Abstract
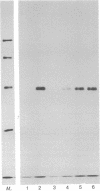
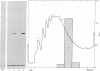
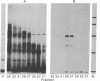
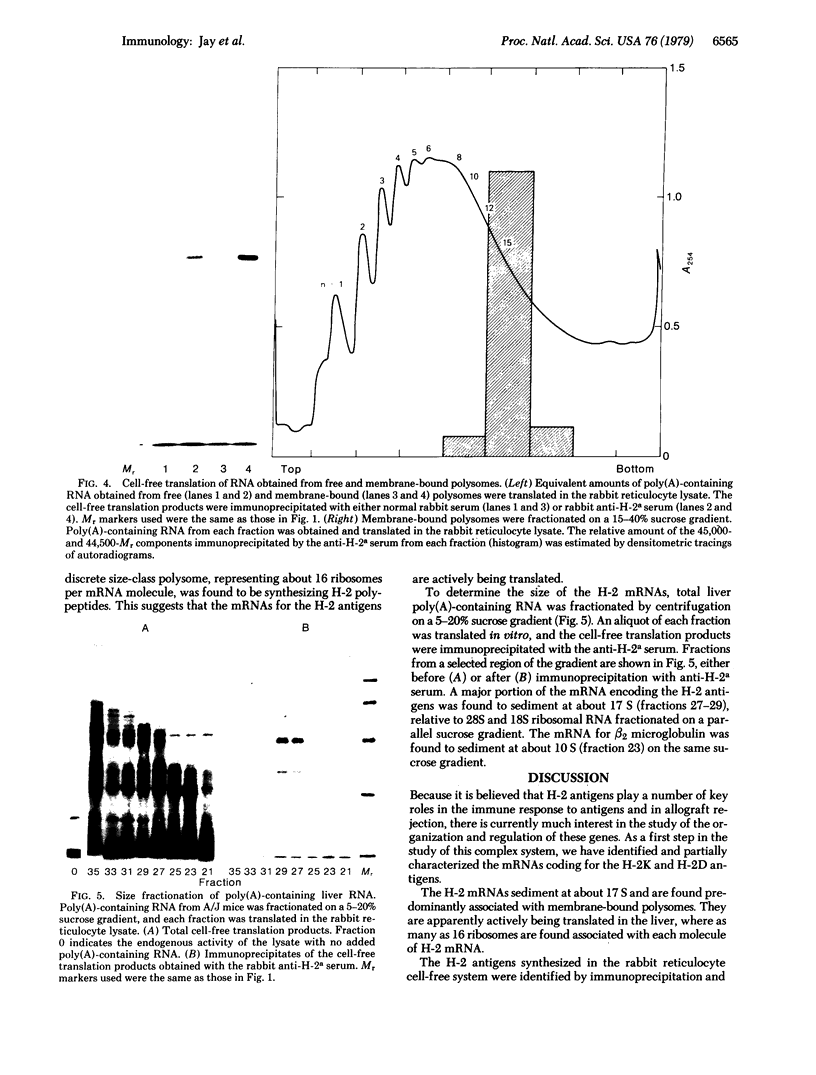
The mRNAs coding for the histocompatibility (H-2) antigens of the mouse have been identified by cell-free translation of poly(A)-containing RNA obtained from the livers of mice (strain A/J), followed by immunoprecipitation of the cell-free products by using an antiserum directed against purified H-2a. Unlike the 47,000- and 46,000-Mr H-2 glycoproteins synthesized in splenic lymphocytes, the cell-free translation products have Mrs of 45,000 and 44,500, representing the unglycosylated forms of these antigens. The cell-free products are shown to be related to the H-2 antigens by competition immunoprecipitation with purified H-2a and by two-dimensional tryptic peptide mapping. The H-2 mRNAs which sediment at 17 S are found associated predominantly with membrane-bound polysomes and are actively translated in the liver where as many as 16 ribosomes are associated with each molecule of H-2 mRNA. The implications of these studies for molecular cloning and for an understanding of the organization and expression of the genes encoding these H-2 antigens are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E., Henriksen O., Natori T., Tanigaki N., Law L. W., Pressman D. Basic structure of mouse histocompatibility antigens. Transplant Proc. 1975 Jun;7(2):191–194. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D. L., Perham R. N., Coggins J. R. Methods for obtaining peptide maps of proteins on a subnanomole scale. Anal Biochem. 1975 Sep;68(1):175–184. doi: 10.1016/0003-2697(75)90692-2. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Primary structures of N-terminal extra peptide segments linked to the variable and constant regions of immunoglobulin light chain precursors: implications on the organization and controlled expression of immunoglobulin genes. Biochemistry. 1978 Jun 13;17(12):2392–2400. doi: 10.1021/bi00605a022. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Ewenstein B. M., Uehara H., Nisizawa T., Nathenson S. G. Primary structure of murine major histocompatibility complex alloantigens: amino acid sequence studies of the cyanogen bromide fragments of the H-2Kb glycoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3390–3394. doi: 10.1073/pnas.75.7.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Henning R., Milner R. J., Reske K., Ziffer J. A., Edelman G. M. Structure of murine histocompatibility antigens. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):351–362. doi: 10.1101/sqb.1977.041.01.042. [DOI] [PubMed] [Google Scholar]
- Ewenstein B. M., Freed J. H., Mole L. E., Nathenson S. G. Localization of the papain cleavage site of H-2 glycoproteins. Proc Natl Acad Sci U S A. 1976 Mar;73(3):915–918. doi: 10.1073/pnas.73.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning R., Milner R. J., Reske K., Cunningham B. A., Edelman G. M. Subunit structure, cell surface orientation, and partial amino-acid sequences of murine histocompatibility antigens. Proc Natl Acad Sci U S A. 1976 Jan;73(1):118–122. doi: 10.1073/pnas.73.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen O., Robinson E. A., Appella E. Purification and chemical characterization of papain-solubilized histocompatibility-2 antigens from mouse liver. J Biol Chem. 1979 Aug 25;254(16):7651–7658. [PubMed] [Google Scholar]
- Jay G., Shiu R. P., Jay F. T., Levine A. S., Pastan I. Identification of a transformation-specific protein induced by a Rous sarcoma virus. Cell. 1978 Mar;13(3):527–534. doi: 10.1016/0092-8674(78)90326-4. [DOI] [PubMed] [Google Scholar]
- Jones P. P. Analysis of H-2 and Ia molecules by two-dimensional gel electrophoresis. J Exp Med. 1977 Nov 1;146(5):1261–1279. doi: 10.1084/jem.146.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem. 1978 Dec 25;253(24):8667–8670. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Cannon L. E., Strominger J. L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. doi: 10.1073/pnas.76.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. A procedure for the quantitative recovery of homogeneous populations of undegraded free and bound polysomes from rat liver. Biochemistry. 1976 Apr 20;15(8):1704–1712. doi: 10.1021/bi00653a018. [DOI] [PubMed] [Google Scholar]
- Shreffler D. C., David C. S. The H-2 major histocompatibility complex and the I immune response region: genetic variation, function, and organization. Adv Immunol. 1975;20:125–195. doi: 10.1016/s0065-2776(08)60208-4. [DOI] [PubMed] [Google Scholar]
- Silver J., Hood L. Detergent-solubilised H-2 alloantigen is associated with a small molecular weight polypeptide. Nature. 1974 Jun 21;249(459):764–765. doi: 10.1038/249764a0. [DOI] [PubMed] [Google Scholar]
- Silver J., Hood L. Structure and evolution of transplantation antigens: partial amino-acid sequences of H-2K and H-2D alloantigens. Proc Natl Acad Sci U S A. 1976 Feb;73(2):599–603. doi: 10.1073/pnas.73.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974 Oct 11;251(5475):547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]