Tonoplast water channels contribute to Tomato yellow leaf curl virus resistance through hormone homeostasis and sugar signaling.
Abstract
Vacuolar water movement is largely controlled by membrane channels called tonoplast-intrinsic aquaporins (TIP-AQPs). Some TIP-AQP genes, such as TIP2;2 and TIP1;1, are up-regulated upon exposure to biotic stress. Moreover, TIP1;1 transcript levels are higher in leaves of a tomato (Solanum lycopersicum) line resistant to Tomato yellow leaf curl virus (TYLCV) than in those of a susceptible line with a similar genetic background. Virus-induced silencing of TIP1;1 in the tomato resistant line and the use of an Arabidopsis (Arabidopsis thaliana) tip1;1 null mutant showed that resistance to TYLCV is severely compromised in the absence of TIP1:1. Constitutive expression of tomato TIP2;2 in transgenic TYLCV-susceptible tomato and Arabidopsis plants was correlated with increased TYLCV resistance, increased transpiration, decreased abscisic acid levels, and increased salicylic acid levels at the early stages of infection. We propose that TIP-AQPs affect the induction of leaf abscisic acid, which leads to increased levels of transpiration and gas exchange, as well as better salicylic acid signaling.
Plants manage their water balance in different ways. Some plants maintain a strict transpiration rate, ensuring nearly constant leaf water potential and relative water content (RWC); this conservative strategy is referred to as isohydric. Others allow leaf water potential to decrease while transpiration increases during the day; this less conservative strategy is referred to as anisohydric (Tardieu and Simonneau, 1998; Sade et al., 2012b). Compared to isohydric plants, anisohydric plants exhibit differential stomatal regulation and higher rates of transpiration and photosynthesis. Anisohydric plants generally exhibit mild to moderate resistance to abiotic stress (McDowellet al., 2008; Sade et al., 2012b).
The movement of water in a plant is largely controlled by membrane channels called aquaporins (AQPs; Tyerman et al., 2002; Kaldenhoff et al., 2007; Maurel et al., 2008). AQPs regulate cellular water transport (Chrispeels and Maurel, 1994; Knepper, 1994; Heymann and Engel, 1999) and transpiration (Aharon et al., 2003; Parent et al., 2009). Tonoplast-intrinsic proteins (TIPs), a family of proteins belonging to the AQP superfamily associated with large vacuoles, play a major role in cell water balance (Maurel et al., 2008; Reuscher et al., 2013). The unique roles of the cell vacuole and TIP-AQPs in the regulation of water homeostasis have been demonstrated by the overexpression of these proteins in plants. For example, Arabidopsis (Arabidopsis thaliana) plants expressing the ginseng (Panax ginseng) TIP-AQP gene PgTIP1 showed altered drought and salt tolerance (Peng et al., 2007).
Recently, we have shown that overexpression of the TIP-AQP gene SlTIP2;2 in an isohydric tomato (Solanum lycopersicum) genotype modified the plant’s water management so that it became anisohydric. This switch was accompanied by increased transpiration and fruit set under mild to moderate (but not severe) drought conditions (Sade et al., 2009). Moreover, in an analysis of the expression levels of different AQP clusters in the presence of different types of biotic stress (elicitors and pathogens), the homologous genes SlTIP2;2 and SlTIP1;1 exhibited 2- and 3.5-fold increases in expression, respectively (Sade et al., 2009). According to comparisons of their amino acid sequences, all TIPs belong to a single phylogenetic group of tonoplast major intrinsic proteins, which includes four TIP subfamilies (TIP1–TIP4). Interestingly, although the expression of both SlTIP1;1 and SlTIP2;2 is affected by biotic stress in tomato (Eybishtz et al., 2009; Sade et al., 2009), these two genes are the most distantly related members of their subfamily (Reuscher et al., 2013).
Tomato yellow leaf curl virus (TYLCV) is a begomovirus transmitted by the whitefly Bemisia tabaci, which threatens tomato crops around the world (Navot et al., 1991). Breeders have been working to develop lines that are resistant to TYLCV using the wild tomato species Solanum habrochaites as a source of resistance. Two lines that differ only in their susceptibility to TYLCV (susceptible [S] and resistant [R]) were developed by Vidavsky and Czosnek (1998). It has been postulated that the genes that are expressed at higher levels in R plants (as compared to S plants) are related to resistance and that silencing these genes will lead to the collapse of resistance (Eybishtz et al., 2009). This concept has been verified for a number of genes, including the tomato hexose transporter LeHT1 (Eybishtz et al., 2010; Sade et al., 2012a). Among the genes preferentially expressed in R plants upon TYLCV infection is the tomato TIP-AQP gene SlTIP1;1 (Eybishtz et al., 2009).
Here, we used molecular and physiological tools to investigate whether TIP-AQPs contribute to TYLCV resistance by cross-talk regulation of water, hormone balance, and hexose signaling upon virus infection. We compared R tomato plants in which the transcript level of the AQP gene SlTIP1;1 was up-regulated, R plants in which SlTIP1;1 had been silenced using Tobacco rattle virus (TRV)-based virus-induced gene silencing (VIGS), and a TYLCV-susceptible tomato genotype rendered TYLCV resistant by constitutive overexpression of the AQP gene SlTIP2;2. The results obtained with tomato were confirmed using an Arabidopsis mutant in which the endogenous AtTIP1;1 (the tomato ortholog; Sade et al., 2009; Reuscher et al., 2013) gene has been knocked out and transgenic Arabidopsis plants in which the tomato SlTIP2;2 gene was overexpressed. The results presented here indicate that TIP-AQPs are involved in TYLCV resistance, which is controlled by cellular water balance and transpiration, with subsequent effects on hormonal balance and sugar signaling.
RESULTS
TYLCV-R and TYLCV-S Tomato Plants Present Different Water Regulation Patterns: Higher Transpiration in R Tomato Plants Correlates with Higher Levels of TIP1;1 Transcription
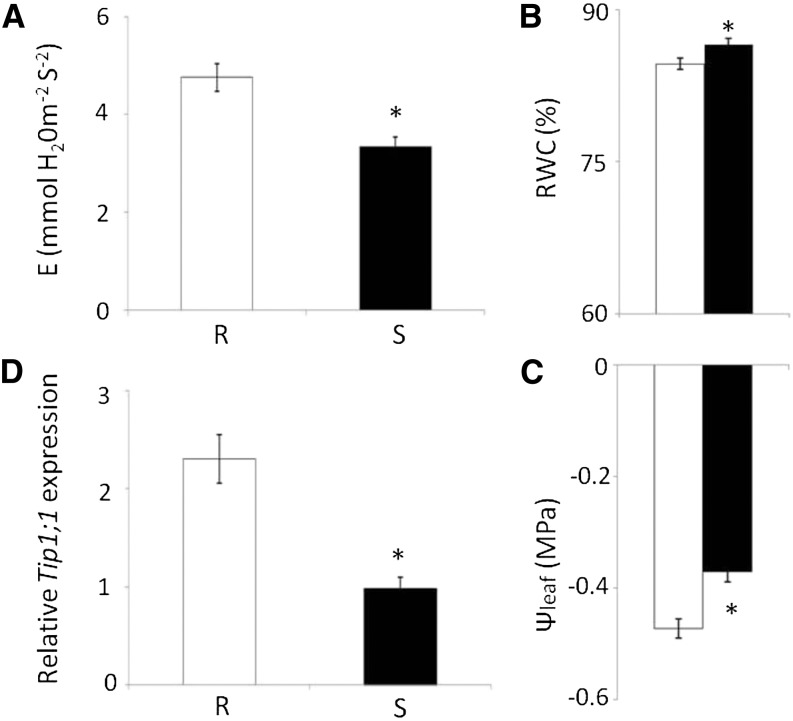
R plants exhibited higher levels of leaf transpiration than S plants (Fig. 1A). Leaf RWC and midday water potential (ψleaf) were lower in the R plants than in the S plants (Fig. 1, B and C). In parallel, the amount of TIP1;1 transcripts was higher in R plants than in S plants (Fig. 1D). Analysis of the water status of the plants as the soil water content decreased showed that R plants exhibited anisohydric behavior (Supplemental Fig. S1). These results indicate that high TIP1;1 expression is correlated with enhanced transpiration and attenuated conservative water regulation. Similar behavior was observed among transgenic tomato plants overexpressing the TIP2;2 gene (Tom-TIP2;2 plants), underscoring the role of TIP-AQPs in water management (Sade et al., 2012b).
Figure 1.
Comparison of water management in R (white bars) and S (black bars) tomato plants. A, Leaf transpiration (E). B, Leaf RWC. C, SlTIP1;1 expression as measured by quantitative PCR (qPCR). D, ψleaf. Asterisk indicates a significant difference (Student’s t test, P < 0.05). Data points are means ± se (n = 7–30).
TIP-AQPs Are Involved in Resistance to TYLCV in Tomato and Arabidopsis
The putative role of TIP-AQPs was investigated by (1) silencing the TIP1;1 gene in tomato (by TRV-VIGS) and Arabidopsis (using a transfer DNA insertion mutant) and (2) constitutively overexpressing the TIP2;2 gene in transgenic tomato and transgenic Arabidopsis. The behavior of these plants was assessed following TYLCV infection.
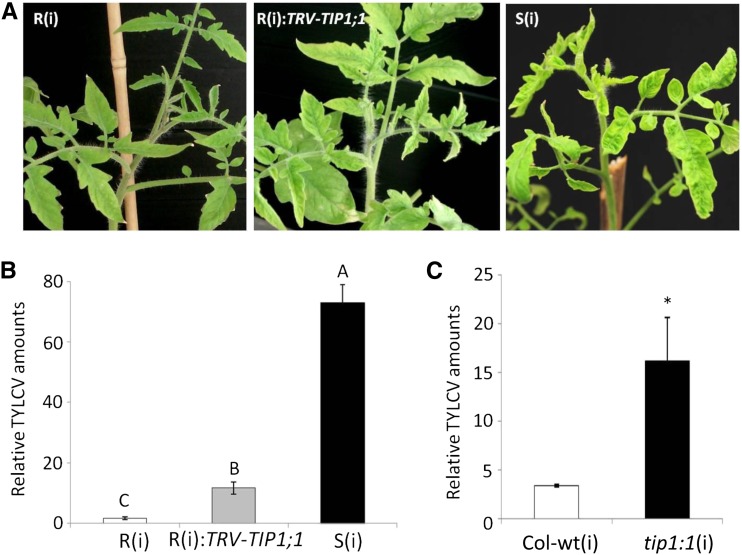
The TIP-AQP gene TIP1;1 is expressed at higher levels in R plants than in S plants (Eybishtz et al., 2009). The putative involvement of TIP1;1 in TYLCV resistance was assessed by gene silencing. In R plants in which TIP1;1 was VIGS silenced {none infected with TYLCV [R(0)]:TRV-TIP1;1}, the expression of TIP1;1, but not TIP1;2, was significantly down-regulated, confirming the specificity of TRV-based silencing (Supplemental Fig. S2). Thirty days post inoculation (dpi), infected R [R(i)] plants remained free of symptoms, while infected silenced R [(R(i):TRV-TIP1;1] plants exhibited the leaf yellowing and curling associated with infected S [S(i)] plants (Fig. 2A). At 7 dpi, the amount of virus in the R(i):TRV-TIP1;1 plants was already significantly higher than the amount of virus in the R(i) plants but lower than in the S(i) plants (Fig. 2B). At 21 dpi, the amount of virus in the R(i):TRV-TIP1;1 plants was similar to that in the S(i) plants, about 100 times higher than in the R(i) tomato plants (Supplemental Fig. S3A). These results indicate that once TIP1;1 is silenced, the resistance of R plants is broken.
Figure 2.
Effect of SlTIP1;1 silencing on the responses of tomato and Arabidopsis plants to TYLCV infection. A, In tomato at 30 dpi, the S [S(i)] plants and the R plants in which the TIP1;1 gene had been silenced [R(i):TRV-TIP1;1] showed symptoms of infection, but the R [R(i)] plants did not. B, qPCR estimation of relative amounts of TYLCV in tomato plants at 7 dpi. S(i), Black bar; R(i):TRV-TIP1;1, gray bar; R(i), white bar. Tomato β-actin was used as the calibrating gene. Different letters above the columns indicate significant differences (Student’s t test, P < 0.05). C, qPCR estimation of relative amounts of TYLCV in Arabidopsis at 7 dpi. Wild-type Col-wt(i), White bar; mutant tip1;1(i), black bar. Arabidopsis UBQ10 was used as the calibrating gene. Asterisk indicates a significant difference (Student’s t test, P < 0.05). Data points are means ± se (n = 7–9). [See online article for color version of this figure.]
The role played by TIP-AQPs in the resistance phenomenon was confirmed using the Arabidopsis mutant tip1;1. This transfer DNA insertion mutant does not express detectable levels of TIP1;1 (Supplemental Fig. S4A; Schüssler et al., 2008). Upon TYLCV inoculation, the tip1;1 mutant was more susceptible to whitefly-mediated virus infection than the wild-type plants. The infected mutant plants [tip1;1(i)] remained stunted, and at 7 dpi, the amount of virus in their leaves was at least 5 times higher than that observed in the infected ecotype Columbia wild-type [Col-wt(i)] plants (Fig. 2C).
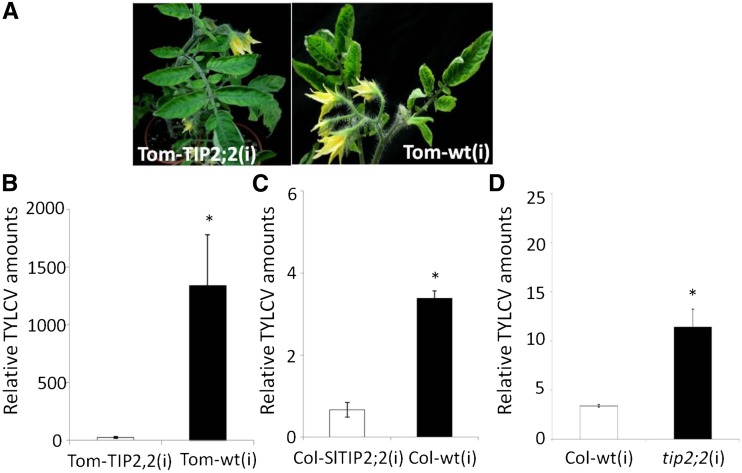
Another TIP-AQP gene, TIP2;2, has been found to be involved in tomato resistance to a variety of biotic agents (Sade et al., 2009, 2012b). The TYLCV-susceptible tomato genotype Tom-TIP2;2 (F1 hybrid cv M82 × cv MicroTom; Sade et al., 2009), in which the endogenous SlTIP2;2 gene is constitutively overexpressed (Supplemental Fig. S4D), was tested for TYLCV resistance. Following TYLCV inoculation, the transgenic tomato plants overexpressing TIP2;2 were more vigorous than the nontransgenic control plants (Tom-wt) and presented attenuated symptoms (Fig. 3A). At 7 dpi, the infected Tom-TIP2;2(i) plants contained about 50 times less virus than the nontransgenic Tom-wt(i) plants. At 21 dpi, the infected Tom-TIP2;2(i) plants contained about 200 times less virus than the nontransgenic Tom-wt(i) plants (Fig. 3B; Supplemental Fig. S3B). The specific role of TIP-AQPs was demonstrated by overexpressing another Solanaceae AQP that localizes to the plasma membrane and not the tonoplast. Overexpression of this AQP, Nicotiana tabacum AQP1 (NtAQP1), was previously reported to improve tomato plant abiotic stress tolerance (these mutant tomato plants were referred to as Tom-NtAQP; Sade et al., 2010). Here, these Tom-NtAQP1 plants did not show any resistance to TYLCV upon inoculation (Supplemental Fig. S5).
Figure 3.
Effect of SlTIP2;2 overexpression on TYLCV infection of tomato and Arabidopsis. A, Appearance of symptoms in transgenic tomato plants overexpressing the tomato gene TIP2;2 [Tom-TIP2;2(i)] and in nontransgenic control plants [Tom-wt(i)] at 30 dpi. Note that the transgenic tomato grew better than the wild type and exhibited less severe symptoms. B to D, qPCR estimations of relative amounts of TYLCV in tomato and Arabidopsis plants at 7 dpi. Tomato β-actin and Arabidopsis UBQ10 were used as calibrating genes. B, Tom-TIP2;2(i), White bar; Tom-wt(i) plants, black bar. C, Col-SlTIP2;2(i), White bar; Col-wt(i), black bar. D, Col-wt(i), White bar; mutant tip2;2, black bar. Asterisk indicates a significant difference (Student’s t test, P < 0.05). Data points are means ± se (n = 5–10). [See online article for color version of this figure.]
The results obtained with tomato plants were confirmed using transgenic Arabidopsis constitutively expressing the tomato gene SlTIP2;2 (these mutants are referred to as Col-SlTIP2;2; Supplemental Fig. S4C). Upon infection, the Arabidopsis Col-SlTIP2;2(i) plants behaved like the Tom-SlTIP2;2 tomato plants. At 7 dpi, the Col-SlTIP2;2(i) plants contained less viral DNA than the wild-type Arabidopsis plants, Col-wt(i; Fig. 3C). In addition, at 7 dpi, the Arabidopsis mutants tip2;2, which does not express TIP2;2 (Supplemental Fig. S4B), contained significantly more viral DNA than the infected wild-type plants (Fig. 3D).
ABA/SA Homeostasis Is Involved in Resistance to TYLCV in Tomato and in Arabidopsis
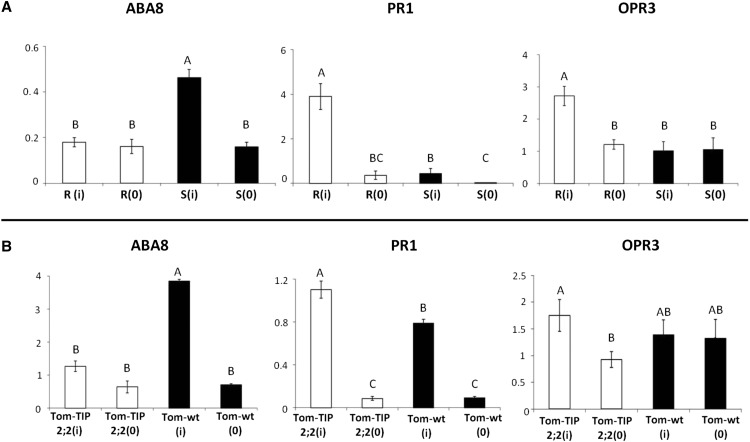
The transcription level of TIPs is known to be regulated by external stresses, as well as phytohormones (Maurel and Chrispeels, 2001). We first examined the transcript levels of hormone-related marker genes in the tomato and Arabidopsis genotypes that present different patterns of TIP expression. At 7 dpi, a difference in the transcript levels of abscisic acid (ABA)- and salicylic acid (SA)-responsive sentinel genes including ABA8 and Arabidopsis CAROTENOID CLEAVAGE DIOXYGENASE1 (AtNCED1; Neill et al., 1998; Saito et al., 2004) and PATHOGEN RESISTANCE1 (PR1) and Arabidopsis ISOCHORISMATE SYNTHASE1 (AtICS1; Durrant and Dong, 2004; Chen et al., 2009) was observed in the R plants as compared to the S plants. By contrast, jasmonic acid (JA) transcript (OXOPHYTODIENOATE-REDUCTASE3 [OPR3] and AtOPR3; Stintziet al., 2001) levels were the same in the different genotypes (Supplemental Fig. S6). The transcript levels of these hormone-related marker or hormone biosynthesis genes in tomato and Arabidopsis are known to be correlated with actual hormone levels. These associations were confirmed in this study (Fig. 4; Supplemental Fig. S6).
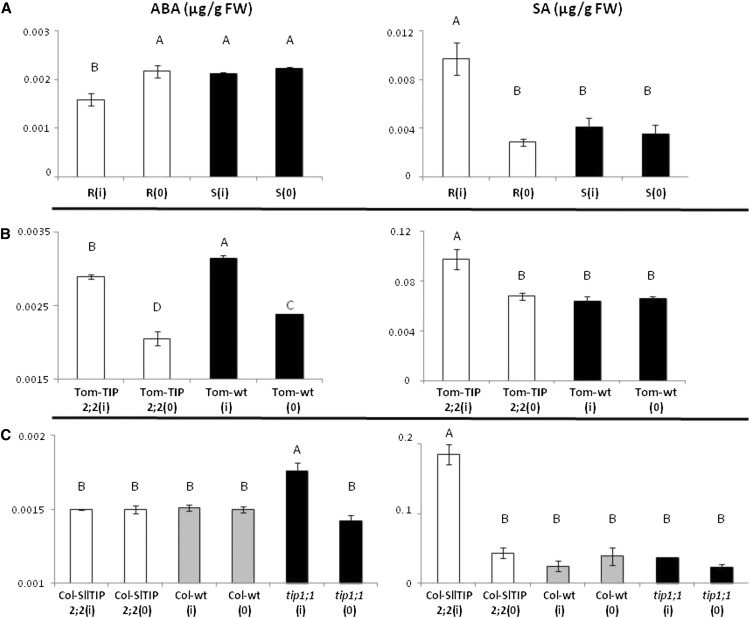
Figure 4.
Amounts of ABA and SA in TYLCV-infected tomato and Arabidopsis plants at 7 dpi. A, R (white bars) and S tomatoes (black bars) before [R(0) and S(0)] and after [R(i) and S(i)] infection. B, Tom-TIP2;2 tomato plants (white bar) and Tom-wt (black bar) before [Tom-Tip2;2(0) and Tom-wt(0)] and after infection [Tom-Tip2;2(i) and Tom-wt (i)]. C, Arabidopsis Col-SlTIP2;2 (white bar), Col-wt (gray bar), and tip1;1 mutant plants (black bar) before [Col-SlTIP2;2(0), Col-wt(0), and tip1;1(0)] and after infection [Col-SlTIP2;2(i), Col-wt(i), and tip1;1(i)]. Hormone levels are expressed as micrograms per gram leaf fresh weight. Data points are means ± se (n = 3–6). Different letters above the columns indicate significant differences (Student’s t test, P < 0.05).
Determination of ABA and SA levels by gas chromatography (GC)-mass spectrometry (MS) revealed no significant differences between noninfected tomato and Arabidopsis leaves (Fig. 4). At 7 dpi, the level of ABA in the R(i) leaves was significantly lower than in the S(i) leaves (Fig. 4A). By contrast, the level of SA at 7 dpi was significantly higher in R(i) than in S(i) leaves (Fig. 4A). In addition, while S plants did not exhibit any significant change in hormone levels upon infection {noninfected [S(0)] versus infected [S(i)]}, R plants showed a significant increase in SA levels and a significant decrease in ABA levels following infection (Fig. 4). Infected overexpressing TIP2;2 tomato plants and nontransgenic plants (Tom-TIP2;2 and Tom-wt) exhibited trends similar to those observed among the R and S plants (Fig. 4B). Although ABA levels increased upon infection in both Tom-TIP2;2 and Tom-wt, the Tom-TIP2;2 plants exhibited a milder increase than the Tom-wt plants, which was accompanied by a significant increase of SA in Tom-TIP2;2 plants (Fig. 4B).
Hormone levels were also compared in Arabidopsis plants upon TYLCV infection (Fig. 4C). The TIP1;1 mutant (tip1;1), the overexpressing Tom-TIP2;2 (Col-SlTIP2;2), and the wild-type (Col-wt) plants exhibited behavior similar to that of their tomato counterparts. Following inoculation, tip1;1 plants displayed the highest levels of ABA. There was little difference between the ABA levels of the Col-SlTIP2;2 plants and the Col-wt plants. At 7 dpi, the Col-SlTIP2;2 plants had higher levels of SA than the other plants.
The changes in hormone homeostasis genes in tomato plants were already noticeable at 1 dpi (Fig. 5). Interestingly, in contrast to what was observed at 7 dpi, at 1 dpi, the JA biosynthesis gene OPR3 transcript level was higher in the R plants than in the S plants.
Figure 5.
Expression of hormone-related marker or biosynthesis genes in tomato (ABA: ABA8; SA: PR1; and JA: OPR3) at 1 dpi. A, Tomato, S line (black bar, i = infected; 0 = uninfected) and R line (white bar, i = infected; 0 = uninfected). B, Tom-wt tomato plants (black bar, i = infected; 0 = uninfected) and Tom-TIP2;2 tomato plants (white bar, i = infected; 0 = uninfected). Data points are means ± se (n = 4–7). Different letters above the columns indicate significant differences (Student’s t test, P < 0.05).
To further confirm the involvement of ABA and SA in the accumulation of viral amounts in the plants, we conducted an experiment using Arabidopsis mutants that were either ABA deficient (aba1; Koornneef et al., 1982) or SA activated (CONSTITUTIVE IMMUNITY10 [cim10]; Maleck et al., 2002). These plants were infected with viruliferous whiteflies. Compared with wild-type plants, the aba1 and cim10 plants (with low ABA and high SA levels, respectively) exhibited stronger resistance at 7 dpi (Supplemental Fig. S7).
Enhancing the SA Pathway by Treating TYLCV-Susceptible Plants with Acibenzolar-S-Methyl Enhances Their Resistance to the Virus
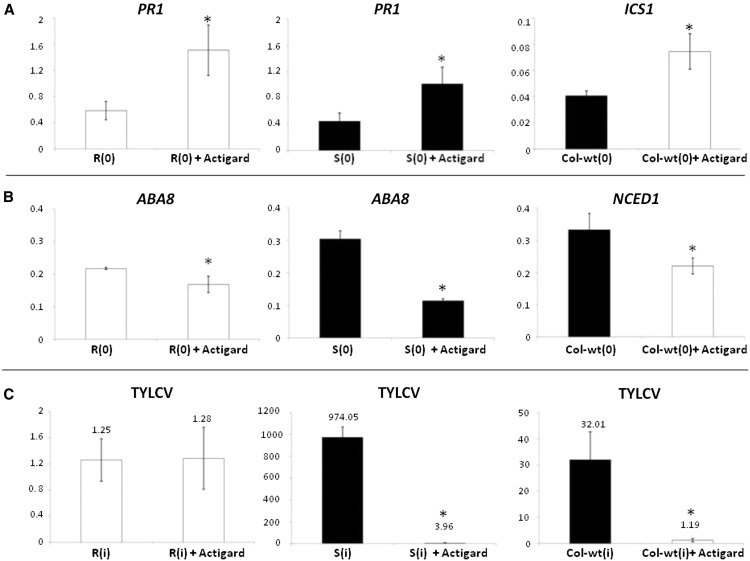
To further establish the link between TYLCV resistance and ABA/SA homeostasis, the SA pathway was up-regulated by treating TYLCV-S plants (S tomato plants and wild-type Arabidopsis plants) with acibenzolar-S-methyl (Actigard), a compound that mimics SA in the plants’ natural systemic activated resistance (SAR) response (Walters et al., 2013). The effects of this treatment on the transcript levels of the tomato hormone synthesis genes PR1 (a marker of SA) and ABA8 (a marker of ABA biosynthesis) and on the Arabidopsis genes ICS1 (SA) and NCED1 (ABA) indicated that, as expected, the SA genes were up-regulated while the ABA genes were down-regulated (Fig. 6, A and B, respectively). In this regard, the treated S plants behaved like R plants. Seven days after the acibenzolar-S-methyl treatment, the plants were inoculated with TYLCV. At 7 dpi, S tomato and wild-type Arabidopsis plants contained significantly less virus than the untreated control plants, and the R tomato plants contained about the same amounts of virus as the untreated plants (Fig. 6C). These results confirm that SA is part of the TYLCV resistance network.
Figure 6.
Effects of acibenzolar-S-methyl (Actigard) on the expression of hormone marker genes (SA: PR1 and AtICS1 and ABA: ABA8 and AtNCED1) and on resistance to TYLCV in R and S tomato and wild-type Arabidopsis plants. A and B, qPCR analysis of hormone-related marker or biosynthesis genes associated with SA (A) and ABA (B) in uninfected tomato and Arabidopsis plants (0 dpi) that had or had been treated with Actigard, as compared with untreated control plants. The tomato β-actin gene and the Arabidopsis Actin2 or UBQ10 genes were used as calibrators. C, qPCR estimation of relative amounts of TYLCV in infected, Actigard-treated tomato and Arabidopsis plants at 7 dpi as compared with untreated control plants. The numbers above the columns represent the values. Data points are means ± se (n = 4–10). Asterisk indicates a significant difference (Student’s t test, P < 0.05).
Hierarchy of Genes Involved in TYLCV Resistance in R Tomato Plants: TIP1;1 Is Upstream of the Extracellular Invertase6 Gene and LeHT1, whereas Extracellular Invertase6 Is Upstream of LeHT1
It has been postulated that the genes up-regulated upon TYLCV infection of R plants are part of an interconnected hierarchical network that confers resistance (Eybishtz et al., 2009). We have recently shown that Extracellular Invertase6 (Lin6) and LeHT1 are involved in the resistance to TYLCV in R plants, likely leading to enhanced carbon utilization and photosynthesis (Sade et al., 2013). In this work, we hypothesized that the silencing of one of these genes will cause a decrease in the transcript levels of genes downstream in the network.
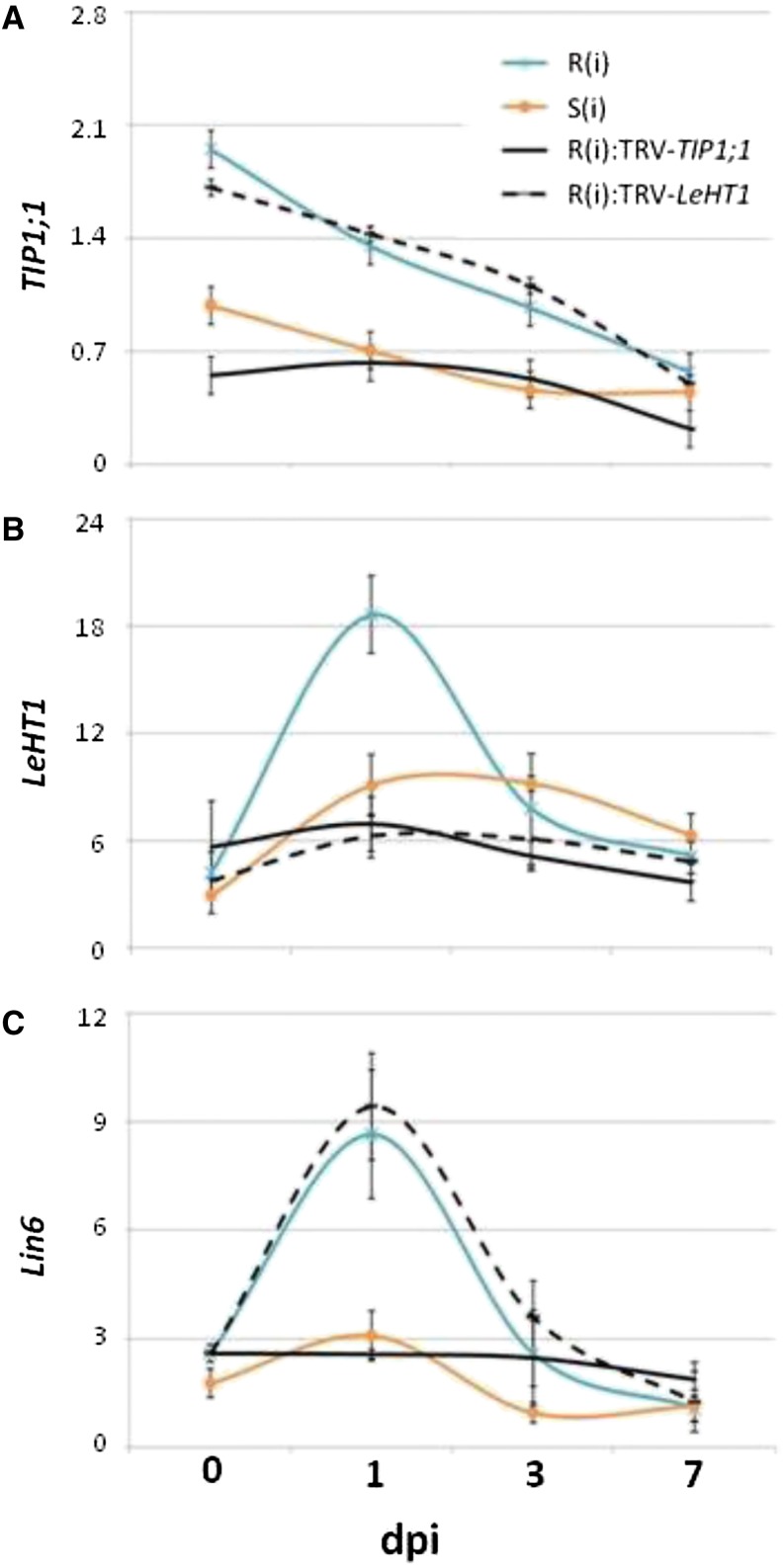
TIP1;1 transcript levels were examined in infected R plants in which LeHT1 had been silenced [R(i):TRV-LeHT1]. We found that in the LeHT1-silenced R plants, the transcript level of TIP1;1 was not affected (Fig. 7A). By contrast, the LeHT1 transcript levels were decreased in R plants in which TIP1;1 had been silenced [R(i):TRV-TIP1;1; Fig. 7B]. Moreover, the transcript level of Lin6 was strongly down-regulated in R(i):TRV-TIP1;1 plants but was not altered in LeHT1-silenced R plants [R(i):TRV-LeHT1; Fig. 7C]. These results indicate that in the hierarchy of the gene network conferring TYLCV resistance, these three genes are interconnected: TIP1;1 is upstream of Lin6 and LeHT1, and Lin6 is upstream of LeHT1.
Figure 7.
Hierarchy in the expression of TIP1;1, LeHT1, and Lin6. Gene expression in infected R [R(i)] and S [S(i)] tomato plants (blue and orange lines) and in infected R plants in which TIP1;1 and LeHT1 had been silenced [R(i):TRV-TIP1;1, black line, and R(i):TRV-LeTH1, black broken line, respectively]. A, Expression of TIP1;1. B, Expression of LeHT1. C, Expression of Lin6. qPCR analysis was performed at 0, 1, 3, and 7 dpi. Tomato β-actin was used as the calibrator gene. Note that the expression of LeHT1 and Lin6 was inhibited in TIP1;1-silenced R plants, but the expression of TIP1;1 and Lin6 was not inhibited in LeHT1-silenced R plants. Data points are means ± se (n = 4–9).
Pearson Analyses in Arabidopsis Revealed a Strong Correlation between the Expression of TIP-AQPs, Sugar Metabolism, and Hormone Homeostasis during Early Stages of Biotic Stress
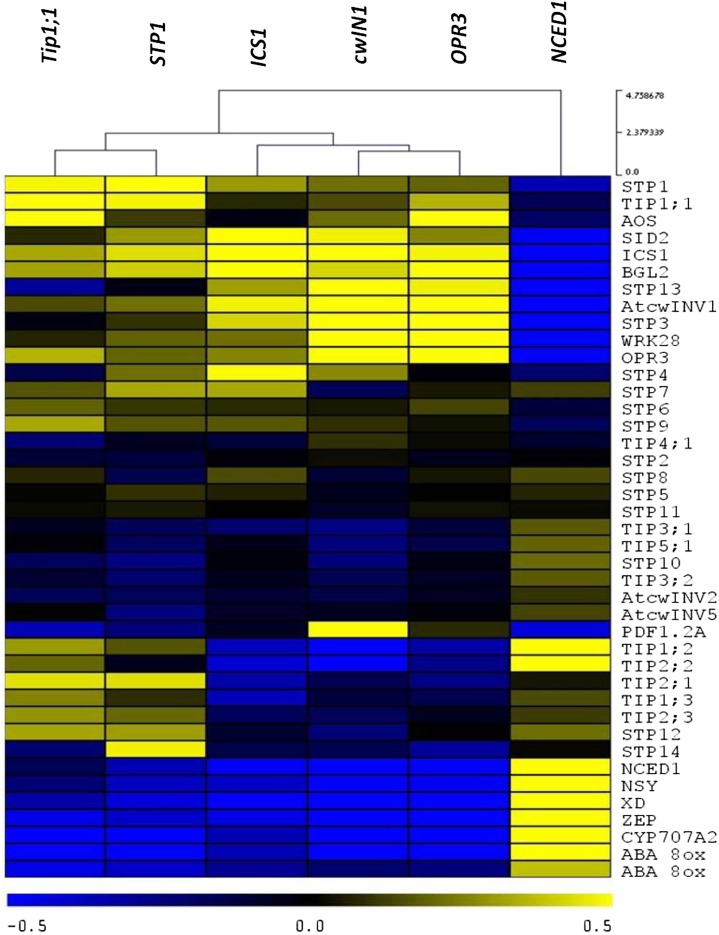
We have previously shown (Sade et al., 2013) and confirmed here (Figs. 5–7) that TIP-AQP genes, LeHT1, Lin6, and hormone homeostasis genes are all involved in the resistance of R tomatoes to TYLCV. To ascertain whether the transcript levels of these genes are affected by pathogens, we data-mined Arabidopsis microarray analyses (there is not enough available data for tomato) upon infestation with fungi and bacteria (there is not enough data for viruses; NASCArray, http://affymetrix.arabidopsis.info). This analysis included gene members of the Arabidopsis AQP TIP family, of the sugar transporter STP, and of the cell wall invertase AtcwINVs. We also included hormone-signaling genes of the SA (ISOCHORISMATE SYNTHASE1 [ICS1], SALICYLIC ACID INDUCTION DEFICIENT2 [SID2], BETA-1,3-GLUCANASE2, and WRKY DNA-BINDING PROTEIN28), JA (OPR3, ALLENE OXIDE SYNTHASE, and PLANT DEFENSIN 1.2A), and ABA (NCED1, CYP707A2 [for CYTOCHROME P450, FAMILY707, SUBFAMILY A, POLYPEPTIDE2], ZEAXANTHIN EPOXIDASE, NEOXANTHIN SYNTHASE, XANTHOXIN, and ABA 8′-HYDROXYLASE) pathways (Fig. 8; Supplemental Table S1). The hierarchical clustering of the correlation intensities (Fig. 8) showed that, in most instances, the correlation patterns of members of a specific gene family were not similar. However, there were correlations between the behavior of Arabidopsis genes and that of their tomato homologs. TIP1;1 expression was positively correlated with STP1 expression (the tomato LeHT1 homolog) and with specific cwINV1 expression at an early stage of infection (24 h after inoculation). Moreover, TIP1;1, STP1, and cwINV1 expression levels were positively correlated with the SA and JA pathways and negatively with the ABA pathway. These analyses strongly suggest that TIP1;1 AQPs, sugar metabolism, and hormone homeostasis are linked.
Figure 8.
Hierarchical clustering of coexpression microarray data derived from Arabidopsis in the presence of several sources of biotic stress (the different pathogens listed below). Blue represents a negative correlation, and yellow represents a positive correlation. The heat map shows the correlations between the genes TIP1;1, STP1, cwINV1, OPR3, and ICS1. NCED1 shows an opposite trend. Pearson correlation coefficient values were calculated for all of the gene pairs in the matrix using the Web-based tool Expression Angler. A subset of expression values derived from the AtGenExpress Pathogen Compendium was used for the calculation. This subset included all of the expression values recorded for experiments in which there was a response to infection by one or more of four different pathogens (P. infestans, B. cinerea, Pseudomonas spp., and Erysiphe orontii).
DISCUSSION
Plants’ water balance management is carefully regulated via stomatal conductance and changes in response to changing environmental conditions. Different plants manage their water budgets differently, and the molecular and physiological mechanisms of this regulation are not yet fully understood. In addition to abiotic stresses, biotic stresses are also known to affect plant water regulation (Gudesblat et al., 2009), raising the possibility that these two types of stress activate the same signal transduction pathways (Fujita et al., 2006). Plant AQPs are involved in regulating both transpiration and hydraulic conductivity (Aharon et al., 2003; Boursiac et al., 2005; Postaire et al., 2010; Pou et al., 2013). Differential levels of AQP expression correlate with differences in the rate of leaf transpiration under normal and abiotic/biotic stress conditions (Siefritz et al., 2002; Parent et al., 2009; Sade et al., 2010, 2012b).
Plants’ responses to stress involve changes in phytohormones and protein interactions (Fujita et al., 2006), which lead to reduced stomatal conductance, decreased CO2 assimilation, and changes in water utilization (Gudesblat et al., 2009; Kyseláková et al., 2011). Resistance to biotic stress is strongly associated with homeostasis of hormones such as SA and JA (Glazebrook, 2005; Thaler et al., 2010). Tomato plants grown in a CO2-enriched atmosphere exhibited increased resistance to the begomovirus TYLCV, as well as increased SA levels (Huang et al., 2012). Infection of Arabidopsis plants with the begomovirus Cabbage leaf curl virus (CaLCuV) led to an early pathogen response involving the SA pathway (Ascencio-Ibáñez et al., 2008). Moreover, antagonistic cross talk between ABA (associated with abiotic stress) and SA and JA (both associated with biotic stress) has been observed upon bacterial, fungal, and viral infection (Audenaert et al., 2002; Yasuda et al., 2008; de Torres Zabala et al., 2009). Hence, it has been suggested that carbon surpluses (McDowell et al., 2008; McDowell, 2011) and hormone homeostasis are involved in resistance to stress (Audenaert et al., 2002; Ascencio-Ibáñez et al., 2008; Huang et al., 2012).
These findings led us to hypothesize that vacuole water channels may be involved in plant resistance to TYLCV (Fig. 1). We suggest that greater permeability of the vacuole membrane to water during the onset of viral infection will continue to buffer the osmotic and mineral concentration in the cytoplasm and thus delay the initiation of a stress signal in the cytoplasm. Hence, a slower stomatal response is expected to be controlled by a mechanism similar to that suggested for isohydric/anisohydric behavior (Sade et al., 2009). The prolonged stress signal might be a result of lower levels of ABA (a phytohormone extensively involved in responses to abiotic stress) acting as a negative regulator of disease resistance (Mauch-Mani and Mauch, 2005). By contrast, SA plays a central role in signaling upon pathogen infection. SA and ABA have antagonistic effects. Thus, the ABA/SA cross talk will result in higher SA levels, leading to resistance to TYLCV (Huang et al., 2012).
By screening tomato ESTs from TYLCV-R and -S genotypes, we found that TIP1;1 was up-regulated upon TYLCV infection (Fig. 1; Eybishtz et al., 2009). R tomato plants displayed less conservative water management than S plants, maintaining relatively high transpiration and lower ψleaf and RWC than S plants (Fig. 1; Supplemental Fig. S1; Sade et al., 2013). This resembles the anisohydric behavior observed in tomato lines transformed with SlTIP2;2 (Sade et al., 2009, 2012b). The unique role of TIP-AQPs in regulating whole-plant water balance and abiotic stress resistance has been demonstrated in other studies as well (Lin et al., 2007; Peng et al., 2007; Pou et al., 2013).
The role of TIP-AQPs was demonstrated in both tomato and Arabidopsis. When a reverse-genetic approach (TRV-VIGS in tomato and transposon-mediated knock out in Arabidopsis) targeting TIP1;1 was used, tomato resistance was impaired and Arabidopsis became more susceptible to infection, as compared with untreated control plants (Fig. 2). In addition, anisohydric transgenic Tom-TIP2;2 tomato plants and transgenic Arabidopsis plants overexpressing SlTIP2;2 (Col-SlTIP2;2) displayed enhanced TYLCV resistance (Fig. 3). These results can be compared with those of a previous study in which anisohydric TIP2;2-overexpressing tomato plants exhibited a higher level of resistance to Botrytis cinerea than the isohydric controls (Sade et al., 2012b). By contrast, transgenic tomato plants overexpressing a N. tabacum plasma membrane AQP1 (NtAQP1) did not show any resistance to TYLCV (Supplemental Fig. S5), emphasizing the specific role of the tonoplast AQPs in resistance. Moreover, tomato plants that have mutations in the ABA biosynthesis pathway that prevent them from closing their stomata (and, as such, could referred to as extremely anisohydric) displayed greater resistance to B. cinerea infection, lower ABA levels, and higher levels of SA, as compared with the isohydric controls (Audenaert et al., 2002).
Generally, anisohydric plants display lower amounts of, or sensitivity to, the stress hormone ABA (Loveys and During, 1984; Loewenstein and Pallardy, 1998; Tardieu and Simonneau, 1998; Soar et al., 2006). Low levels of ABA can lead to high levels of SA due to antagonistic interactions between these two phytohormones (Audenaert et al., 2002; Mohr and Cahill, 2007; de Torres Zabala et al., 2009; Jiang et al., 2010). SA is well known for its role in resistance to numerous types of biotic stress (Hammond Kosack et al., 1996; Thomma et al., 1998), including geminiviruses (Ascencio-Ibáñez et al., 2008; Huang et al., 2012). This suggests that anisohydric resistance to biotic stress may be related to a plant defense mechanism regulated by ABA and SA. Analysis of ABA and SA levels at 7 dpi revealed that the anisohydric R and the Tom-2;2 transgenic tomato plants contained larger amounts of SA and smaller amounts of ABA than the isohydric plants (S line and Tom-wt; Fig. 4, A and B).
The same trend was observed when the two TYLCV-infected Arabidopsis plants Col-SlTIP2;2 and tip1;1 were compared. The transgenic plants contained larger amounts of SA and smaller amounts of ABA than the mutant plants (Fig. 4C). A close correlation was also observed between hormone levels and the transcript levels of hormone marker and biosynthesis genes in these plants (with the exception of the amounts of ABA in the Col-SlTIP2;2 plants; Supplemental Fig. S6). It is possible that the heterologous expression of SlTIP2;2 in Arabidopsis caused a bias between ABA hormone level and gene expression at 7 dpi. Additionally, no change was observed in the amounts of JA or the transcript levels of the JA biosynthesis genes in the different lines at 7 dpi (data not shown; Supplemental Fig. S6).
The changes in hormone homeostasis in tomato plants were noticeable as early as at 1 dpi (Fig. 5). Interestingly, at 1 dpi, the JA biosynthesis gene OPR3 was more highly expressed in R plants than in S plants (Fig. 5A), emphasizing the involvement of this hormone in the early stages of infection and the activation of resistance. JA has been shown to be a crucial component of the plant defense response to sucking insects such as whiteflies (Kempema et al., 2007; Zarate et al., 2007). Hence, overexpression of the JA-associated gene may be related to insect feeding rather than to virus infection per se. Moreover, combined applications of exogenous SA and JA induced stronger resistance to TYLCV than the application of either SA or JA alone (Huang et al., 2012). Therefore, it appears that the modulated interaction between SA and JA in the R line may significantly contribute to the observed TYLCV resistance.
As expected, the lower levels of ABA were accompanied by higher transpiration rates in the anisohydric tomato plants Tom-TIP2;2 and R (Fig. 1; Sade et al., 2009). This phenomenon has also been associated with increased photosynthetic activity (Sade et al., 2013). Interestingly, the TYLCV-resistant Arabidopsis Col-SlTIP2;2 plants also exhibited improved photosynthesis compare with the wild type (Supplemental Fig. S8).
It was recently shown (Huang et al., 2012) that tomatoes grown under elevated CO2 show enhanced resistance to TYLCV and to Phytophthora parasitica, a phenomenon likely related to changes in SA/ABA levels (Jwa and Walling, 2001; Zavala et al., 2008). In addition, it has been previously demonstrated that in R plants in which LeHT1 has been silenced, hexoses are not taken up by the cells and, therefore, do not act as defense-signaling molecules, provoking the collapse of TYLCV resistance (Sade et al., 2013). Moreover, both cell wall invertases Lin6 and LeHT1 were differentially expressed in R plants soon after infection (1 dpi; Sade et al., 2013).
We further hypothesized that this interconnecting network is not specific to tomato and can be identified in Arabidopsis as well. The overexpression of TIPs was correlated with TYLCV resistance; whereas down-regulation of TIP expression was correlated with susceptibility (Figs. 2C and 3C). This was further connected to ABA/SA homeostasis (Figs. 4 and 6; Supplemental Fig. S6). Coexpression analysis of Arabidopsis under several types of biotic stress at early stages of infection (<1 dpi) revealed a positive correlation between the TIP-AQP, hexose transport, and hormone pathways (Fig. 8). Interestingly, TIP1;1 was positively correlated with STP1 (homolog of the tomato LeHT1; McCurdy et al., 2010), with cwINV1, and with the SA and JA pathways. By contrast, a negative correlation was observed between TIP1;1 expression and activation of the ABA pathway. This unique pattern was not observed for other TIP genes, other STP genes, or cwINV, emphasizing the specific interconnection of these genes at early stages of infection. Therefore, it is likely that cross talk between sugar and hormone signaling pathways in plants leads to an effective immune response (Herbers et al., 1996; Bolouri Moghaddam and Van den Ende, 2012).
Because TIP1;1, LeHT1, and Lin6 are preferentially expressed in R plants and are overexpressed upon TYLCV infection (Sade et al., 2013; Fig. 7), we postulated that they belong to an interconnected hierarchical network that confers resistance to this virus. We tested the hypothesis that silencing one of these genes would influence the transcript levels of genes downstream in the gene hierarchy but not those upstream. The step-wise silencing and gene transcript level analyses indicated that TIP1;1 is upstream of both Lin6 and LeHT1 and that Lin6 is upstream of LeHT1 (Fig. 7). Therefore, it is likely that endogenous TIP1;1 in R plants leads to more efficient gas exchange (i.e. transpiration and photosynthesis), which induces a subsequent effect on sugar (i.e. hexose transport) and hormone signaling (i.e. SA).
SA plays a major role in resistance to TYLCV at the early stages of infection (7 dpi) and can also act as a resistance signal, activating pathogen resistance genes through the SAR signaling pathway (Vlot et al., 2009). Analysis of gene expression in Arabidopsis plants infected with the geminivirus CaLCuV revealed that virus triggers an early pathogen response via the SA pathway (Ascencio-Ibáñez et al., 2008). Moreover, Arabidopsis constitutive pathogenesis related1 plants that constitutively expressed PR1 (Bowling et al., 1994) were less susceptible to CaLCuV infection than ecotype Columbia wild-type plants; symptoms in those plants were attenuated and developed much later than in the wild-type plants. In our study, Arabidopsis plants with an up-regulated SA pathway were more resistant to TYLCV (Supplemental Fig. S7). These findings and our results indicate that up-regulation of the SA pathway and SAR triggering (and subsequent down-regulation of the ABA pathway) impair geminivirus infection (Fig. 6, B and C; Supplemental Fig. S7).
CONCLUSION
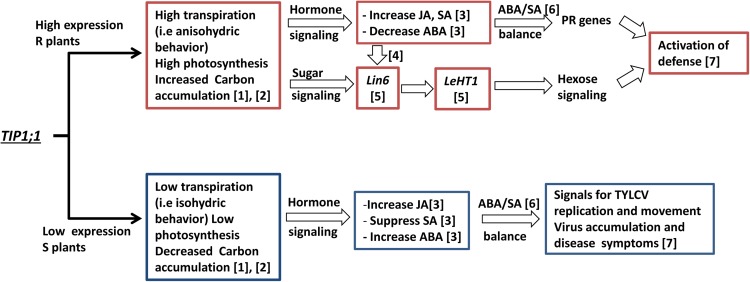
We have shown that TIP-AQPs play a significant role in mediating TYLCV resistance and that this involves changes in water balance regulation, hexose signaling, and hormone homeostasis. A flow chart of events triggered by TIP1;1 in R and S plants is presented in Figure 9. The question of how these pathways are interconnected should be explored in further research.
Figure 9.
Flow chart describing the possible role of TIP1;1 in hormone and sugar signaling leading to resistance to TYLCV in R and S plants. 1, Sade et al. (2012). 2, Figure 1; Supplemental Figure S1. 3, Figures 4 to 6; Supplemental Figure S6; Huang et al. (2012). 4, Proles et al. (2009). 5, Figure 7. 6, Figures 4 to 6; Supplemental Figure S6. 7, Figure 2. [See online article for color version of this figure.]
MATERIALS AND METHODS
Plants
Tomato
Two inbred tomato (Solanum lycopersicum) lines were used, line 902 and line 906-4. These lines were generated in a breeding program aimed at introgressing resistance to TYLCV from Solanum habrochaites (Vidavsky and Czosnek, 1998) into the domesticated tomato. Line 902 is resistant to the virus, whereas line 906-4 is susceptible. Upon infection, R plants developed normally, produced fruit, and contained low amounts of virus. By contrast, S plants exhibited stunted growth, produced small quantities of fruit, and contained large amounts of virus. In addition, two independent lines of the tomato ‘M82’ × ‘MicroTom’ hybrid expressing the tomato AQP gene SlTIP2;2 (called TOM-TIP2;2 plants; Sade et al., 2009) and nontransgenic controls were used. The plants were transplanted into 2-L pots and grown for about 3 weeks in a climate-controlled greenhouse that was kept at 18°C to 25°C and 50% to 60% relative humidity before being infected with the virus via viruliferous whiteflies (Bemisia tabaci). Fertilizer was applied through the automatic irrigation system.
Arabidopsis
Arabidopsis (Arabidopsis thaliana) plants were transformed using the floral-dip method (Clough and Bent, 1998). Two independent T2 transgenic lines (ecotype Columbia) overexpressing the tomato gene TIP2;2 and a line with a transposon insertion mutation in the endogenous TIP1;1 gene (line tip1;1, SM_3_32402 plants; Schüssler et al., 2008) were used, together with their nontransgenic control counterparts. Homozygous mutants tip2;2 (Salk 151945) and aba1-1 were obtained from the Arabidopsis Biological Resource Center. All plants were grown in 200-mL pots in a climate-controlled growth chamber (22°C and 8-h dark/16-h light regimen). After 28 d of growth, these plants were infected using viruliferous whiteflies (about 30 insects per plant).
Whitefly-Mediated Inoculation of Tomato and Arabidopsis Plants with TYLCV
Whiteflies, B. tabaci B biotype, were reared on cotton (Gossypium hirsutum) plants, as described previously (Zeidan and Czosnek, 1991). TYLCV (Navot et al., 1991) was maintained in tomato plants. We inoculated the tomato plants (3 weeks after sowing) and Arabidopsis plants (4 weeks after sowing; Polston and Schuster, 2001) by placing them together with viruliferous whiteflies (approximately 30 insects per plant) in insect-proof, wooden boxes in a growth chamber kept at 24°C to 27°C . After 3 d, the insects were killed with imidacloprid (Bayer Crop Science), and the plants were returned to the greenhouse.
Gas Exchange Measurements
Gas exchange was recorded using a Li-6400 portable gas exchange system (Li-Cor). All measurements were taken between 11 am and 1 pm under saturating light (1,200 μmol m–2 s–1) and with 400 μmol mol–1 CO2 surrounding the leaf. The amount of blue light (signal for stomata opening) was set to 10% photosynthetically active photon flux density to optimize stomatal aperture. The leaf-to-air vapor pressure deficit was kept at 1 to 2.5 kPa while the data were being collected. All measurements were taken at 25°C.
Water Potential
ψleaf was measured between 11 am and 1 pm using a pressure chamber (ARIMAD-3000; MRC, http://www.mrclab.com/htmls/ home.aspx). A leaflet (third to fourth apical) was harvested, placed in the chamber, and observed under a binocular microscope (SZ; Olympus, Intralux 5000; Volpi AG). The N2 pressure in the tightly closed chamber was slowly increased until water oozed out of the petiole cut.
RWC
After ψleaf was determined, the very same leaves were kept in previously weighed, zipper-locked plastic bags to avoid weight loss by evaporation-transpiration and taken to the laboratory for measurement of fresh weight and RWC between 11 am and 1 pm. The sealed plastic bag with the leaf was weighed, and the weight of the bag was subtracted from the total weight to get the fresh weight. Two milliliters of 5 mm CaCl2 was added to each plastic bag, and the leaf petioles were left to soak in the dark at room temperature for 7 to 9 h. Then, the leaves were gently removed from the bags and placed between two paper towels to absorb excess water, and the turgid weight was recorded. The leaves’ total dry weight (DW) was measured after they had been dried in an oven at 60°C for 72 to 84 h. Leaf RWC was calculated as percentage = (fresh weight – DW/turgid weight – DW) × 100.
Silencing of the Tomato AQP Gene TIP1;1
A fragment of the tomato AQP gene TIP1;1 (nucleotides 420–722, TC170408) was cloned into a pDrive vector (Qiagen). The gene fragments were excised from the vector using XbaI and KpnI and ligated to the TRV RNA2 vector (Liu et al., 2002) using the same restriction sites, resulting in the silencing vector TRV-TIP1;1. The plasmid was introduced into Agrobacterium tumefaciens LB4404 cells by electroporation. The agrobacteria containing TRV-TIP1;1 and TRV RNA1 were cultured in Luria-Bertani medium for 48 h at 28°C. A mixture of TRV RNA1 and TRV-TIP1;1 was introduced into 30 R and S tomato seedlings at the six- to eight-leaf stage by agroinoculation. Five days later, 20 silenced plants were inoculated with TYLCV .We did this by placing the plants in cages with viruliferous whiteflies for a 3-d period. In a previous study, we found that inoculation with TRV does not affect TYLCV replication or resistance in R tomato plants (Eybishtz et al., 2009). Samples from at least two independent experiments were analyzed in triplicate.
Analysis of Gene Expression and TYLCV Amounts by qPCR
Gene Transcript Level
At the times indicated, the two youngest leaves of each plant were harvested. Total RNA was extracted using Tri-Reagent (Molecular Research Center) and treated with RNase-Free DNase (Fermentas). Complementary DNA was synthesized using the EZ-First Strand cDNA Synthesis Kit (Biological Industries) according to the manufacturer’s instructions. qPCR was performed in the presence of SYBR Green I (Takara) in a Corbett Research Rotor-Gene 6000 cycler. The tomato β-actin gene (TC198350; Sade et al., 2012a) and the Arabidopsis ACTIN2 (Alexandersson et al., 2005) were used as references for the standardization of complementary DNA quantities. The reaction was carried out as follows: 30 s at 94°C, followed by 40 cycles consisting of 10 s at 94°C, 30 s at 58°C, and 20 s at 72°C.
Virus Amounts
To estimate the relative amount of TYLCV (GenBank accession no. X15656), DNA was extracted from the youngest two leaves (Bernatzky and Tanksley, 1986) and subjected to qPCR with virus-specific primers. Tomato β-actin and Arabidopsis POLYUBIQUITIN10 were used as calibrating genes. The primers used are listed in Supplemental Table S1.
Determination of Hormone Levels by GC-MS
Frozen young leaves were treated with 1.5 mL of 90% (v/v) methanol. The extract was sonicated for 15 min, and cell debris was removed by centrifugation at 13,000g for 10 min. The supernatant was dried in glass tubes at 40°C. The dried residues were dissolved in 1 mL of 4 m HCl and hydrolyzed at 80°C for 1 h. The hydrolyzed mixtures were treated with cyclopentane:ethyl acetate (1:1, v/v), and the organic phase of each mixture was collected and dried at 40°C under nitrogen. ABA and JA were extracted overnight from 1 g of frozen tissue in 20 mL of 80% (v/v) methanol. After extraction, each sample was reduced in vacuo and diluted with 20 mL of water. The pH of the aqueous phase was adjusted to pH 2.8 with 1 m HCl and partitioned four times with equal volumes of ethyl acetate. The ethyl acetate extracts were combined and evaporated to dryness. The residues were then dissolved in 1 mL of 10% (v/v) methanol and applied to a preequilibrated C18 cartridge (http://www.phenomenex.com/). The column was washed with aqueous acetic acid (pH 3.0), and JA and ABA were then eluted with 80% (v/v) methanol. After evaporation to dryness, the samples from the SA, ABA, and JA analyses were dissolved in 40 µL of 20 mg mL–1 methoxyamine hydrochloride in pyridine for 2 h at 37°C to protect the carbonyl moieties. Acidic protons were derivatized by treatment with 70 µL of N-methyl-N-(trimethylsilyl)-trifluoracetamide for 30 min at 37°C. A volume of 1 μL of each sample was injected into a GC-time of flight-MS system (Pegasus III, Leco) using an autosampler system (PAL Agilent). GC was performed on a 30-m MDN-35 column. The injection temperature was 230°C, and the transfer line and ion source temperatures were set to 250°C. The initial oven temperature (85°C) was gradually increased (15°C min–1) to a final temperature of 360°C. The samples were analyzed by GC-MS and quantified using external SA, ABA, and JA standards.
Acibenzolar-S-Methyl Treatment
Acibenzolar-S-methyl (Actigard 50WG, Syngenta) was applied to tomato plants (3 weeks after sowing) and Arabidopsis plants (4 weeks after sowing) by twice spraying a mixture of 98 μL L–1 on the plants. The two spray applications were made 3 d apart.
Pearson Correlation Coefficient
Pearson correlation coefficient values were calculated for all of the gene pairs in the matrix using the Web-based tool Expression Angler (Toufighi et al., 2005). A subset of expression values derived from the AtGenExpress Pathogen Compendium was used for the calculation. This subset included all the expression values of experiments in response to infection by four different pathogens (Phytophthora infestans, B. cinerea, Pseudomonas spp., and Erysiphe orontii).
A list of all the accession numbers is provided in Supplemental Tables S1 and S2.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. RWC of leaves and leaf water potential at decreasing relative soil volumetric water content levels in S and R plants.
Supplemental Figure S2. SlTIP1;1 and SlTIP1;2 expression in R(0) plants and R(0):TRV-TIP1;1 plants as measured by qPCR.
Supplemental Figure S3. Effects of the expression of the tomato genes TIP1;1 and TIP2;2 on TYLCV infection of tomato plants at 21 dpi.
Supplemental Figure S4. Relative expression levels of the Arabidopsis TIP1;1, Arabidopsis TIP2;2, and tomato TIP2;2 genes in plants with different genotypes.
Supplemental Figure S5. Effect of the expression of the N. tabacum plasma membrane AQP gene NtAQP1 on TYLCV infection of tomato plants transformed with NtAQP1 (Tom-NtAQP1 plants), as compared with nontransgenic tomato plants (wild type).
Supplemental Figure S6. Expression of hormone-related marker and biosynthesis genes (ABA: ABA8 and AtNCED1; SA: PR1 and AtICS1; and JA: OPR3 and AtOPR3) in tomato and Arabidopsis plants at 7 dpi.
Supplemental Figure S7. Effects of hormone levels on TYLCV infection of Arabidopsis mutants at 7 dpi.
Supplemental Figure S8. Photosynthesis and transpiration of wild-type Arabidopsis plants (Col-wt) and Arabidopsis plants expressing SlTIP2;2.
Supplemental Table S1. List of primers used for quantitative reverse transcription-PCR analyses of gene expression.
Supplemental Table S2. Analysis of the coexpression of several Arabidopsis genes (sorted by family) in the presence of biotic stress (P. infestans, B. cinerea, Pseudomonas spp., and Erysiphe orontii).
Supplementary Material
Acknowledgments
We thank David Baulcombe (the Gatsby Charitable Foundation, and the Sainsbury Laboratory) for providing the TRV vectors, Dr. Vered Caspi and Inbar Plaschkes (Bioinformatics Core Facility, National Institute of Biotechnology in the Negev, Ben-Gurion University of the Negev), and Linda Walling (Botany and Plant Sciences, University of California) for providing the cim10 mutant.
Glossary
- TIP-AQP
tonoplast-intrinsic aquaporin
- TYLCV
Tomato yellow leaf curl virus
- ABA
abscisic acid
- SA
salicylic acid
- JA
jasmonic acid
- AQP
aquaporin
- TIP
tonoplast-intrinsic protein
- S
susceptible
- R
resistant
- RWC
relative water content
- ψleaf
midday water potential
- dpi
days after inoculation
- GC
gas chromatography
- MS
mass spectrometry
- SAR
systemic activated resistance
- CaLCuV
Cabbage leaf curl virus
- DW
dry weight
- qPCR
quantitative PCR
Footnotes
This work was supported by the Deutsch Forschungsgemeinschaft Trilateral Collaboration Program (grant no. SCHA 591/5–1 to H.C.), the Middle East Regional Cooperation Program (grant no. M21–037 to H.C.), the Chief Scientist of the Israel Ministry of Agriculture (grant no. 837–0135–13 to H.C.), and the German-Israeli Project Cooperation (grant nos. FE 552/12–1 to A.R.F. and OR309/1–1 to M.M.).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G. (2003) Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15: 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersson E, Fraysse L, Sjövall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P. (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59: 469–484 [DOI] [PubMed] [Google Scholar]
- Ascencio-Ibáñez JT, Sozzani R, Lee TJ, Chu TM, Wolfinger RD, Cella R, Hanley-Bowdoin L. (2008) Global analysis of Arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol 148: 436–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert K, De Meyer GB, Höfte MM. (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol 128: 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatzky R, Tanksley S. (1986) Methods for detection of single or low copy sequences in tomato on southern blots. Plant Mol Biol Rep 4: 37–41 [Google Scholar]
- Bolouri Moghaddam MR, Van den Ende W. (2012) Sugars and plant innate immunity. J Exp Bot 63: 3989–3998 [DOI] [PubMed] [Google Scholar]
- Boursiac Y, Chen S, Luu DT, Sorieul M, van den Dries N, Maurel C. (2005) Early effects of salinity on water transport in Arabidopsis roots: molecular and cellular features of aquaporin expression. Plant Physiol 139: 790–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X. (1994) A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6: 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zheng Z, Huang J, Lai Z, Fan B. (2009) Biosynthesis of salicylic acid in plants. Plant Signal Behav 4: 493–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels MJ, Maurel C. (1994) Aquaporins: the molecular basis of facilitated water movement through living plant cells? Plant Physiol 105: 9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- de Torres Zabala M, Bennett MH, Truman WH, Grant MR. (2009) Antagonism between salicylic and abscisic acid reflects early host-pathogen conflict and moulds plant defence responses. Plant J 59: 375–386 [DOI] [PubMed] [Google Scholar]
- Durrant WE, Dong X. (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185–209 [DOI] [PubMed] [Google Scholar]
- Eybishtz A, Peretz Y, Sade D, Akad F, Czosnek H. (2009) Silencing of a single gene in tomato plants resistant to Tomato yellow leaf curl virus renders them susceptible to the virus. Plant Mol Biol 71: 157–171 [DOI] [PubMed] [Google Scholar]
- Eybishtz A, Peretz Y, Sade D, Gorovits R, Czosnek H. (2010) Tomato yellow leaf curl virus infection of a resistant tomato line with a silenced sucrose transporter gene LeHT1 results in inhibition of growth, enhanced virus spread, and necrosis. Planta 231: 537–548 [DOI] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9: 436–442 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Gudesblat GE, Torres PS, Vojnov AA. (2009) Stomata and pathogens: warfare at the gates. Plant Signal Behav 4: 1114–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Silverman P, Raskin I, Jones J. (1996) Race-specific elicitors of Cladosporium fulvum induce changes in cell morphology and the synthesis of ethylene and salicylic acid in tomato plants carrying the corresponding Cf disease resistance gene. Plant Physiol 110: 1381–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Métraux JP, Sonnewald U. (1996) Salicylic acid-independent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett 397: 239–244 [DOI] [PubMed] [Google Scholar]
- Heymann JB, Engel A. (1999) Aquaporins: phylogeny, structure, and physiology of water channels. News Physiol Sci 14: 187–193 [DOI] [PubMed] [Google Scholar]
- Huang L, Ren Q, Sun Y, Ye L, Cao H, Ge F. (2012) Lower incidence and severity of tomato virus in elevated CO2 is accompanied by modulated plant induced defence in tomato. Plant Biol (Stuttg) 14: 905–913 [DOI] [PubMed] [Google Scholar]
- Jiang CJ, Shimono M, Sugano S, Kojima M, Yazawa K, Yoshida R, Inoue H, Hayashi N, Sakakibara H, Takatsuji H. (2010) Abscisic acid interacts antagonistically with salicylic acid signaling pathway in rice-Magnaporthe grisea interaction. Mol Plant Microbe Interact 23: 791–798 [DOI] [PubMed] [Google Scholar]
- Jwa NS, Walling LL. (2001) Influence of elevated CO2 concentration on disease development in tomato. New Phytol 149: 509–518 [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R, Bertl A, Otto B, Moshelion M, Uehlein N. (2007) Characterization of plant aquaporins. Methods Enzymol 428: 505–531 [DOI] [PubMed] [Google Scholar]
- Kempema LA, Cui X, Holzer FM, Walling LL. (2007) Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs: similarities and distinctions in responses to aphids. Plant Physiol 143: 849–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepper MA. (1994) The aquaporin family of molecular water channels. Proc Natl Acad Sci USA 91: 6255–6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Jorna ML, Brinkhorst-van der Swan DL, Karssen CM. (1982) The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet 61: 385–393 [DOI] [PubMed] [Google Scholar]
- Kyseláková H, Prokopová J, Nauš J, Novák O, Navrátil M, Safářová D, Spundová M, Ilík P. (2011) Photosynthetic alterations of pea leaves infected systemically by pea enation mosaic virus: a coordinated decrease in efficiencies of CO2 assimilation and photosystem II photochemistry. Plant Physiol Biochem 49: 1279–1289 [DOI] [PubMed] [Google Scholar]
- Lin W, Peng Y, Li G, Arora R, Tang Z, Su W, Cai W. (2007) Isolation and functional characterization of PgTIP1, a hormone-autotrophic cells-specific tonoplast aquaporin in ginseng. J Exp Bot 58: 947–956 [DOI] [PubMed] [Google Scholar]
- Loewenstein NJ, Pallardy SG. (1998) Drought tolerance, xylem sap abscisic acid and stomatal conductance during soil drying: a comparison of canopy trees of three temperate deciduous angiosperms. Tree Physiol 18: 431–439 [DOI] [PubMed] [Google Scholar]
- Loveys BR, During H. (1984) Diurnal changes in water relations and abscisic-acid in field-grown Vitis vinifera cultivars. 2. Abscisic acid changes under semi-arid conditions. New Phytol 97: 37–47 [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP. (2002) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Maleck K, Neuenschwander U, Cade RM, Dietrich RA, Dangl JL, Ryals JA. (2002) Isolation and characterization of broad-spectrum disease-resistant Arabidopsis mutants. Genetics 160: 1661–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch-Mani B, Mauch F. (2005) The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 8: 409–414 [DOI] [PubMed] [Google Scholar]
- Maurel C, Chrispeels MJ. (2001) Aquaporins: a molecular entry into plant water relations. Plant Physiol 125: 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59: 595–624 [DOI] [PubMed] [Google Scholar]
- McCurdy DW, Dibley S, Cahyanegara R, Martin A, Patrick JW. (2010) Functional characterization and RNAi-mediated suppression reveals roles for hexose transporters in sugar accumulation by tomato fruit. Mol Plant 3: 1049–1063 [DOI] [PubMed] [Google Scholar]
- McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, et al. (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178: 719–739 [DOI] [PubMed] [Google Scholar]
- McDowell NG. (2011) Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol 155: 1051–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr PG, Cahill DM. (2007) Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes in Arabidopsis infected with Pseudomonas syringae pv. tomato. Funct Integr Genomics 7: 181–191 [DOI] [PubMed] [Google Scholar]
- Navot N, Pichersky E, Zeidan M, Zamir D, Czosnek H. (1991) Tomato yellow leaf curl virus: a whitefly-transmitted geminivirus with a single genomic component. Virology 185: 151–161 [DOI] [PubMed] [Google Scholar]
- Neill SJ, Burnett EC, Desikan R, Hancock JT. (1998) Cloning of a wilt-responsive cDNA from an Arabidopsis thaliana suspension culture cDNA library that encodes a putative 9-cis-epoxy-carotenoid dioxygenase. J Exp Bot 49: 1893–1894 [Google Scholar]
- Parent B, Hachez C, Redondo E, Simonneau T, Chaumont F, Tardieu F. (2009) Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiol 149: 2000–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Lin W, Cai W, Arora R. (2007) Overexpression of a Panax ginseng tonoplast aquaporin alters salt tolerance, drought tolerance and cold acclimation ability in transgenic Arabidopsis plants. Planta 226: 729–740 [DOI] [PubMed] [Google Scholar]
- Polston JE, Schuster D J (2001) Evaluation and development of tactics to reduce or eliminate infection of transplants by Tomato yellow leaf curl virus and other whitefly-transmitted geminiviruses. http://www.pestcompact.org/reports/tylc%20fl%20.pdf (July 18, 2014)
- Postaire O, Tournaire-Roux C, Grondin A, Boursiac Y, Morillon R, Schäffner AR, Maurel C. (2010) A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiol 152: 1418–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou A, Medrano H, Flexas J, Tyerman SD. (2013) A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant Cell Environ 36: 828–843 [DOI] [PubMed] [Google Scholar]
- Proels RK, Roitsch T. (2009) Extracellular invertase LIN6 of tomato: a pivotal enzyme for integration of metabolic, hormonal, and stress signals is regulated by a diurnal rhythm. J Exp Bot 60: 1555–1567 [DOI] [PubMed] [Google Scholar]
- Reuscher S, Akiyama M, Mori C, Aoki K, Shibata D, Shiratake K. (2013) Genome-wide identification and expression analysis of aquaporins in tomato. PLoS ONE 8: e79052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade D, Brotman Y, Eybishtz A, Cuadros-Inostroza A, Fernie AR, Willmitzer L, Czosnek H. (2013) Involvement of the hexose transporter gene LeHT1 and of sugars in resistance of tomato to Tomato yellow leaf curl virus. Mol Plant 6: 1707–1710 [DOI] [PubMed] [Google Scholar]
- Sade D, Eybishtz A, Gorovits R, Sobol I, Czosnek H. (2012a) A developmentally regulated lipocalin-like gene is overexpressed in Tomato yellow leaf curl virus-resistant tomato plants upon virus inoculation, and its silencing abolishes resistance. Plant Mol Biol 80: 273–287 [DOI] [PubMed] [Google Scholar]
- Sade N, Gebremedhin A, Moshelion M. (2012b) Risk-taking plants: anisohydric behavior as a stress-resistance trait. Plant Signal Behav 7: 767–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade N, Gebretsadik M, Seligmann R, Schwartz A, Wallach R, Moshelion M. (2010) The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol 152: 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade N, Vinocur BJ, Diber A, Shatil A, Ronen G, Nissan H, Wallach R, Karchi H, Moshelion M. (2009) Improving plant stress tolerance and yield production: is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol 181: 651–661 [DOI] [PubMed] [Google Scholar]
- Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M. (2004) Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol 134: 1439–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüssler MD, Alexandersson E, Bienert GP, Kichey T, Laursen KH, Johanson U, Kjellbom P, Schjoerring JK, Jahn TP. (2008) The effects of the loss of TIP1;1 and TIP1;2 aquaporins in Arabidopsis thaliana. Plant J 56: 756–767 [DOI] [PubMed] [Google Scholar]
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R. (2002) PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell 14: 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soar CJ, Speirs J, Maffei SM, Penrose AB, McCarthy MG, Loveys BR. (2006) Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: apparent links with ABA physiology and gene expression in leaf tissue. Aust J Grape Wine Res 12: 2–12 [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE. (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA 98: 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu F, Simonneau T. (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot 49: 419–432 [Google Scholar]
- Thaler JS, Agrawal AA, Halitschke R. (2010) Salicylate-mediated interactions between pathogens and herbivores. Ecology 91: 1075–1082 [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF. (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufighi K, Brady SM, Austin R, Ly E, Provart NJ. (2005) The Botany Array Resource: e-Northerns, Expression Angling, and promoter analyses. Plant J 43: 153–163 [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Niemietz CM, Bramley H. (2002) Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 25: 173–194 [DOI] [PubMed] [Google Scholar]
- Vidavsky F, Czosnek H. (1998) Tomato breeding lines resistant and tolerant to Tomato Yellow leaf curl virus issued from Lycopersicon hirsutum. Phytopathology 88: 910–914 [DOI] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Walters DR, Ratsep J, Havis ND. (2013) Controlling crop diseases using induced resistance: challenges for the future. J Exp Bot 64: 1263–1280 [DOI] [PubMed] [Google Scholar]
- Yasuda M, Ishikawa A, Jikumaru Y, Seki M, Umezawa T, Asami T, Maruyama-Nakashita A, Kudo T, Shinozaki K, Yoshida S, et al. (2008) Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell 20: 1678–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate SI, Kempema LA, Walling LL. (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Casteel CL, Delucia EH, Berenbaum MR. (2008) Anthropogenic increase in carbon dioxide compromises plant defense against invasive insects. Proc Natl Acad Sci USA 105: 5129–5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan M, Czosnek H. (1991) Acquisition of Tomato yellow leaf curl virus by the whitefly Bemisia tabaci. J Gen Virol 72: 2607–2614 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.