Phytochrome B nuclear bodies perceive not only the low red to far-red ratio, but also the low irradiance of canopy shade.
Abstract
The current consensus is that plant responses to canopy shade involve the perception of low red to far-red ratios (R:FRs) by phytochrome B (phyB), which leads to the direct activation of auxin synthesis genes by PHYTOCHROME INTERACTING FACTORs (PIFs). In addition to its effect on R:FRs, shade also reduces irradiance, but whether shade-induced drops in irradiance affect phyB activity has not been demonstrated. To address this issue, we investigated whether irradiance and R:FRs have similar effects on the nuclear distribution of phyB in petiole cells of light-grown plants. Under high-irradiance white light, phyB formed large nuclear bodies. Lowering irradiance without changing R:FRs or lowering R:FRs by adding far-red light led to the appearance of small nuclear bodies containing phyB. Large nuclear bodies remained but with some concomitant reduction in diameter. The appearance of small nuclear bodies was rapid, stable, and reversible upon the return to high irradiance and high R:FRs. High levels of red light but not of blue light were enough to restrain the formation of small phyB nuclear bodies. Irradiance was effective within the range found in natural canopies and even under relatively low R:FRs. The promotion of leaf hyponasty by lowering irradiance was impaired in phyB and pif mutants, as previously reported for the response to R:FRs. The expression of auxin-related genes showed a similar hierarchy of response to low R:FRs and low irradiance. We propose that phyB is able to perceive not only the low R:FRs, but also the low irradiance of shade.
Because green leaves absorb more red light (600–700 nm) than far-red light (700–800 nm), the understory of plant canopies is characterized by reduced red to far-red ratios (R:FRs). The dynamic balance between the active Pfr and the inactive Pr depends on the R:FR (Holmes and Smith, 1977; Smith et al., 1990). The low R:FR perceived by phytochrome initiates a series of shade avoidance responses, including enhanced elongation of stems and petioles, reorientation of the leaves toward a more vertical position (leaf hyponasty), reduced branching, and accelerated flowering, which tend to reduce the magnitude of present or future shade (Smith, 1982; Martínez-García et al., 2010; Casal, 2013). Phytochrome B (phyB) is the main photoreceptor of the R:FR of plant canopies (Franklin et al.., 2003). Phytochrome is synthesized in the cytosol in the inactive Pr form, which upon conversion to the active Pfr form by red light migrates to the nucleus, where it forms phyB-containing nuclear bodies (phyB-NBs; Sakamoto and Nagatani, 1996; Kircher et al., 1999, 2002; Yamaguchi et al., 1999; Chen et al., 2003; Chen, 2008). The phyB-NBs are stabilized after prolonged exposure to high R:FR (Kevei et al., 2007), but the impact of a subsequent shift to low R:FR on the subcellular localization of phyB has not been established.
Under low R:FR, low phyB Pfr levels favor the activity of PHYTOCHROME INTERACTING FACTOR (PIF) basic helix-loop-helix transcription factors, which bind auxin synthesis genes, increase auxin, and cause shade avoidance responses (Hornitschek et al., 2012; Li et al., 2012). Low R:FR also causes the accumulation of CONSTITUTIVE PHOTOMORPHOGENIC1 in the nucleus (Pacín et al., 2013), which would also contribute to shade avoidance (McNellis et al., 1994; Rolauffs et al., 2012; Pacín et al., 2013).
In sparse canopies, before mutual shading among plants is established, far-red light reflected on the green leaves of neighboring vegetation is enough to elicit shade avoidance responses (Ballaré et al., 1987). However, under dense canopies, there is a reduction in irradiance in addition to a low R:FR. The reduced blue-light irradiance of shade contributes to shade avoidance reactions (Casal and Alvarez, 1988.; Pierik et al., 2004), and this signal is perceived mainly by cryptochromes (Sellaro et al., 2010; Keller et al., 2011; Keuskamp et al., 2011). In addition, lowering red plus far-red light reaching the stem of Datura ferox or Sinapis alba plants (without reducing the R:FR) also promotes stem growth (Ballaré et al., 1991). Because this response is reduced in the aurea mutant of tomato (Solanum lycopersicum; Casal and Kendrik, 1993), which is deficient in the synthesis of phytochrome chromophore (Terry and Kendrick, 1996), phytochromes could also be involved in the perception of irradiance signals of shade.
phyB could be involved in the perception of the low irradiance of crowded compared with sparse plant canopies. In favor of this hypothesis, when Arabidopsis (Arabidopsis thaliana) rosettes are transferred from a higher to a lower irradiance of white light (without changing light quality), the leaves show a robust hyponastic response that is reduced in the phyB mutant (Vandenbussche et al., 2003; Mullen et al., 2006; Millenaar et al., 2009; Dornbusch et al., 2012). However, it is not clear whether phyB actually perceives the signal or, alternatively, its absence conditions the response to other receptors involved in the perception of irradiance.
The level of phyB Pfr is irradiance dependent due to the phototransformation of Pr to Pfr in the presence of Pfr-to-Pr thermal reversion, which competes with light reactions and increases the irradiance required to establish a given level of Pfr (Elich and Chory, 1997; Sweere et al., 2001; Rausenberger et al., 2010; Medzihradszky et al., 2013). For this reason, during deetiolation of young seedlings, the inhibition of hypocotyl growth by phyB is irradiance dependent and reaches saturation between 1 and 10 µmol m–2 s–1 of continuous red light (Chen et al., 2003; Rausenberger et al., 2010). Under canopy conditions, irradiance is reduced compared with sunlight but often well above the latter levels. This indicates that phyB can act as a sensor of irradiance but perhaps out of the range required for effective shade avoidance reactions.
A cornerstone that supports the model involving phyB perception of canopy R:FR is the knowledge that phytochrome photoequilibrium depends on R:FR (Holmes and Smith, 1977; Smith, 1982, 2000; Smith et al., 1990). To provide similar support to the idea that phyB also perceives changes in irradiance, it would be necessary to demonstrate that changes in irradiance within the range of natural shade affect some aspect of the dynamics of phyB. The aim of this article is to test several predictions of the hypothesis that the reduced irradiance of plant canopies perceived by phyB initiates shade avoidance reactions. We used genetic, molecular, and cellular approaches; in particular, we investigated whether irradiance changes in the range of sparse versus dense canopies affect phyB-NBs.
RESULTS
Leaf Hyponastic Response to Low Irradiance Is Impaired in phyB and pif Mutants
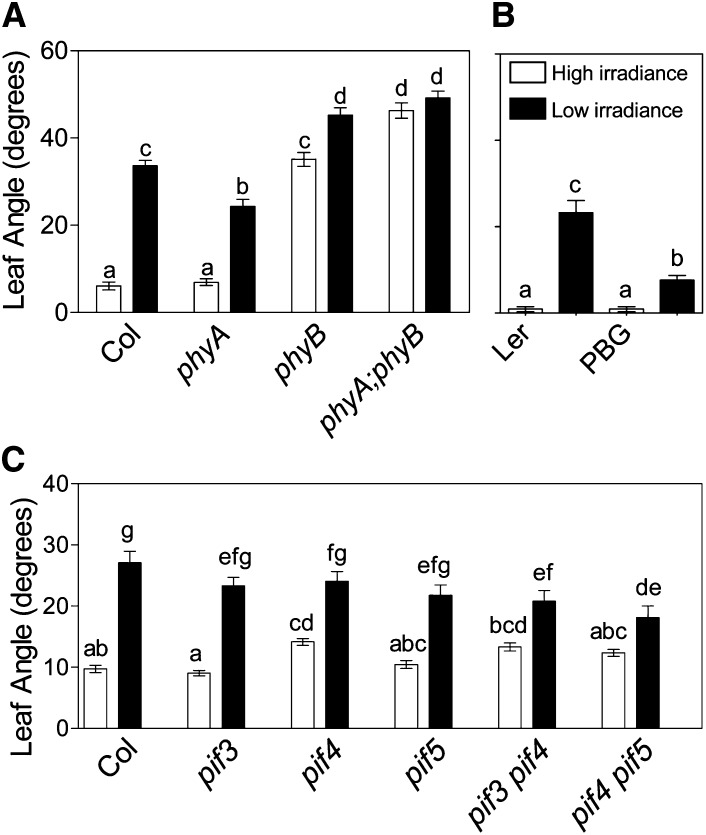
A first prediction of the hypothesis that phyB can perceive changes in irradiance associated to canopy shade is that mutations at PHYB or at the downstream PIF genes should impair the response to irradiance. Arabidopsis plants of the ecotype Columbia wild-type and of the phyB mutant were grown under controlled conditions (16-h white light/8-h darkness) under relatively high irradiance (200 µmol m–2 s–1 of photosynthetically active radiation) to reach the rosette stage and either transferred to low irradiance (25 µmol m–2 s–1 of photosynthetically active radiation) or left as high irradiance controls. The wild type showed hyponasty in response to low irradiance, but in the phyB mutant, the leaves were hyponastic even under high irradiance (Fig. 1A; Vandenbussche et al., 2003; Mullen et al., 2006; Millenaar et al., 2009). Complementary, plants bearing the phyB-GFP fusion in addition to endogenous phyB (Yamaguchi et al., 1999) showed reduced hyponasty under low irradiance (Fig. 1B), indicating that phyB-GFP used for subsequent studies is biologically active. Therefore, phyB is required for high light suppression of hyponasty. Conversely, neither cryptochrome1 (cry1) nor cry2 was required for suppression of hyponasty under high irradiance (mean leaf angle under high irradiance in degrees ± se: the wild type = 8 ± 1; cry1 = 5 ± 1; cry2 = 10 ± 1; and cry1 cry2 = 6 ± 1; P > 0.10). There are other reports showing weak shade avoidance phenotypes of cry1 (Ballaré and Scopel, 1997; Mullen et al., 2006).
Figure 1.
Impaired hyponastic response in phyB and phyA mutants (A), in transgenic seedlings expressing phyB-GFP (PBG; B), and in pif3, pif4, and pif5 mutants (C). Arabidopsis plants were grown under high-irradiance white light for 2 weeks, transferred to low irradiance 4 h after the beginning of the photoperiod, and measured 24 h later. Control plants remained under high irradiance. Data are means ± se of at least 10 plants. In all cases, the interaction between genotype and irradiance is significant at P < 0.0001 (factorial ANOVA). Different letters denote significant differences among means (P < 0.05) in Bonferroni posttests.
Compared with the wild type, the phyA mutant showed weak hyponasty under low irradiance (Fig. 1A). This pattern is interesting because the phyA mutation had been shown to reduce the response to low R:FR perceived by phyB (Casal, 1996; Cole et al., 2011; Sellaro et al., 2012). The reduced hyponastic response of phyA is consistent with the enhanced phyB-mediated signaling observed in this mutant (Cerdán et al., 1999). In accordance with this interpretation, the phyA mutation did not reduce hyponasty in the phyB background. On the contrary, compared with the phyB mutant, the phyA phyB mutant showed enhanced hyponasty under high irradiance (Fig. 1A), which is consistent with a direct action of phyA on the repression of hyponasty as reported for other shade avoidance responses (Casal, 2013). A dual action of phyA (positive and negative) has also been reported for deetiolating seedlings (Mazzella et al., 1997).
The pif3 pif4 and pif4 pif5 double mutants showed an attenuated hyponastic response (Fig. 1C). To estimate the contribution of each PIF gene to hyponasty under low irradiance, we used multiple linear regression with dichotomous variables (also known as categorical or dummy variables; x = 1 for wild-type allele and x = 0 for mutant alleles). The resulting estimates (degrees ± se) were: PIF3 = 5.5 ± 0.8, PIF4 = 7.5 ± 0.8, and PIF5 = 3.7 ± 1.0. In other experiments, we observed that leaf hyponasty under low irradiance was similarly affected in the pif3 pif4 (degrees, 22 ± 2) and the quadruple mutant pif1pif3 pif4 pif5 (21 ± 1) compared with the wild type (42 ± 2), while the three genotypes were similar under high irradiance (pif3 pif4: 7 ± 1; pif1pif3 pif4 pif5: 9 ± 1; and the wild type: 10 ± 2; P > 0.05). These results provide genetic evidence in favor of a role of phyB in the perception of irradiance signals of shade.
Low Irradiance and Low R:FR Similarly Increase the Number of Small phyB-NBs
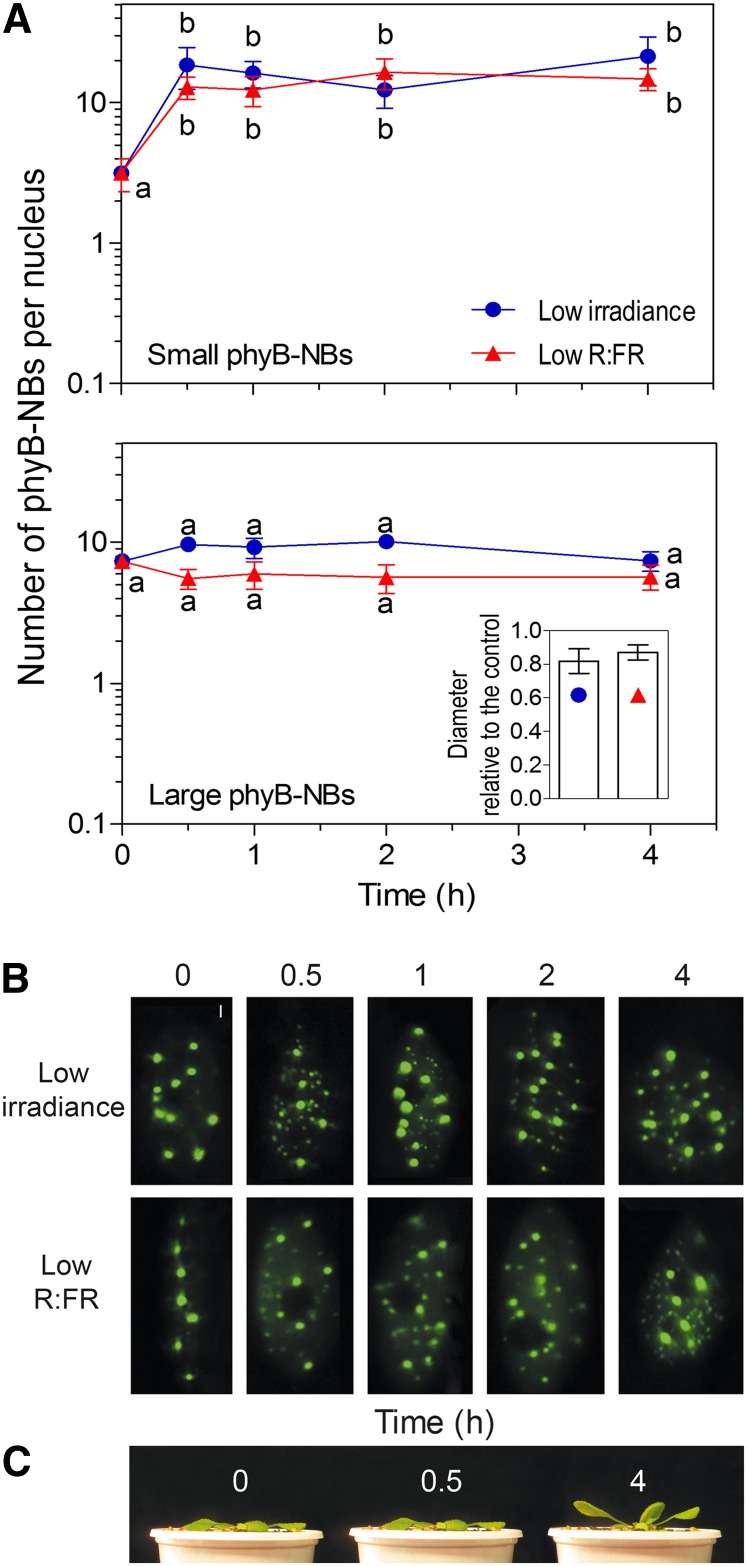
The dynamics of phyB cellular localization during deetiolation, i.e. during the first dark-to-light transition, has been extensively characterized, but the response to shade signals is not established. We decided to investigate the dynamics of nuclear phyB in the abaxial cells of the petiole of fully deetiolated plants bearing the phyB-GFP fusion line (Yamaguchi et al., 1999) grown for 2 weeks under high-irradiance white light and transferred to either low-irradiance white light or high-irradiance white plus far-red light (i.e. R:FR reduced from 4.3 to 0.8). After exposure to low irradiance or low R:FR, the number of small phyB-NBs significantly increased within the first 30 min and remained stable for at least 4 h (Fig. 2, A and B). The number of large phyB-NBs showed no changes (Fig. 2, A and B), but the diameter was somewhat reduced (Fig. 2A, inset). Lowering irradiance or R:FR respectively increased leaf angle in 9 ± 1 and 8 ± 1 degrees (mean ± se of at least 10 plants) during the first 4 h of treatment. The increase in the number of small phyB-NBs preceded the physiological response (Fig. 2C).
Figure 2.
Low irradiance and low R:FR increase the number of small phyB-NBs in leaf petiole cells. A, Time course of small (diameter ≤ 0.4 µm) and large (diameter > 0.4 µm) phyB-NBs upon transfer to either low irradiance or low R:FR. Data are means ± se of eight plant replicates. The effects of irradiance and R:FR were significant at P < 0.01 and P < 0.0001, respectively (ANOVA). Different letters denote significant differences among means (P < 0.05) in Bonferroni posttests. The inset shows the diameter of the large phyB-NBs after 2-h low irradiance or low R:FR relative to the control under high irradiance and R:FR (in both cases, P < 0.05 compared with the control). B, Representative pictures of phyB-NBs. Bar = 2 µm. C, Plants exposed to low irradiance. Note that changes in phyB-NBs in A and B anticipate leaf angle responses in C. Arabidopsis plants were grown under high-irradiance white light for 2 weeks and transferred to either low irradiance or low R:FR 4 h after the beginning of the photoperiod (time = 0). [See online article for color version of this figure.]
To investigate whether the formation of small phyB-NBs is specific of the abaxial cells of the petiole or a more general feature observed when plants grown at high irradiance are transferred to low irradiance, we characterized the response in cells of the adaxial petiole surface, leaf lamina, and hypocotyls of young seedlings. A similar response was observed in these different developmental contexts (Supplemental Fig. S1), confirming that the response is general.
In some experiments, the confocal plane was selected by irradiating a leaf area distant from the site of observation. Then, the position was changed, and a picture was taken without any previous irradiation. Small phyB-NBs were present in plants grown under low irradiance for 2 h (Supplemental Fig. S2A). In additional experiments, repeated irradiation with the laser source did not increase the number of small phyB-NBs (this procedure actually caused some bleaching; Supplemental Fig. S2B). These observations indicate that the small phyB-NBs were formed as a result of the shift from high to low growth irradiance and not as a result of sample irradiation during confocal microscopy.
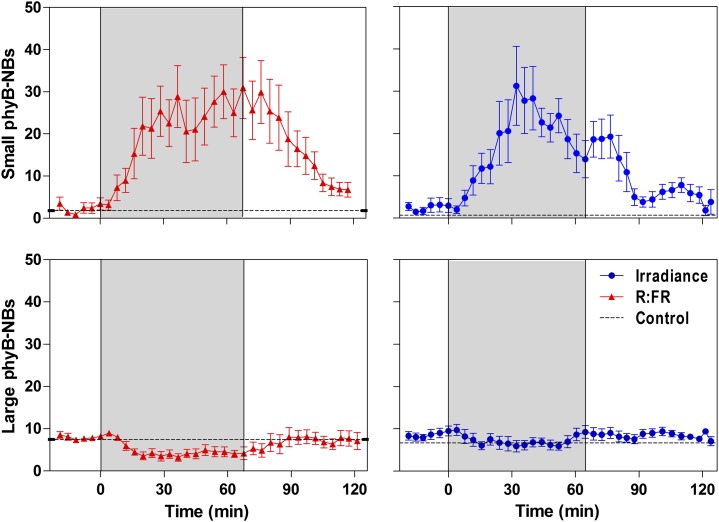
Kinetics of the Small phyB-NBs in Response to Fluctuating Shade Signals
To investigate the kinetics of phyB-NBs in further detail, we recorded the number of small and large phyB-NBs under high irradiance and high R:FR, transferred the seedlings to either low irradiance or low R:FR for 1 h, and returned them to the control conditions for another hour. Different seedlings were analyzed every 4 min, and the three-point running average was calculated to smooth out short-term fluctuations due to plant variability and to highlight the trends. The number of small phyB-NBs increased steadily during the first 0.5 h under low irradiance or low R:FR, without any obvious lag (Fig. 3). The number of phyB-NBs remained elevated during the low irradiance or R:FR treatments and gradually returned to the prestimulation values following the transfer back to high irradiance and R:FR conditions (Fig. 3). The number of large phyB-NBs remained relatively stable.
Figure 3.
Rapid and reversible response of the small phyB-NBs to changes in irradiance or R:FR. Arabidopsis plants were grown under high-irradiance white light for 2 weeks. The number of phyB-NBs per nucleus was recorded under high irradiance and high R:FR for 25 min (white background), transferred to low irradiance or low R:FR for 65 min (gray background), and returned to high irradiance and high R:FR for an additional 60 min (white background). Confocal images were recorded from different plants every 4 min in three independent experiments. Data show the moving average of three successive time points ± se. The dotted lines show the number of phyB-NBs in plants kept under high irradiance and high R:FR during the whole experiment (recorded at the end of each experiment). [See online article for color version of this figure.]
Spectral Dependence of the phyB-NBs Response to Irradiance
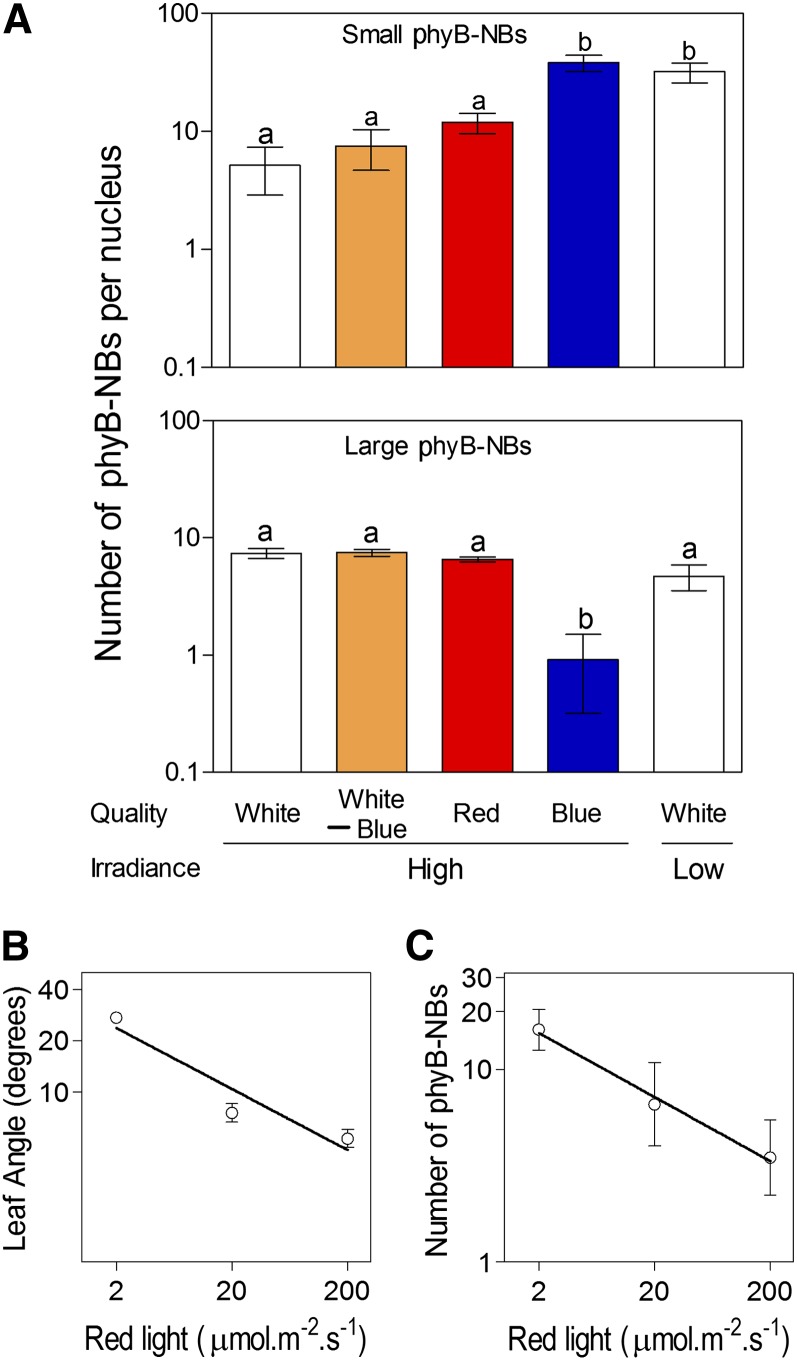
Cryptochromes can be irradiance sensors during shade avoidance (Sellaro et al., 2010; Keller et al., 2011; Keuskamp et al., 2011), show signaling convergence with phyB (Sellaro et al., 2009), and can share physical interaction partners with phyB (Jarillo et al., 2001). Therefore, the increased number of small phyB-NBs in response to a reduction in white light irradiance could be either the direct consequence of reduced light absorption by phyB or the indirect consequence of reduced blue light perceived by cryptochromes affecting phyB. To discriminate between these two possibilities, white-light-grown plants were transferred to orange (white minus blue), red, or blue light without changing irradiance (200 µmol m–2 s–1 in all the cases). The numbers of small and large phyB-NBs were unaffected by orange or red light, compared with the controls under high-irradiance white light (Fig. 4A). However, under blue light, the number of small phyB-NBs increased to the level observed in plants transferred to low-irradiance white light. Actually, blue light affected even the number of large phyB-NBs (Fig. 4). In conclusion, lowering irradiance affects phyB-NBs because it reduces phyB-absorbable radiation (as blue light does when compared with white light) and not because it affects cryptochrome-absorbable radiation (as orange and red light do compared with white light). Both the number of small phyB-NBs and leaf angle responded to the irradiance of red light (Fig. 4, B and C).
Figure 4.
Spectral dependence of the number of small and large phyB-NBs in leaf petiole cells. A, Arabidopsis plants were grown under high-irradiance white light for 2 weeks, transferred to high-irradiance white minus blue (orange), high-irradiance red, high-irradiance blue, or low-irradiance white light 2 h after the beginning of the photoperiod and analyzed 4 h later. Controls remained under high-irradiance white light. Data are means ± se of seven plant replicates. Different letters denote significant differences among means (P < 0.05) in Bonferroni posttests. B and C, Plants were grown under high-irradiance white light for 2 weeks and transferred to the indicated irradiance of red light. The angle of the leaves (B) and the number of phyB-NBs (C) were measured 2 h later. Data are means ± se of 18 (A) or six (B) plant replicates. Linear regression analysis indicates significant slope deviation from zero (B, P < 0.0001; C, P < 0.05). [See online article for color version of this figure.]
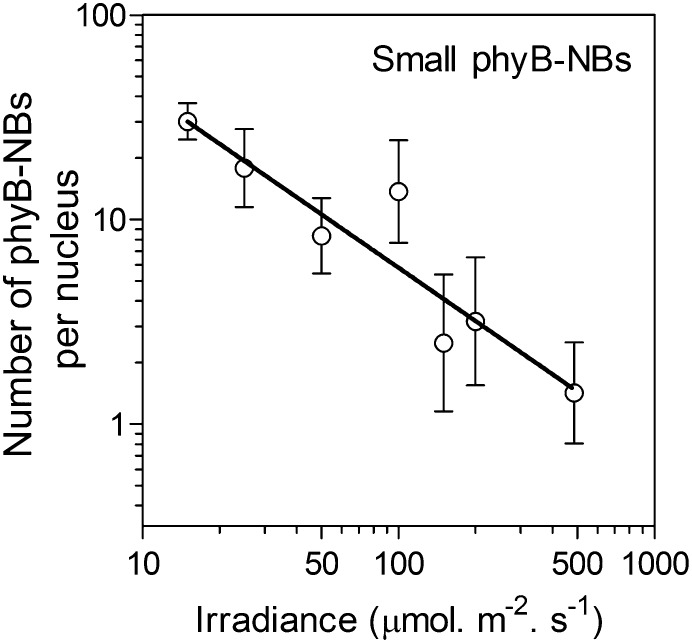
The Number of Small phyB-NBs Responds to the Range of Canopy Irradiances
The aforementioned experiments involve an 8-fold decrease in white-light irradiance and do not exclude the possibility that only the severe shade of very dense canopies changes the number of phyB-NBs. Therefore, we investigated the number of small phyB-NBs in plants grown as described in previous experiments but transferred to a range of irradiances (15 to 485 µmol m–2 s–1). A significant linear relationship between log number of small phyB-NBs and log irradiance was observed through the whole range of irradiances used here (Fig. 5). This indicates that phyB cellular status is able to reflect typical changes in irradiance within a natural canopy. The number of large phyB-NBs did not respond to irradiance (P > 0.7).
Figure 5.
Inverse log-log linear relationship between the number of small phyB-NBs and irradiance levels. Arabidopsis plants were grown under high-irradiance white light for 2 weeks, transferred to a range of irradiances (15–485 µmol m–2 s–1) 4 h after the beginning of the photoperiod, and measured 2 h later. Data are means ± se of six plant replicates. Linear regression analysis indicates significant slope deviation from zero (P < 0.0001).
Low Irradiance Can Increase the Number of Small phyB-NBs Even under Low R:FR
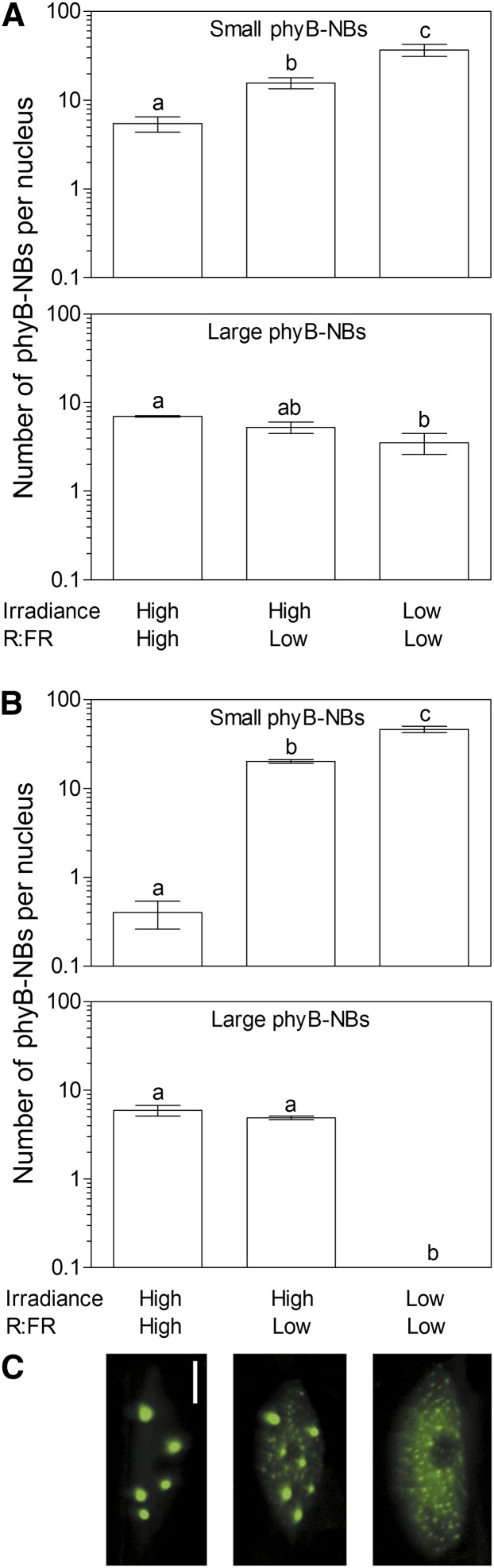
The above experiments demonstrate that the low irradiance of plant canopies can change the subcellular status of phyB. However, this is also the case with low R:FR (Figs. 2 and 3). In growing (green) canopies, the R:FR can be reduced without changes in irradiance, but the reduction of irradiance by mutual shading inevitably comes with a reduction in R:FR. Therefore, we investigated whether changes in irradiance are still effective when the R:FR is lower than that provided by unfiltered sunlight in experiments conducted either outdoors or under controlled conditions.
Plants bearing the phyB-GFP fusion protein were grown under sunlight during 15 summer days. Then, one group remained as control under sunlight (midday irradiance: 1,360 µmol m–2 s–1; R:FR = 1.2), another group was transferred to full sunlight (irradiance: 1,360 µmol m–2 s–1) supplemented with far-red light to lower the R:FR (0.8), and the third group was transferred to the natural shade of a tree canopy, i.e. reduced irradiance (110 µmol m–2 s–1) and reduced R:FR (0.8). The status of nuclear phyB in leaf petioles was investigated 2 h later. Lowering the R:FR at high irradiances increased the number of small phyB-NBs (Fig. 6A). However, lowering irradiance in addition to the R:FR further increased the number of small phyB-NBs, indicating that irradiance is effective even when the R:FR is in itself low enough to affect phyB-NBs.
Figure 6.
Low irradiances increase the number of small phyB-NBs even under low R:FR. A, Response of phyB-NBs under natural radiation. Plants were grown under natural photoperiods (14-h light, 10-h darkness) during early summer in Buenos Aires. At midday of day 15, plants were left as high-irradiance and high-R:FR controls under unfiltered sunlight (1,360 µmol m–2 s–1 of photosynthetically active radiation, R:FR = 1.2) or transferred to either low R:FR (1,360 µmol m–2 s–1 photosynthetically active radiation plus supplementary far-red light, R:FR = 0.8) or low irradiance and low R:FR under the natural shade provided by a canopy of Tipuana tipu trees (110 µmol m–2 s–1 photosynthetically active radiation, R:FR = 0.8). Confocal images were taken 2 h later. B, Response of phyB-NBs under controlled conditions. Plants were grown at high irradiance and high R:FR (200 µmol m–2 s–1 photosynthetically active radiation, R:FR = 4.3). At 4 h of the photoperiod, plants were left as controls or transferred to either high irradiance and low R:FR (0.8) or low irradiance and low R:FR (25 µmol m–2 s–1 photosynthetically active radiation, R:FR = 0.8). Confocal images were taken 2 h later. C, Representative confocal images showing nuclei under controlled light conditions. Bar = 5 µm. Data are means ± se of three plants. Different letters denote significant differences among means (P < 0.05) in ANOVA followed by Bonferroni posttests. [See online article for color version of this figure.]
Plants were also grown under white light (200 µmol m–2 s–1; R:FR = 4.3) under controlled conditions and transferred to white light of either the same irradiance and a lower R:FR (0.8) or a lower irradiance (25 µmol m–2 s–1) and R:FR (0.8). Lowering the R:FR at high irradiances increased the number of small phyB-NBs, but lowering irradiance in addition to the R:FR further increased the number of small phyB-NBs (Fig. 6B), indicating that irradiance is effective even under a low R:FR. Of note, the combined reduction of both irradiance and R:FR also strongly reduced the number of large phyB-NBs (Fig. 6B).
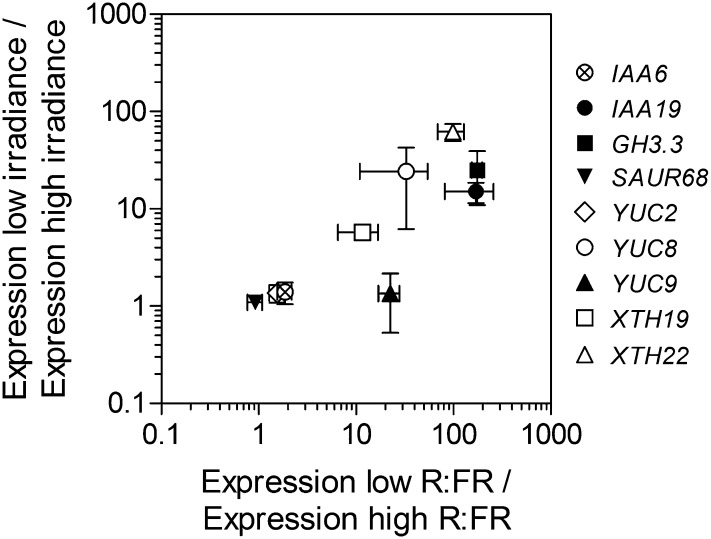
Convergent Control of Gene Expression by Low R:FR and Low Irradiance
We reasoned that if the status of phyB can be affected by light quantity and light quality signals of neighbors, both light signals should show at least partial convergence in the control of target genes. Plants were transferred from high-irradiance white light to either low R:FR or low-irradiance white light and harvested to analyze the expression of genes previously described to be affected by the R:FR in the petiole of Arabidopsis leaves (Kozuka et al.., 2010). Confirming the prediction, most genes showed a strong correlation between the effect of lowering irradiance and that of lowering the R:FR (Fig. 7). The only exception was YUCCA9, which increased its expression only under low R:FR conditions but not in response to low irradiance. One possible explanation for the latter pattern might be that to reduce irradiance without changing light quality, all spectral regions must be reduced, and this can affect other photoreceptors, which could condition the response of YUCCA9 a change in phyB status.
Figure 7.
Correlation between the effects of low irradiance and low R:FR on gene expression. Arabidopsis plants were grown under high-irradiance white light for 2 weeks and transferred to either low irradiance or low R:FR 4 h after the beginning of the photoperiod, and leaves were harvested 3 h later. Data are means ± se of three biological replicates. Linear regression analysis shows significant slope deviation from zero (P < 0.05).
Leaf Position Follows Daily Changes in Irradiance But Has Reduced Sensitivity to the Darkness of Night
Irradiance is affected not only by shade but also by time of day and cloudiness. To characterize the function of leaf position responses to irradiance, we investigated its kinetics in further detail. When the plants were transferred from high to low irradiance, they retained the hyponastic response despite an age-dependent decrease in leaf angle (Supplemental Fig. S3). However, under fluctuating light environments, leaf angle showed a dynamic response to irradiance. When plants were transferred from high to low irradiance and back to high irradiance, leaf angle first increased and then returned to the original values (Supplemental Fig. S3). Leaf angle was even able to follow the simulated fluctuations in irradiance typical of a sunny day (Supplemental Fig. S3).
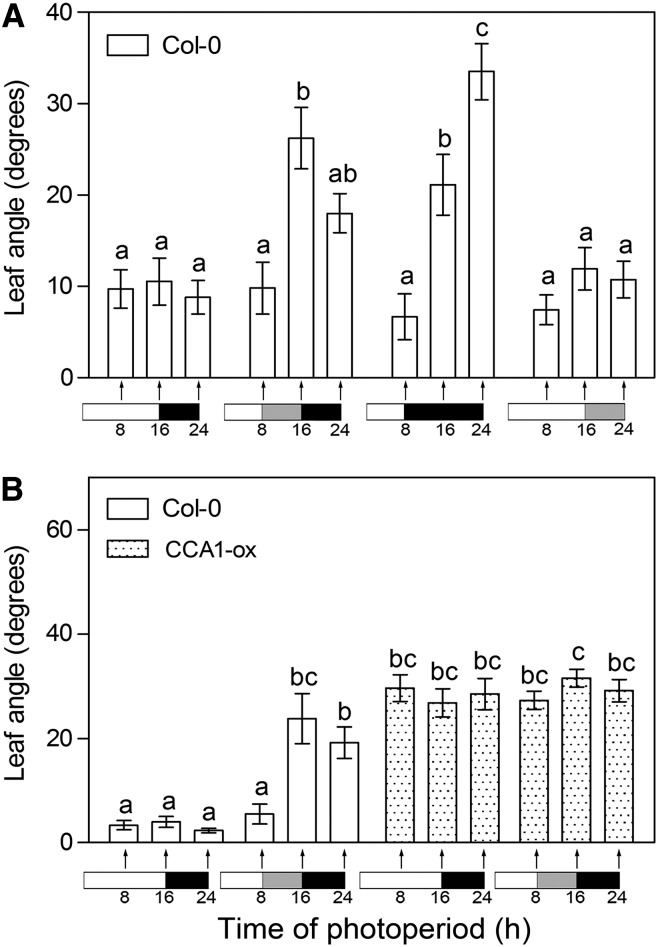
Given the ability of leaf position to dynamically adjust to irradiance, we decided to investigate whether leaf angle also responds to the darkness of the night. In plants grown under 16-h light and 8-h darkness, leaf angle at the end of the night was similar to that observed at the end of the photoperiod, despite the 8 h of full darkness (Fig. 8A). However, 8 h of low irradiance or 8 h of darkness applied during daytime (or subjective daytime) did promote leaf angle. Furthermore, low irradiance during subjective nighttime also failed to enhance leaf angle (Fig. 8A). Therefore, plants were more sensitive to low irradiance or darkness during subjective daytime than during the subjective night. In other experiments, a constitutive hyponastic response was observed in overexpressors of CIRCADIAN CLOCK ASSOCIATED1 (CCA1; Fig. 8B). Taken together, these observations indicate that leaf angle responses to low irradiance depend on time of day, likely due to a clock-dependent control.
Figure 8.
Diurnal sensitivity of leaf angle to low irradiance. A, Reduced response to the darkness of the night. B, Constitutive hyponasty in plants overexpressing CCA1. Arabidopsis plants were grown under high-irradiance white light for 2 weeks, and leaf angle was measured 8, 16, and 24 h after the beginning of the photoperiod in the controls (A and B), in plants transferred to reduced irradiance at 8 h (A and B), in plants transferred to darkness at 8 h (A), and in plants transferred to low irradiance at 16 h (A). The light protocols are indicated under the relevant data: white, gray, and black rectangles represent high irradiance, low irradiance, and darkness, respectively. Data are means ± se of at least 10 plants. Different letters denote significant differences among means (P < 0.05) in ANOVA followed by Bonferroni posttests.
phyA Mutants Show a Reduced Low-Irradiance Hyponasty, Which Is Correlated with a Reduced Auxin Signaling Status in the Leaves
The hyponastic phenotype of the phyB mutant under high irradiance is consistent with a role of phyB in irradiance perception. The reduced hyponastic phenotype of the phyA mutant observed only in the presence of phyB suggests that phyA could condition the phyB-mediated response to irradiance. Reduced leaf angle under low irradiance was observed in phyA mutants of Columbia as well as Landsberg erecta backgrounds (Supplemental Fig. S4). Lowering phyB activity by far-red light does not cause detectable increments in auxin levels in the leaves, but leaf growth responses are auxin dependent (Kozuka et al.., 2010). Because low-irradiance-induced leaf hyponasty is also auxin dependent (Vandenbussche et al., 2003), we decided to investigate the auxin signaling status in the wild type and phyA mutants using an auxin reporter gene (pDR5:GUS), whose activity correlates with auxin signaling status (Casimiro et al.., 2001). Leaf distribution of GUS activity was consistent with previous reports (Aloni et al., 2003). The phyA mutant showed reduced GUS staining driven by DR5 (Supplemental Fig. S4). This is consistent with the idea that in phyA, low background levels of auxin under high irradiance limit the hyponastic response when the plant is exposed to the low-irradiance signal.
Regarding the possible function of phyA in the wild type, we hypothesized that long-term perception of high irradiance by phyA could enhance auxin signaling, leading to a system more sensitive to a reduction of irradiance perceived by phyB. Therefore, we tested whether growth irradiance affects auxin signaling status in the wild type in a phyA-dependent manner. Consistently with the proposed interpretation, GUS activity was significantly higher in wild-type plants grown under high than under low irradiances, and the phyA mutant showed low GUS activity under both conditions (Supplemental Fig. S4).
DISCUSSION
In dense canopies, mutual shading among plants reduces both the R:FR and irradiance. The perception of low R:FR by phyB is considered the main input leading to shade avoidance responses. Here, we provide several lines of evidence to support the contention that phyB also perceives the low irradiance of shade light: first, lowering irradiance without changing R:FR modified the size distribution of phyB-NBs, indicating that irradiance affects phyB dynamics (Figs. 2 and 3). This effect depended on phyB-absorbable radiation (i.e. red rather than blue light; Fig. 4). Irradiance was effective even within the range typical of canopy shade (Fig. 5) and under low R:FR, which affected phyB-NBs (Fig. 6). Second, the phyB mutant showed shade avoidance responses (leaf hyponasty in adult rosettes) under high irradiance (Fig. 1A; Vandenbussche et al., 2003; Mullen et al., 2006; Millenaar et al., 2009). Third, pif mutations that affect shade avoidance responses to low R:FR or natural shade (Lorrain et al., 2008; Leivar et al., 2012a, 2012b; Sellaro et al., 2012) also impaired leaf hyponasty to reduced irradiance (Fig. 1C). Fourth, the magnitude of gene expression responses to irradiance and R:FR showed a strong correlation (Fig. 7).
During deetiolation of young seedlings, phyB physiological activity and phyB-NB patterns are irradiance dependent but within a range that would be poorly relevant for shade avoidance in light-grown plants (typically saturated by less than 10 µmol m–2 s–1; Chen et al., 2003; Rausenberger et al., 2010). This scenario justifies why the perception of irradiance by phyB was normally not considered to be related to shade avoidance in the literature. Although, in light-grown plants, irradiance was effective at substantially higher levels (Fig. 5), the cause of the irradiance dependency of phyB activity is not necessarily different from that in etiolated seedlings and could be associated either to the thermal instability of phyB Pfr or to the rate of cycling between phyB Pr and Pfr. In the presence of thermal reversion of Pfr to Pr, higher red-light levels are required to establish a given level of Pfr (Elich and Chory, 1997; Sweere et al., 2001; Rausenberger et al., 2010; Medzihradszky et al., 2013). phyB mutations affecting the rate of Pfr-to-Pr thermal reversion rates cause changes in the patterns of phyB-NBs (Ádám et al., 2011; Medzihradszky et al., 2013; Zhang et al., 2013).
In dark-grown seedlings, most phyB is diffusely present in the cytosol, and red- or white-light exposure causes a gradual accumulation in the nucleus, reaching saturation in 6 to 8 h (Kircher et al., 2002). A pulse of red light causes nuclear accumulation, but this effect is cancelled if red light is immediately followed by a pulse of far-red light to back transform phyB from the active Pfr form to Pr (Kircher et al., 1999). In the nucleus, phyB forms speckles or NBs (Kircher et al., 1999; Yamaguchi et al., 1999). During the dark-to-light transitions, transient phyB-NBs are observed after 2 to 3 min of red light and disappear after 15 min of red light, and stable phyB-NBs appear and persist after 2 to 3 h of continuous red light (Kevei et al., 2007). In light-grown plants, phyB localizes to the nucleus, but phyB nuclear levels decrease after prolonged darkness (night), particularly if far-red light is given at the beginning of the dark period (Sakamoto and Nagatani, 1996). Nuclear phyB reaccumulates a few hours before the beginning of the day, suggesting a control by the circadian clock (Kircher et al., 2002). Despite extensive information on phyB nuclear dynamics, the response to changes between sunlight and shade conditions had not been specifically addressed.
Here, we show that transfer of light-grown plants to conditions that simulate shade reductions in either irradiance or R:FR led to the appearance of small phyB-NBs (Fig. 2). The number of large phyB-NBs present under high irradiance and high R:FR was not necessarily reduced (Fig. 2), but their diameter was, suggesting that large phyB-NBs could be the origin of the new small phyB-NBs. More intense shade signals did reduce the number of large phyB-NBs (Fig. 6, A and B). The appearance of small phyB-NBs occurred during the first 30 min after transfer to shade conditions and showed no apparent lag (Fig. 3). The opposite pattern was observed when the plants were transferred back from low to high irradiance or R:FR (Fig. 3). This indicates that the pattern of phyB-NBs is dynamic in a time range compatible with physiological responses to shade signals.
During deetiolation, phyB-NBs are formed in response to light conditions that increase phyB activity, whereas, here, we show that new small phyB-NBs are formed by shade signals that actually reduce phyB activity. However, during deetiolation, the steady-state condition of nuclear phyB depends on irradiance, with large phyB-NBs forming at the highest red-light inputs, small NBs at lower red-light inputs (both NBs are present at intermediate red light), and diffuse nuclear phyB at the lowest irradiance (Chen et al., 2003). This suggests that when the plants are transferred either from darkness or from high irradiance to low (intermediate) irradiance conditions, phyB-NBs would converge to similar patterns, where large and small phyB-NBs are observed. Noteworthy, red light terminated with a far-red light pulse followed by darkness also leads to the formation of new small phyB-NBs in the hypersensitive phyB-401 mutant (bearing a G-to-E amino acid change at position 564), which retains some activity after far-red light (Ádám et al., 2011). Taken together, these data are consistent with a scenario where small phyB-NBs are formed under conditions that establish intermediate levels of active phyB (and shade is an intermediate condition between the sunlight and full-darkness extremes). During deetiolation, transient small phyB-NBs contain PIF3, which is not present in later and more stable large phyB-NBs (Bauer et al., 2004). The small phyB-NBs reported here are more stable than those formed transiently during deetiolation and appear under conditions where reduced Pfr levels would not favor interaction with PIF proteins; however, the presence of PIF proteins in small phyB-NBs cannot be ruled out. It is tempting to speculate that these small phyB-NBs could serve as stores allowing rapid reassembling of active phyB complexes under the fluctuating shade-sunlight conditions.
The phyA mutant showed reduced hyponasty under low irradiance in the presence of phyB (Fig. 1A). This phenotype adds support to the proposal of irradiance perception by phyB because phyA had previously been shown to reduce plant responses to R:FR mediated by phyB (Casal, 1996; Cole et al., 2011; Sellaro et al., 2012). Intact auxin signaling is essential for correct leaf hyponasty under both low R:FR and low irradiance (Vandenbussche et al., 2003; Tao et al., 2008; Millenaar et al., 2009; Keuskamp et al., 2010), and the phyA mutant showed reduced response to irradiance and a reduced auxin signaling status (Supplemental Fig. S4).
The elegant simplicity of monitoring plant canopy status via the perception of R:FR by phyB rests on the fact that R:FR is largely unaffected by other factors (Smith, 1982). Some reduction in R:FR unrelated to shade can be observed at the extremes of the photoperiod, but they have no major consequence (Casal et al., 1990). Therefore, it is, to some extent, disconcerting that phyB status depends not only on R:FR, but also on irradiance, which changes with time of day, cloudiness, and time of the year, in addition to canopy shade. Two observations provide cues concerning the significance of the irradiance dependency of phyB activity. First, leaf hyponasty is a rather dynamic response, which reversibly follows changes in irradiance during the photoperiod (Supplemental Fig. S4). A more erectophile position of the leaves in response to reductions in irradiance toward the extremes of the photoperiod or during winter would increase light interception due to the low solar elevation at these times of the photoperiod or of the year (Falster and Westoby, 2003). Having these different conditions (shade, time of day, and time of year) integrated at the level of phyB could optimize the response. Second, the hyponastic response to reduced irradiance occurred during daytime but not during the night (Fig. 8), suggesting that the clock controls sensitivity to the irradiance signal to avoid taking the darkness of the night as a signal of shade. The occurrence of this type of fine control of sensitivity argues against the idea that the phyB-mediated hyponastic response to reduced irradiance is a maladaptive feature of the phyB perception system. Rather, it supports the view that the perception of neighbors involves sensing and integrating diverse signals (Pierik and de Wit, 2013).
MATERIALS AND METHODS
Plant Material
We compared the wild-type Arabidopsis (Arabidopsis thaliana) accession Columbia with the phyB-9 (Reed et al., 1993), phyA-211 (Reed et al., 1994), cry1-304 (Mockler et al., 1999), cry2-1, cry1-304 cry2-1 (Guo et al., 1998), pif3-7, pif3-7 pif4-2, pif1-1 pif3-7 pif4-2 pif5-3 (Leivar et al., 2008), pif5-3 (pil6-1; Fujimori et al., 2004), pif4-101, and pif4-101 pif5-3 (Lorrain et al., 2008) mutants and with the CCA1 OVEREXPRESSOR (CCA1-OX) transgenic line in the same background. We compared the wild-type accession Landsberg erecta with the phyA-201 (Nagatani et al., 1993) and phyB-5 (Reed et al., 1993) mutants in the same background. For confocal microscopy, we used the phyB-GFP line (Yamaguchi et al., 1999). For GUS activity, we used the DR5:GUS line provided by the Arabidopsis Biological Resource Center, which was introgressed into the phyA-211 background.
Growth Conditions and Light Treatments
Seeds were sown on agar, and 4-day-old single seedlings were transplanted to pots containing perlite, vermiculite, and sphagnum peat moss (1:1:1). Plants were grown in a growth room under white-light photoperiods (16-h light/8-h darkness) provided by high-pressure sodium lamps (400-W Philips SON) at 25°C, until they reached the rosette stage 3.5 (Boyes et al., 2001). For the experiments shown in Figure 6, the plants were grown under natural radiation. Irradiance was adjusted by means of neutral filters and by changing the distance to the source. In some experiments, artificial white light or sunlight was supplemented with far-red light (maximum 740 nm) provided by light-emitting diode lamps (LumiBulb-FR, LumiGrow, http://www.lumigrow.com). Photosynthetically active radiation (400–700 nm) and R:FR were measured with an SKR-1850/SS2 sensor (Skye Instruments, http://www.skyeinstruments.com). Orange (white minus blue), red, and blue light were as described (Casal, 1996; Strasser et al., 2010).
Leaf Angle
The angle formed between the leaf and the horizontal vector was measured with a protractor. In plants with approximately 10 rosette leaves, the first two leaves and the two to four youngest leaves (smaller than 70% of the size reached by adult leaves) were not measured. The remaining four to six leaves were measured and averaged to generate one replicate. The phyB mutant produced less leaves, and data are the average of three to five adult leaves.
Confocal Microscopy
Confocal fluorescence images were taken with a LSM5 Pascal laser-scanning microscope (Zeiss) with a water immersion objective lens (C-Apochromat 40×/1,2; Zeiss). For phyB-GFP fusion protein visualization, probes were excited with an argon laser (wavelength 488 nm), and fluorescence was detected using a BP 505-530 filter. Images were taken from the epidermis and the first subepidermal layers in the abaxial surface of the basal portion of the petiole, which is important for the hyponastic response (Polko et al., 2013). The sampling process (starting with the plant under the indicated light conditions and ending with the mounted leaf ready for confocal microscopy) typically took approximately 3 min. Two leaves (five nuclei per leaf) were averaged for each plant replicate. The number and diameter of the NBs were obtained by using Zeiss LSM Image Browser.
Real-Time PCR
Leaves were harvested in liquid nitrogen (samples from three plants were pooled per replicate). The RNeasy Plant Mini Kit (Qiagen) was used for total RNA extraction followed by DNase treatment. Complementary DNA derived from this RNA was synthesized using Invitrogen SuperScript III and an oligo(dT) primer (Supplemental Table S1) and amplified with FastStart Universal SYBR Green Master (Roche) using the 7500 Real Time PCR System cycler (Applied Biosystems). Annealing and extension (1 min) were at 60°C. The PCR-minus-template controls were routinely included and showed negative results. Each primer pair yielded a single peak in melting curves, and a single product was confirmed on agarose gels. Contamination by DNA was ruled out by PCR analysis after DNase treatment. Furthermore, two of the gene primers flanked sequences containing one or three introns, and the PCR-amplified products of the real-time reaction of these genes showed only the size corresponding to spliced transcripts in agarose gels.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Reduced irradiance increases the number of small phyB-NBs in different parts of the leaf and in the hypocotyl of young seedlings.
Supplemental Figure S2. Small phyB-NBs are not an artifact caused by sample irradiation during confocal microscopy.
Supplemental Figure S3. Dynamic leaf position in response to changes in irradiance.
Supplemental Figure S4. Reduced hyponastic response correlates with reduced auxin signalling status in the phyA mutant.
Supplemental Table S1. Primers used in the analysis of gene expression by quantitative PCR.
Supplementary Material
Acknowledgments
We thank the Arabidopsis Biological Resource Center for the provision of seed stocks.
Glossary
- R:FR
red to far-red ratio
- Pfr
far red-absorbing form of phytochrome
- Pr
red-absorbing form of phytochrome
- phyB-NB
phytochrome B-containing nuclear body
- NB
nuclear body
Footnotes
This work was supported by the University of Buenos Aires (grant no. 20020100100437 to J.J.C.) and the Agencia Nacional de Promoción Científica y Tecnológica (grants nos. PICT 1819 and PICT 1444 to J.J.C.).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Ádám É, Hussong A, Bindics J, Wüst F, Viczián A, Essing M, Medzihradszky M, Kircher S, Schäfer E, Nagy F. (2011) Altered dark- and photoconversion of phytochrome B mediate extreme light sensitivity and loss of photoreversibility of the phyB-401 mutant. PLoS ONE 6: e27250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R, Schwalm K, Langhans M, Ullrich CI. (2003) Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 216: 841–853 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Sánchez RA, Scopel AL, Casal JJ, Ghersa CM. (1987) Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ 10: 551–557 [Google Scholar]
- Ballaré CL, Scopel AL. (1997) Phytochrome signalling in plant canopies: testing its population-level implications with photoreceptor mutants of Arabidopsis. Funct Ecol 11: 441–450 [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA. (1991) Photocontrol of stem elongation in plant neighbourhoods: effects of photon fluence rate under natural conditions of radiation. Plant Cell Environ 14: 57–65 [Google Scholar]
- Bauer D, Viczián A, Kircher S, Nobis T, Nitschke R, Kunkel T, Panigrahi KCS, Adám E, Fejes E, Schäfer E, et al. (2004) Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16: 1433–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J. (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. (1996) Phytochrome A enhances the promotion of hypocotyl growth caused by reductions of phytochrome B Pfr levels in light-grown Arabidopsis thaliana. Plant Physiol 112: 965–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. (2013) Photoreceptor signaling networks in plant responses to shade. Annu Rev Plant Biol 64: 403–427 [DOI] [PubMed] [Google Scholar]
- Casal JJ, Alvarez MA. (1988) Blue light effects on the growth of Lolium multiflorum Lam leaves under natural radiation. New Phytol 109: 41–45 [Google Scholar]
- Casal JJ, Kendrik RE. (1993) Impaired phytocrome-mediated shade avoidance responses in the aurea mutant of tomato. Plant Cell Environ 16: 703–710 [Google Scholar]
- Casal JJ, Sánchez RA, Gibson D. (1990) The significance of changes in the red/far-red ratio, associated with either neighbour plants or twilight, for tillering in Lolium multiflorum Lam. New Phytol 116: 565–572 [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdán PD, Yanovsky MJ, Reymundo FC, Nagatani A, Staneloni RJ, Whitelam GC, Casal JJ. (1999) Regulation of phytochrome B signaling by phytochrome A and FHY1 in Arabidopsis thaliana. Plant J 18: 499–507 [DOI] [PubMed] [Google Scholar]
- Cole B, Kay SA, Chory J. (2011) Automated analysis of hypocotyl growth dynamics during shade avoidance in Arabidopsis. Plant J 65: 991–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. (2008) Phytochrome nuclear body: an emerging model to study interphase nuclear dynamics and signaling. Curr Opin Plant Biol 11: 503–508 [DOI] [PubMed] [Google Scholar]
- Chen M, Schwab R, Chory J. (2003) Characterization of the requirements for localization of phytochrome B to nuclear bodies. Proc Natl Acad Sci USA 100: 14493–14498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch T, Lorrain S, Kuznetsov D, Fortier A, Liechti R, Xenarios I, Fankhauser C. (2012) Measuring the diurnal pattern of leaf hyponasty and growth in Arabidopsisis—a novel phenotyping approach using laser scanning. Funct Plant Biol 39: 860–869 [DOI] [PubMed] [Google Scholar]
- Elich TD, Chory J. (1997) Biochemical characterization of Arabidopsis wild-type and mutant phytochrome B holoproteins. Plant Cell 9: 2271–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falster DS, Westoby M. (2003) Leaf size and angle vary widely across species: what consequences for light interception? New Phytol 158: 509–525 [DOI] [PubMed] [Google Scholar]
- Franklin KA, Praekelt U, Stoddart WM, Billingham OE, Halliday KJ, Whitelam GC. (2003) Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol 131: 1340–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori T, Yamashino T, Kato T, Mizuno T. (2004) Circadian-controlled basic/helix-loop-helix factor, PIL6, implicated in light-signal transduction in Arabidopsis thaliana. Plant Cell Physiol 45: 1078–1086 [DOI] [PubMed] [Google Scholar]
- Guo H, Yang H, Mockler TC, Lin C. (1998) Regulation of flowering time by Arabidopsis photoreceptors. Science 279: 1360–1363 [DOI] [PubMed] [Google Scholar]
- Holmes MG, Smith H. (1977) The function of phytochrome in the natural environment. III. Measurement and calculation of phytochrome photoequilibria. Photochem Photobiol 25: 547–550 [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, López-Vidriero I, Franco-Zorrilla JM, Solano R, Trevisan M, Pradervand S, et al. (2012) Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J 71: 699–711 [DOI] [PubMed] [Google Scholar]
- Jarillo JA, Capel J, Tang RH, Yang HQ, Alonso JM, Ecker JR, Cashmore AR. (2001) An Arabidopsis circadian clock component interacts with both CRY1 and phyB. Nature 410: 487–490 [DOI] [PubMed] [Google Scholar]
- Keller MM, Jaillais Y, Pedmale UV, Moreno JE, Chory J, Ballaré CL. (2011) Cryptochrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially independent hormonal cascades. Plant J 67: 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R. (2010) Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci USA 107: 22740–22744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp DH, Sasidharan R, Vos I, Peeters AJM, Voesenek LACJ, Pierik R. (2011) Blue-light-mediated shade avoidance requires combined auxin and brassinosteroid action in Arabidopsis seedlings. Plant J 67: 208–217 [DOI] [PubMed] [Google Scholar]
- Kevei E, Schäfer E, Nagy F. (2007) Light-regulated nucleo-cytoplasmic partitioning of phytochromes. J Exp Bot 58: 3113–3124 [DOI] [PubMed] [Google Scholar]
- Kircher S, Gil P, Kozma-Bognár L, Fejes E, Speth V, Husselstein-Muller T, Bauer D, Adám E, Schäfer E, Nagy F. (2002) Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell 14: 1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S, Kozma-Bognar L, Kim L, Ádám E, Harter K, Schäfer E, Nagy F. (1999) Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 11: 1445–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka T, Kobayashi J, Horiguchi G, Demura T, Sakakibara H, Tsukaya H, Nagatani A. (2010) Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol 153: 1608–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Monte E, Cohn MM, Quail PH. (2012a) Phytochrome signaling in green Arabidopsis seedlings: impact assessment of a mutually negative phyB-PIF feedback loop. Mol Plant 5: 734–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, Huq E, Quail PH. (2008) Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol 18: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Tepperman JM, Cohn MM, Monte E, Al-Sady B, Erickson E, Quail PH. (2012b) Dynamic antagonism between phytochromes and PIF family basic helix-loop-helix factors induces selective reciprocal responses to light and shade in a rapidly responsive transcriptional network in Arabidopsis. Plant Cell 24: 1398–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ljung K, Breton G, Schmitz RJ, Pruneda-Paz J, Cowing-Zitron C, Cole BJ, Ivans LJ, Pedmale UV, Jung HS, et al. (2012) Linking photoreceptor excitation to changes in plant architecture. Genes Dev 26: 785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Martínez-García JF, Galstyan A, Salla-Martret M, Cifuentes-Esquivel N, Gallemí M, Bou-Torrent J. (2010) Regulatory components of shade avoidance syndrome. Adv Bot Res 53: 65–116 [Google Scholar]
- Mazzella MA, Alconada Magliano TM, Casal JJ. (1997) Dual effect of phytochrome A on hypocotyl growth under continuous red light. Plant Cell Environ 20: 261–267 [Google Scholar]
- McNellis TW, von Arnim AG, Araki T, Komeda Y, Miséra S, Deng XW. (1994) Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell 6: 487–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzihradszky M, Bindics J, Ádám É, Viczián A, Klement É, Lorrain S, Gyula P, Mérai Z, Fankhauser C, Medzihradszky KF, et al. (2013) Phosphorylation of phytochrome B inhibits light-induced signaling via accelerated dark reversion in Arabidopsis. Plant Cell 25: 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar FF, van Zanten M, Cox MCH, Pierik R, Voesenek LACJ, Peeters AJM. (2009) Differential petiole growth in Arabidopsis thaliana: photocontrol and hormonal regulation. New Phytol 184: 141–152 [DOI] [PubMed] [Google Scholar]
- Mockler TC, Guo H, Yang H, Duong H, Lin C. (1999) Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development 126: 2073–2082 [DOI] [PubMed] [Google Scholar]
- Mullen JL, Weinig C, Hangarter RP. (2006) Shade avoidance and the regulation of leaf inclination in Arabidopsis. Plant Cell Environ 29: 1099–1106 [DOI] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J. (1993) Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol 102: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacín M, Legris M, Casal JJ. (2013) COP1 re-accumulates in the nucleus under shade. Plant J 75: 631–641 [DOI] [PubMed] [Google Scholar]
- Pierik R, de Wit M. (2013) Shade avoidance: phytochrome signalling and other aboveground neighbour detection cues. J Exp Bot 65: 2815– 2824 [DOI] [PubMed] [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, de Kroon H, Visser EJW. (2004) Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. Plant J 38: 310–319 [DOI] [PubMed] [Google Scholar]
- Polko JK, Pierik R, van Zanten M, Tarkowská D, Strnad M, Voesenek LACJ, Peeters AJM. (2013) Ethylene promotes hyponastic growth through interaction with ROTUNDIFOLIA3/CYP90C1 in Arabidopsis. J Exp Bot 64: 613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausenberger J, Hussong A, Kircher S, Kirchenbauer D, Timmer J, Nagy F, Schäfer E, Fleck C. (2010) An integrative model for phytochrome B mediated photomorphogenesis: from protein dynamics to physiology. PLoS ONE 5: e10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. (1994) Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol 104: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolauffs S, Fackendahl P, Sahm J, Fiene G, Hoecker U. (2012) Arabidopsis COP1 and SPA genes are essential for plant elongation but not for acceleration of flowering time in response to a low red light to far-red light ratio. Plant Physiol 160: 2015–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Nagatani A. (1996) Nuclear localization activity of phytochrome B. Plant J 10: 859–868 [DOI] [PubMed] [Google Scholar]
- Sellaro R, Crepy M, Trupkin SA, Karayekov E, Buchovsky AS, Rossi C, Casal JJ. (2010) Cryptochrome as a sensor of the blue/green ratio of natural radiation in Arabidopsis. Plant Physiol 154: 401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellaro R, Hoecker U, Yanovsky M, Chory J, Casal JJ. (2009) Synergism of red and blue light in the control of Arabidopsis gene expression and development. Curr Biol 19: 1216–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellaro R, Pacín M, Casal JJ. (2012) Diurnal dependence of growth responses to shade in Arabidopsis: role of hormone, clock, and light signaling. Mol Plant 5: 619–628 [DOI] [PubMed] [Google Scholar]
- Smith H. (1982) Light quality, photoperception and plant strategy. Annu Rev Plant Physiol 33: 481–518 [Google Scholar]
- Smith H. (2000) Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407: 585–591 [DOI] [PubMed] [Google Scholar]
- Smith H, Casal JJ, Jackson GM. (1990) Reflection signals and the perception by phytochrome of the proximity of neighbouring vegetation. Plant Cell Environ 13: 73–78 [Google Scholar]
- Strasser B, Sánchez-Lamas M, Yanovsky MJ, Casal JJ, Cerdán PD. (2010) Arabidopsis thaliana life without phytochromes. Proc Natl Acad Sci USA 107: 4776–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweere U, Eichenberg K, Lohrmann J, Mira-Rodado V, Bäurle I, Kudla J, Nagy F, Schäfer E, Harter K. (2001) Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 294: 1108–1111 [DOI] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al. (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MJ, Kendrick RE. (1996) The aurea and yellow-green-2 mutants of tomato are deficient in phytochrome chromophore synthesis. J Biol Chem 271: 21681–21686 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Vriezen WH, Smalle J, Laarhoven LJJ, Harren FJM, Van Der Straeten D. (2003) Ethylene and auxin control the Arabidopsis response to decreased light intensity. Plant Physiol 133: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Nakamura M, Mochizuki N, Kay SA, Nagatani A. (1999) Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J Cell Biol 145: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Stankey RJ, Vierstra RD. (2013) Structure-guided engineering of plant phytochrome B with altered photochemistry and light signaling. Plant Physiol 161: 1445–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.