured around 10% of the total glucans in this mutant to be soluble (Fig. 2). In addition, TEM analysis of leaves showed that the mesophyll contains a mixture of unusually big starch granules, intermediate particles, and phytoglycogen. In the epidermis and vasculature, we observed intermediate particles that are likely to be insoluble but are small and have an uneven surface (Fig. 9). The altered starch granule structure and/or morphology is consistent with reports of ss2 mutants of other species (Buléon et al., 1997; Craig et al., 1998; Edwards et al., 1999; Yamamori et al., 2000; Umemoto et al., 2002; Morell et al., 2003; Zhang et al., 2008; Szydlowski et al., 2011); however, the presence of a soluble polysaccharide was not thoroughly investigated in these reports. To our knowledge, soluble polysaccharides were only quantified in sugary2 (i.e. SSIIa-deficient) maize and in Arabidopsis ss2 mutants, in which it was reported that levels were unchanged relative to the respective wild types (Creech, 1965; Zhang et al., 2008).
Upon additional loss of SS3, the phytoglycogen-accumulating phenotype becomes clearer. Almost one-half of the measured glucans in the ss2ss3 mutant were soluble (Fig. 2) even though the total levels were strongly reduced. No starch granules were observed by TEM; only phytoglycogen or intermediate particles (Fig. 8) were observed, consistent with the absence of iodine staining of the rosettes (Fig. 1). Elevated levels of water-soluble polysaccharides were noted in an earlier report of the same mutant line under some growth conditions (Zhang et al., 2008), but the nature of this glucan was not investigated in depth. We reproducibly measured and visualized phytoglycogen accumulation and confirmed its presence in two other ss2ss3 lines with different ecotype backgrounds (Supplemental Fig. S2).
The relative increase of very short external chains and the depletion of longer external chains (Figs. 3 and 4) is likely to be a major factor in the increased solubility of glucans in ss2 and ss2ss3. That said, the CLD profiles of ss2 and ss2ss3 did not differ very much from each other, even though the two lines had contrasting starch- and phytoglycogen-accumulating phenotypes overall. A conspicuous difference was detected in the β-limit CLDs, however, with the ss2 mutant having a wild type-like β-limit CLD, whereas the ss2ss3 double mutant had a β-limit CLD enriched in short chains, indicating branch points more close together. In this respect, the glucans appeared more similar to those in the isa mutants. As discussed above, the likely explanation for this is that the loss of SS (in this case SS3) has a critical impact on the production of substrates for the BEs. This is consistent with the idea that SS3, which has tandem carbohydrate binding modules, may preferentially synthesize longer chains. Thus, we suggest that the strong impact on the solubility of glucan in ss2ss3 is attributable to both CLD and branch point distribution.
The fact that ss2 and ss2ss3 are phytoglycogen-accumulating mutants has been previously overlooked, presumably because only small amounts of phytoglycogen are present. These low amounts are most likely due to the turnover of the soluble glucans by starch-degrading enzymes including α- and β-amylases and, ironically, ISA1-ISA2 itself. The involvement of ISA1-ISA2 is indicated by the fact that the ss2ss3isa triple mutant has high glucan levels, implying that the low levels in ss2ss3 are not due to an incapacity for synthesis per se. This idea is supported by the low levels of the SS substrate ADP-Glc: Despite being three times elevated in ss2ss3 compared with the wild type, this increase is small compared to other mutants, such as ss4 and ss3ss4 (ADP-Glc 60 and 170 times elevated, respectively; Crumpton-Taylor et al., 2013; Ragel et al., 2013) or ss1ss2ss3 mutants (35 times elevated; this work). Therefore, we argue that in the ss2ss3 mutant, the combination of SS1 and SS4 results in a glucan that ISA1-ISA2 can readily debranch but without giving rise to a crystallization-competent structure. This hypothesis is supported by the recent expression of the ISA1-ISA2 enzyme in Escherichia coli, which had the effect of suppressing glycogen production rather than promoting the synthesis of a more amylopectin-like polymer (Sundberg et al., 2013).
Our measurements of maltose levels provide further evidence for glucan turnover. Maltose is present at low levels when starch is synthesized, suggesting that there are relatively few soluble substrates for β-amylase when amylopectin synthesis proceeds efficiently. Presumably, as glucans crystallize, they become relatively inaccessible. A maltose pool is seen whenever soluble glucans accumulate (e.g. in all lines deficient in ISA1-ISA2). In the isa lines, maltose levels tend to reflect the extent of soluble glucan accumulation, probably because as the amount of phytoglycogen increases, so does the number of accessible external chains (i.e. the β-amylase substrate; Delatte et al., 2005). However, ss2 and ss2ss3 represent exceptions, having high maltose levels despite the low levels of soluble glucan. We propose that this is because ISA1-ISA2-mediated debranching of the soluble glucans results in linear chains that can be degraded to maltose. This may happen to an extent in ss2; however, because this line still makes significant amounts of starch, it suggests that debranching still facilitates the crystallization (or partial crystallization) of most of the glucans made by the other SSs. However, if the product of SS1 and SS4 cannot crystallize, ISA1-ISA2-mediated debranching may happen to a much greater extent in ss2ss3. This would explain why ss2ss3 has more maltose than ss2, yet accumulates so little glucan.
In summary, this study demonstrates that both the lengths of external chains (influenced by the SS isoforms) and branching pattern (influenced by ISA1-ISA2) determine whether a crystallization-competent glucan is produced. Our findings support conclusions derived from observations made on Cecropia peltata, a plant that can make both starch (in leaves) and glycogen (in specialized myrmecophytic Müllerian bodies) depending on the differential expression of SS, BE, and ISA (Bischof et al., 2013). Together these data provide an important step forward toward understanding how plants produce semicrystalline starch granules.
Finally, many of the mutants analyzed here exhibit growth defects. These defects may be caused by one or more factors. First, the production of soluble glucans rather than starch leads to turnover during the day and thus to lower glucan levels. In extreme cases, this could result in starvation at night. Second, the accumulation of the ADP-Glc precursor (e.g. in ss1ss2ss3) can unbalance the plastidial adenylate pool, causing impaired photosynthesis (Ragel et al., 2013). Third, the accumulation of starch degradation intermediates such as maltose may disturb the osmotic balance between the plastid and cytosol, as observed when maltose or Glc export to the cytosol is impaired (Niittylä et al., 2004). Thus, our data reinforce the importance of a normal glucan metabolism on plant growth and vitality.
MATERIALS AND METHODS
Growth Conditions and Plant Material
Plants were grown in Percival growth chambers (CLF Plant Climatics) under a 12-h dark/12-h light regime as described in Streb et al. (2008). Sown seeds were stratified for 2 to 3 d at 4°C in the dark prior to light exposure. Multiple ssisa mutants were generated by crossing the homozygous mutants ss1ss2ss3 (WS) and isa1isa2 (Col-0). The alleles used were ss1-1, ss2-3, ss3-2, isa1-1, and isa2-1, which were all previously shown to be null mutants (Delatte et al., 2005; Szydlowski et al., 2011). For ss2ss3, we analyzed three lines of different ecotypic backgrounds (WS, Col-0, or a mix; Supplemental Tables S1 and S2). Unless otherwise indicated, the results are from the ss2ss3 mutant with Col-0 background (ss2-1 and ss3-1; Zhang et al., 2008). All mutations used, except for isa2-1, arise from transfer DNA insertions. The isa2-1 allele is a single base-pair deletion that can be identified via a specific cleavage of the PCR amplicon. Homozygous mutant alleles were selected in the segregating F2 to F4 generations by PCR-based and cleaved-amplified polymorphic sequence-based genotyping. Detailed information about the mutants used in this study concerning genotype, ecotype, alleles, primers used for their identification, and references is provided in Supplemental Tables S1 and S2.
Starch and Phytoglycogen Extraction and Quantification
Whole rosettes from 30-d-old plants were harvested at the end of the photoperiod or at the end of the night and immediately frozen in liquid nitrogen. The plant material was homogenized in 1.12 m perchloric acid using an all-glass homogenizer. The homogenate was centrifuged for 10 min at 3,000g to separate the insoluble fraction (containing starch and starch-like glucans) and the soluble fraction (containing sugars and soluble glucans). The insoluble fraction was washed once with water and four times with 80% (v/v) ethanol, dried, resuspended in water, and stored at −20°C. The soluble fraction was neutralized to pH 6 by adding neutralization buffer (2 m KOH, 0.4 m MES, and 0.4 m KCl) to the soluble fraction. Insoluble salts (KClO4) were precipitated by centrifugation at 3,000g for 10 min. The remaining supernatant was subjected to phytoglycogen precipitation by adding methanol until a final concentration of 75% (v/v) was reached and incubation at −20°C for at least 2 h. After centrifugation at 3,000g for 10 min, the pellet was washed once with 75% (v/v) methanol, dried, and resuspended in water. Glucans in the insoluble fraction and the phytoglycogen fraction were quantified as described in Hostettler et al. (2011).
The percentage of insoluble glucans from total glucans (i.e. insoluble and soluble glucans) was calculated individually for each biological replicate.
Structural Analysis of Starch and Phytoglycogen
For iodine staining, whole rosettes of 30-d-old plants were harvested at the end of the day, decolorized in hot 80% (v/v) ethanol, stained with Lugol solution (Sigma-Aldrich), and destained again in water.
For obtaining the normal and β-limit CLDs from starch and phytoglycogen, the insoluble and phytoglycogen fractions from the perchloric-acid extraction (above) were used. In the case of starch, three or four samples from individual plants were processed individually in all lines, except for ss2ss3, ss1ss2ss3, and ss1ss2ss3isa, in which two plants were pooled three or four times to reach appropriate amounts. In the case of phytoglycogen, all mean values arise from three or four samples from individual plants without pooling, except for ss2 and ss2ss3, in which three plants were pooled. Further sample preparation and HPAEC-PAD (Dionex) was performed as described by Streb et al. (2008).
Quantification of Maltose
The neutralized soluble fraction obtained from the perchloric-acid extraction (above) was spiked with 5 nmol cellobiose per 20 mg of original plant material as an internal standard. Phytoglycogen was precipitated as described above, and the remaining methanol supernatant (containing sugars) was dried and resuspended in water. Further sample preparation, including columns of Dowex 50W and Dowex 1 (Sigma-Aldrich), chromatography (Dionex), and evaluation, was performed as described in Egli et al. (2010), except for slightly modified gradients: 100% eluent A (100 mm NaOH) from 0 to 7 min; a concave gradient from 100% A to 20% (v/v) A and 80% (v/v) eluent B (150 mm NaOH and 500 mm sodium acetate) from 7 to 26.5 min; 20% (v/v) A and 80% (v/v) B from 26.5 min to 32 min (cleaning of the column); and 100% A from 32 to 40 min (equilibration of the column). A standard containing maltose was used to identify the maltose peak. All mean values are from three to five biological replicates arising from individual plants.
Quantification of ADP-Glc
ADP-Glc was measured in methanol-chloroform extracts by high-performance anion-exchange chromatography coupled to tandem mass spectrometry as described by Lunn et al. (2006). Plants were grown under a 12-h day/12-h night cycle for 4 to 5 weeks and were harvested after 11 h of light. Mean values ± sd of four to five replicate plant samples are given.
TEM
Mature leaves from 31-d-old plants were cut with a razor blade. The leaf sections were fixed in 2% (v/v) glutaraldehyde in 0.05 m sodium cacodylate buffer, pH 7.4, for 4 h at 4°C. After washing three times with 0.1 m sodium cacodylate buffer, pH 7.4, the samples were incubated overnight in 1% (w/v) osmium tetroxide in 0.1 m sodium cacodylate buffer, pH 7.4, at 4°C. Samples were then washed three times in ice-cold 0.1 m sodium cacodylate buffer, pH 7.4, and once with water. The samples were dehydrated in a series of aqueous ethanol solutions from 50% (v/v) to 100% ethanol and were embedded in Spurr epoxy resin (Agar Scientific). Ultrathin sections were cut with a diamond knife and stained sequentially with uranyl acetate and Reynold lead citrate. Stained sections were photographed with an FEI Morgagni 268 electron microscope. Images are representative pictures from analyzing sections from two individual plants per genotype. Exceptions are the wild type (WS) and the isa1isa2 controls, in which only sections from one plant were imaged.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At5g24300 (SS1), At3g01180 (SS2), At1g11720 (SS3), At2g39930 (ISA1), and At1g03310 (ISA2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Native PAGE of ssisa mutant extracts.
Supplemental Figure S2. Three ss2ss3 lines have identical phenotypes.
Supplemental Figure S3. The loss of individual SSs results in distinct CLD patterns.
Supplemental Figure S4. Effects of losing SS1, SS2, or SS3 on CLDs are similar in phytoglycogen and insoluble glucans.
Supplemental Table S1. SS and DBE genes, respective mutant alleles, and primers used for their identification.
Supplemental Table S2. Genotypes and ecotypes of the mutant lines used in this study.
Supplemental Table S3. Starch and phytoglycogen content of ssisa mutants at the end of the night.
Supplemental Methods S1. Native PAGE (Zymograms).
Supplementary Material
Acknowledgments
We thank Dr. Michaela Stettler for technical advice, Andrea Ruckle for assistance with plant cultivation, the ScopeM staff for advice on electron microscopy, and Christophe D’Hulst, Dr. Nicolas Szydlowski, Alan Myers, and Dr. Xiaoli Zhang for seeds of SS and BE mutants.
Glossary
- SS
starch synthase
- BE
branching enzyme
- DBE
debranching enzyme
- ACL
average chain length
- CLD
chain length distribution
- DP
degree of polymerization
- WS
ecotype Wassilewskija of Arabidopsis
- Col-0
ecotype Columbia-0 of Arabidopsis
- FW
fresh weight
- TEM
transmission electron microscopy
- HPAEC-PAD
high-performance anion-exchange chromatography pulsed-amperometric detection
Footnotes
This work was supported by the Swiss National Science Foundation Bonus of Excellence Award (grant no. 31003A–131074) and by ETH Zurich.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Ball S, Guan HP, James M, Myers A, Keeling P, Mouille G, Buléon A, Colonna P, Preiss J. (1996) From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell 86: 349–352 [DOI] [PubMed] [Google Scholar]
- Bischof S, Umhang M, Eicke S, Streb S, Qi W, Zeeman SC. (2013) Cecropia peltata accumulates starch or soluble glycogen by differentially regulating starch biosynthetic genes. Plant Cell 25: 1400–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buléon A, Gallant DJ, Bouchet B, Mouille G, D’Hulst C, Kossmann J, Ball S. (1997) Starches from A to C: Chlamydomonas reinhardtii as a model microbial system to investigate the biosynthesis of the plant amylopectin crystal. Plant Physiol 115: 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Jenner H, Carrangis L, Fahy B, Fincher GB, Hylton C, Laurie DA, Parker M, Waite D, van Wegen S, et al. (2002) Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity. Plant J 31: 97–112 [DOI] [PubMed] [Google Scholar]
- Bustos R, Fahy B, Hylton CM, Seale R, Nebane NM, Edwards A, Martin C, Smith AM. (2004) Starch granule initiation is controlled by a heteromultimeric isoamylase in potato tubers. Proc Natl Acad Sci USA 101: 2215–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JW, Cole DA. (1954) Effects of the genes su1, su2, and du on carbohydrates in developing maize kernels. Agron J 51: 424–427 [Google Scholar]
- Caspar T, Lin TP, Monroe J, Bernhard W, Spilatro S, Preiss J, Somerville C. (1989) Altered regulation of β-amylase activity in mutants of Arabidopsis with lesions in starch metabolism. Proc Natl Acad Sci USA 86: 5830–5833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commuri PD, Keeling PL. (2001) Chain-length specificities of maize starch synthase I enzyme: studies of glucan affinity and catalytic properties. Plant J 25: 475–486 [DOI] [PubMed] [Google Scholar]
- Craig J, Lloyd JR, Tomlinson K, Barber L, Edwards A, Wang TL, Martin C, Hedley CL, Smith AM. (1998) Mutations in the gene encoding starch synthase II profoundly alter amylopectin structure in pea embryos. Plant Cell 10: 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creech RG. (1965) Genetic control of carbohydrate synthesis in maize endosperm. Genetics 52: 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton-Taylor M, Grandison S, Png KMY, Bushby AJ, Smith AM. (2012) Control of starch granule numbers in Arabidopsis chloroplasts. Plant Physiol 158: 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton-Taylor M, Pike M, Lu KJ, Hylton CM, Feil R, Eicke S, Lunn JE, Zeeman SC, Smith AM. (2013) Starch synthase 4 is essential for coordination of starch granule formation with chloroplast division during Arabidopsis leaf expansion. New Phytol 200: 1064–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte T, Trevisan M, Parker ML, Zeeman SC. (2005) Arabidopsis mutants Atisa1 and Atisa2 have identical phenotypes and lack the same multimeric isoamylase, which influences the branch point distribution of amylopectin during starch synthesis. Plant J 41: 815–830 [DOI] [PubMed] [Google Scholar]
- Delatte T, Umhang M, Trevisan M, Eicke S, Thorneycroft D, Smith SM, Zeeman SC. (2006) Evidence for distinct mechanisms of starch granule breakdown in plants. J Biol Chem 281: 12050–12059 [DOI] [PubMed] [Google Scholar]
- Delvallé D, Dumez S, Wattebled F, Roldán I, Planchot V, Berbezy P, Colonna P, Vyas D, Chatterjee M, Ball S, et al. (2005) Soluble starch synthase I: a major determinant for the synthesis of amylopectin in Arabidopsis thaliana leaves. Plant J 43: 398–412 [DOI] [PubMed] [Google Scholar]
- Dumez S, Wattebled F, Dauvillee D, Delvalle D, Planchot V, Ball SG, D’Hulst C. (2006) Mutants of Arabidopsis lacking starch branching enzyme II substitute plastidial starch synthesis by cytoplasmic maltose accumulation. Plant Cell 18: 2694–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A, Fulton DC, Hylton CM, Jobling SA, Gidley M, Ro U, Martin C, Smith AM. (1999) A combined reduction in activity of starch synthases II and III of potato has novel effects on the starch of tubers. Plant J 17: 251–261 [Google Scholar]
- Egli B, Kölling K, Köhler C, Zeeman SC, Streb S. (2010) Loss of cytosolic phosphoglucomutase compromises gametophyte development in Arabidopsis. Plant Physiol 154: 1659–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Hanashiro I, Suzuki S, Higuchi T, Toyosawa Y, Utsumi Y, Itoh R, Aihara S, Nakamura Y. (2012) Elongated phytoglycogen chain length in transgenic rice endosperm expressing active starch synthase IIa affects the altered solubility and crystallinity of the storage α-glucan. J Exp Bot 63: 5859–5872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Yoshida M, Asakura N, Ohdan T, Miyao A, Hirochika H, Nakamura Y. (2006) Function and characterization of starch synthase I using mutants in rice. Plant Physiol 140: 1070–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton DC, Stettler M, Mettler T, Vaughan CK, Li J, Francisco P, Gil M, Reinhold H, Eicke S, Messerli G, et al. (2008) β-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active β-amylases in Arabidopsis chloroplasts. Plant Cell 20: 1040–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley MJ, Bulpin PV. (1987) Crystallisation of malto-oligosaccharides as models of the crystalline forms of starch: minimum chain-length requirement for the formation of double helices. Carbohydr Res 161: 291–300 [Google Scholar]
- Hizukuri S. (1986) Polymodal distribution of the chain lengths of amylopectins, and its significance. Carbohydr Res 147: 342–347 [Google Scholar]
- Hostettler C, Kölling K, Santelia D, Streb S, Kötting O, Zeeman SC. (2011) Analysis of starch metabolism in chloroplasts. Methods Mol Biol 775: 387–410 [DOI] [PubMed] [Google Scholar]
- James MG, Robertson DS, Myers AM. (1995) Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell 7: 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JS, Ryoo N, Hahn TR, Walia H, Nakamura Y. (2010) Starch biosynthesis in cereal endosperm. Plant Physiol Biochem 48: 383–392 [DOI] [PubMed] [Google Scholar]
- Laby RJ, Kim D, Gibson SI. (2001) The ram1 mutant of Arabidopsis exhibits severely decreased β-amylase activity. Plant Physiol 127: 1798–1807 [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sun F, Xu S, Chu X, Mukai Y, Yamamoto M, Ali S, Rampling L, Kosar-Hashemi B, Rahman S, et al. (2003) The structural organisation of the gene encoding class II starch synthase of wheat and barley and the evolution of the genes encoding starch synthases in plants. Funct Integr Genomics 3: 76–85 [DOI] [PubMed] [Google Scholar]
- Lin Q, Huang B, Zhang M, Zhang X, Rivenbark J, Lappe RL, James MG, Myers AM, Hennen-Bierwagen TA. (2012) Functional interactions between starch synthase III and isoamylase-type starch-debranching enzyme in maize endosperm. Plant Physiol 158: 679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M. (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners DJ. (1989) Recent developments in our understanding of amylopectin structure. Carbohydr Polym 11: 87–112 [Google Scholar]
- Marshall J, Sidebottom C, Debet M, Martin C, Smith AM, Edwards A. (1996) Identification of the major starch synthase in the soluble fraction of potato tubers. Plant Cell 8: 1121–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Kawasaki T, Shimada H, Satoh H, Kobayashi E, Okumura S, Arai Y, Baba T. (1993) Alteration of the structural properties of starch components by the lack of an isoform of starch branching enzyme in rice seeds. J Biol Chem 268: 19084–19091 [PubMed] [Google Scholar]
- Morell MK, Kosar-Hashemi B, Cmiel M, Samuel MS, Chandler P, Rahman S, Buleon A, Batey IL, Li Z. (2003) Barley sex6 mutants lack starch synthase IIa activity and contain a starch with novel properties. Plant J 34: 173–185 [DOI] [PubMed] [Google Scholar]
- Mouille G, Maddelein ML, Libessart N, Talaga P, Decq A, Delrue B, Ball S. (1996) Preamylopectin processing: a mandatory step for starch biosynthesis in plants. Plant Cell 8: 1353–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Kubo A, Shimamune T, Matsuda T, Harada K, Satoh H. (1997) Correlation between activities of starch debranching enzyme and α-polyglucan structure in endosperms of sugary-1 mutants of rice. Plant J 12: 143–153 [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC. (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303: 87–89 [DOI] [PubMed] [Google Scholar]
- Pfannemüller B. (1987) Influence of chain length of short monodisperse amyloses on the formation of A- and B-type X-ray diffraction patterns. Int J Biol Macromol 9: 105–108 [Google Scholar]
- Ragel P, Streb S, Feil R, Sahrawy M, Annunziata MG, Lunn JE, Zeeman S, Mérida Á. (2013) Loss of starch granule initiation has a deleterious effect on the growth of Arabidopsis plants due to an accumulation of ADP-glucose. Plant Physiol 163: 75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán I, Wattebled F, Mercedes Lucas M, Delvallé D, Planchot V, Jiménez S, Pérez R, Ball S, D’Hulst C, Mérida A. (2007) The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J 49: 492–504 [DOI] [PubMed] [Google Scholar]
- Schwall GP, Safford R, Westcott RJ, Jeffcoat R, Tayal A, Shi YC, Gidley MJ, Jobling SA. (2000) Production of very-high-amylose potato starch by inhibition of SBE A and B. Nat Biotechnol 18: 551–554 [DOI] [PubMed] [Google Scholar]
- Stinard PS, Robertson DS, Schnable PS. (1993) Genetic isolation, cloning, and analysis of a mutator-induced, dominant antimorph of the maize amylose extender1 locus. Plant Cell 5: 1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb S, Delatte T, Umhang M, Eicke S, Schorderet M, Reinhardt D, Zeeman SC. (2008) Starch granule biosynthesis in Arabidopsis is abolished by removal of all debranching enzymes but restored by the subsequent removal of an endoamylase. Plant Cell 20: 3448–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb S, Zeeman SC. (2012) Starch metabolism in Arabidopsis. The Arabidopsis Book 10: e0160, /10.1199/tab.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg M, Pfister B, Fulton D, Bischof S, Delatte T, Eicke S, Stettler M, Smith SM, Streb S, Zeeman SC. (2013) The heteromultimeric debranching enzyme involved in starch synthesis in Arabidopsis requires both isoamylase1 and isoamylase2 subunits for complex stability and activity. PLoS ONE 8: e75223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowski N, Ragel P, Hennen-Bierwagen TA, Planchot V, Myers AM, Mérida A, d’Hulst C, Wattebled F. (2011) Integrated functions among multiple starch synthases determine both amylopectin chain length and branch linkage location in Arabidopsis leaf starch. J Exp Bot 62: 4547–4559 [DOI] [PubMed] [Google Scholar]
- Szydlowski N, Ragel P, Raynaud S, Lucas MM, Roldán I, Montero M, Muñoz FJ, Ovecka M, Bahaji A, Planchot V, et al. (2009) Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 21: 2443–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatge H, Marshall J, Martin C, Edwards EA, Smith AM. (1999) Evidence that amylose synthesis occurs within the matrix of the starch granule in potato tubers. Plant Cell Environ 22: 543–550 [Google Scholar]
- Tetlow IJ, Emes MJ. (2011) Starch biosynthesis in higher plants: the enzymes of starch synthesis. In Moo-Young M, ed, Comprehensive Biotechnology, Ed 2, Elsevier, Amsterdam, The Netherlands, pp 47–65 [Google Scholar]
- Umemoto T, Yano M, Satoh H, Shomura A, Nakamura Y. (2002) Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties. Theor Appl Genet 104: 1–8 [DOI] [PubMed] [Google Scholar]
- Wang Q, Monroe J, Sjölund RD. (1995) Identification and characterization of a phloem-specific β-amylase. Plant Physiol 109: 743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattebled F, Dong Y, Dumez S, Delvallé D, Planchot V, Berbezy P, Vyas D, Colonna P, Chatterjee M, Ball S, et al. (2005) Mutants of Arabidopsis lacking a chloroplastic isoamylase accumulate phytoglycogen and an abnormal form of amylopectin. Plant Physiol 138: 184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori M, Fujita S, Hayakawa K, Matsuki J, Yasui T. (2000) Genetic elimination of a starch granule protein, SGP-1, of wheat generates an altered starch with apparent high amylose. Theor Appl Genet 101: 21–29 [Google Scholar]
- Zeeman SC, Delatte T, Messerli G, Umhang M, Stettler M, Mettler T, Streb S, Reinhold H, Kötting O. (2007) Starch breakdown: recent discoveries suggest distinct pathways and novel mechanisms. Funct Plant Biol 34: 465–473 [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Kossmann J, Smith AM. (2010) Starch: its metabolism, evolution, and biotechnological modification in plants. Annu Rev Plant Biol 61: 209–234 [DOI] [PubMed] [Google Scholar]
- Zhang X, Colleoni C, Ratushna V, Sirghie-Colleoni M, James MG, Myers AM. (2004) Molecular characterization demonstrates that the Zea mays gene sugary2 codes for the starch synthase isoform SSIIa. Plant Mol Biol 54: 865–879 [DOI] [PubMed] [Google Scholar]
- Zhang X, Myers AM, James MG. (2005) Mutations affecting starch synthase III in Arabidopsis alter leaf starch structure and increase the rate of starch synthesis. Plant Physiol 138: 663–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Szydlowski N, Delvallé D, D’Hulst C, James MG, Myers AM. (2008) Overlapping functions of the starch synthases SSII and SSIII in amylopectin biosynthesis in Arabidopsis. BMC Plant Biol 8: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.