Abstract
Purpose
Bisphosphonates have been shown to inhibit and deplete macrophages. The effects of bisphosphonates on other cell types in the tumor microenvironment have been insufficiently studied. Here, we sought to determine the effects of bisphosphonates on ovarian cancer angiogenesis and growth via their effect on the microenvironment, including macrophage, endothelial and tumor cell populations.
Experimental Design
Using in vitro and in vivo models, we examined the effects of clodronate on angiogenesis and macrophage density, and the overall effect of clodronate on tumor size and metastasis.
Results
Clodronate inhibited the secretion of pro-angiogenic cytokines by endothelial cells and macrophages, and decreased endothelial migration and capillary tube formation. In treated mice, clodronate significantly decreased tumor size, number of tumor nodules, number of tumor-associated macrophages and tumor capillary density.
Conclusions
Clodronate is a potent inhibitor of tumor angiogenesis. These results highlight clodronate as a potential therapeutic for cancer.
Keywords: anti-angiogenesis, bisphosphonate, clodronate, ovarian cancer, tumor angiogenesis, tumor microenvironment, tumor-associated macrophages
Introduction
Current anti-angiogenic treatments target pro-angiogenic growth factors (e.g., vascular endothelial growth factor [VEGF]) or directly disrupt the blood vessel network in tumors.1 Disrupting the disorganized and rapidly proliferating tumor vasculature depletes resources required for tumor growth and expansion.2 However, these treatments are not always successful. The factors limiting universal application of existing anti-angiogenic treatments, including toxicity and eventual drug resistance, are inspiring a wave of research into new alternative strategies to target tumor angiogenesis.2
One potential alternative strategy involves targeting tumor-associated macrophages (TAMs), which are a key component of the tumor microenvironment and contribute to tumor angiogenesis, growth, invasion, and metastasis. TAMs are polarized toward a repair phenotype, and secrete high levels of cytokines that may be pro-angiogenic, anti-inflammatory, or both. High levels of TAMs predict poor prognosis in a variety of cancers, including breast and ovarian malignancies.3 Additionally, elevated levels of TAMs have been linked to the development of resistance to both chemotherapeutic and anti-angiogenic agents through the secretion of survival factors into the tumor microenvironment. Conversely, the depletion of TAMs increases the efficacy of anti-angiogenic therapy and chemotherapy.4,5
Bisphosphonates traditionally have been used to target osteoclasts in bone-degenerating diseases. More recently, these drugs have been used to treat primary tumor metastasis to the bone.6 Among these, clodronate has recently been shown to inhibit tumor growth and vascular network formation in teratocarcinoma; effects thought to result from depletion of TAMs.7 Our study illustrates a more complex mechanism whereby clodronate inhibits both endothelial and macrophage function, thus disrupting a dynamic and synergistic relationship in tumor angiogenesis.
Results
Clodronate treatment decreases tumor burden in a mouse orthotopic model of ovarian cancer
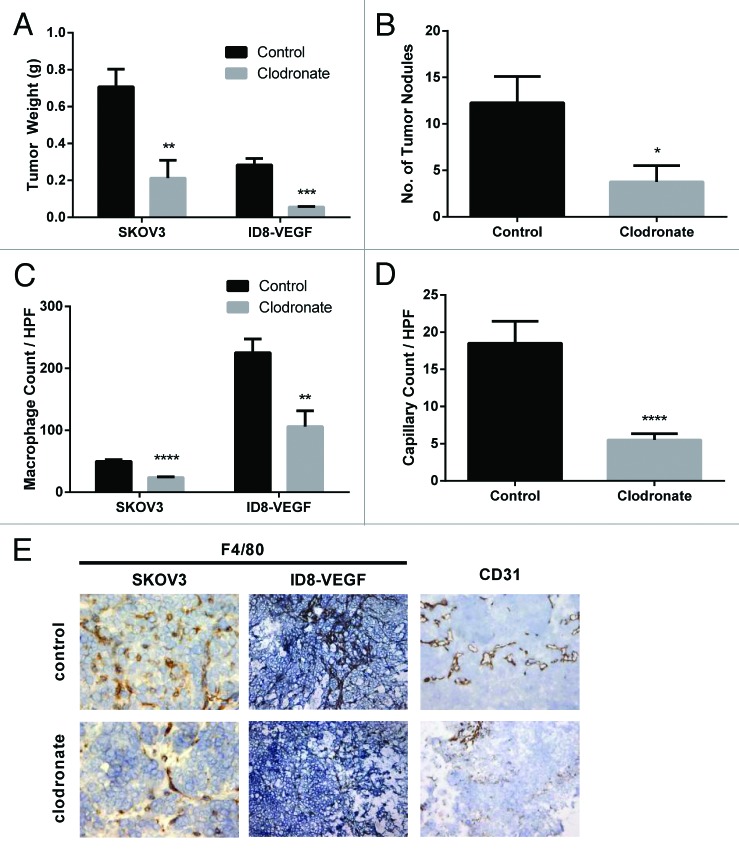
We first examined the effects of clodronate on tumor growth using a syngeneic mouse model of ovarian cancer. In the ID8-VEGF model, mice treated with clodronate had an 80.2% decrease in tumor weight (P < 0.001) relative to the control liposome group (Fig. 1A). To determine whether similar effects would be noted in another model, we next used the SKOV3ip1 model. In this model, there was a 69.9% decrease in tumor weight (P = 0.008) and a 69.3% decrease in the number of tumor nodules (P = 0.03) in the clodronate treatment group compared with the controls (Fig. 1A and B). Mouse body weights did not differ significantly between the clodronate treatment group and controls, suggesting no obvious adverse effects of clodronate.
Figure 1. Clodronate treatment reduces tumor burden, TAM levels, and tumor capillary density. (A) Quantification of tumor weight (g) harvested from female mouse SKOV3ip1 and ID8-VEGF ovarian tumor models treated with 0.2 or 1.0 mg/mouse clodronate-encapsulated liposomes, respectively (n = 4 [SKOV3ip1] and 4 [ID8-VEGF] mice) or equally diluted control liposomes (n = 8 [SKOV3ip1] and 6 [ID8-VEGF] mice) 1 d after last injection showed significantly decreased tumor weight in the clodronate treatment group relative to the control group. **P < 0.01 and ***P < 0.0009 determined by the Student t test. Data are shown as means ± the standard error of the mean (SEM). (B) Quantification of tumor nodules present in the peritoneal cavity of female nude mice in the SKOV3ip1 ovarian tumor model group treated with 0.2 mg/mouse clodronate-encapsulated liposomes (n = 4) or equally diluted control liposomes (n = 8) 1 d after last injection showed significantly fewer number of tumor nodules in the clodronate treatment group relative to the control group. *P < 0.03 determined by the Student t test. Data are shown as means ± SEM. (C) Quantification of F4/80 stained tumor sections taken from female mouse SKOV3ip1 and ID8-VEGF ovarian tumor models treated with 0.2 or 1.0 mg/mouse clodronate-encapsulated liposomes or equally diluted control liposomes showed significantly decreased macrophage density in the clodronate treatment group relative to the control group. n = 5 mice for the SKOV3ip1 group and n = 3 mice for the ID8-VEGF group. ****P < 0.0001 and **P = 0.0014 as determined by the Student t test. Data are shown as means ± SEM. (D) Quantification of CD31-positive cells in the tumor sections taken from female mouse SKOV3ip1ovarian tumor models treated with 0.2 mg/mouse clodronate-encapsulated liposomes or equally diluted control liposomes showed significantly decreased capillary density in the clodronate treatment group relative to the control group (n = 2 mice). ***P = 0.0002 as determined by the Student t test. Data are shown as means ± SEM. (E) Representative histology images of F4/80, and CD31 stained tumor sections taken from female mouse SKOV3ip1 and ID8-VEGF ovarian tumor models treated with 0.2 or 1.0 mg/mouse clodronate-encapsulated liposomes or equally diluted control liposomes show decreased macrophage and capillary density in the clodronate treatment group relative to the control group. F4/80-positive cells (staining darkly) represent macrophages within the tissue section. CD31-positive cells (staining darkly) represent endothelial cells within the tissue section. Images were taken at 200× magnification.
Next, we examined for potential effects of treatment on the tumor microenvironment. Tumor sections from the clodronate treatment groups showed 52.9% (ID8-VEGF model) and 52.4% (SKOV3ip1 model) decrease in macrophage density (P = 0.001 and P < 0.001, respectively) relative to controls (Fig. 1C). On inspection of the tumor sections, the control groups had many macrophages within the tumor islands, while the clodronate treatment groups had predominantly extratumoral macrophages between the tumor islands. Additionally, clodronate treatment resulted in a 70.2% decrease in microvessel density (P < 0.001) relative to controls (Fig. 1D). These results suggest that clodronate treatment alters tumor size, the number of tumor nodules, macrophage density, and capillary density within the microenvironment.
Clodronate treatment decreases angiogenic cytokines
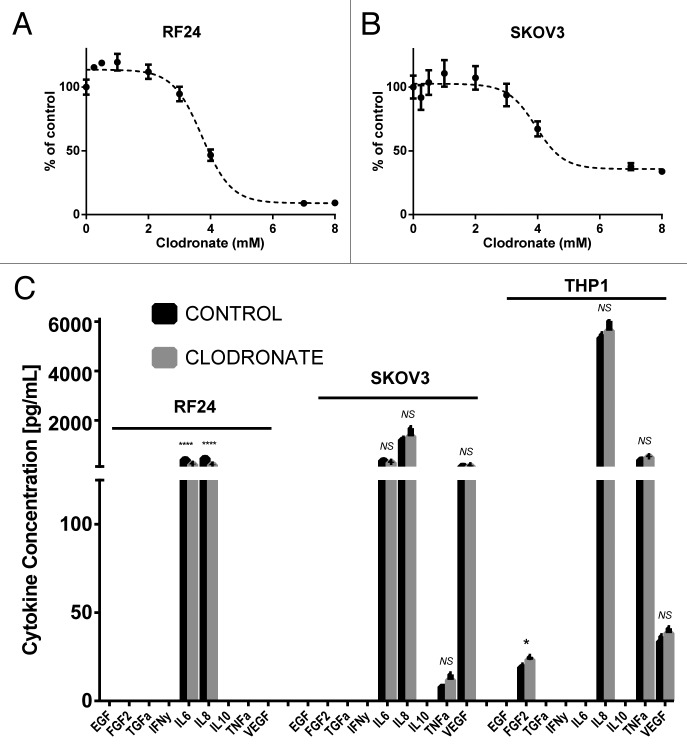
To further explore possible mechanisms underlying the effects of clodronate on tumor growth, we next examined its effects on in vitro cell proliferation and cytokine production. Clodronate inhibited the growth of endothelial cells with an IC50 of 3.54–3.91 mM (95% confidence interval [CI]), and of tumor cells with an IC50 of 3.49–4.39 mM (95% CI) (Fig. 2A and B). From these data, we selected the 2 mM dose of clodronate for use in our subsequent in vitro studies. For our animal studies, the final injection dose of liposomal clodronate was 1.0 mg/mouse (2.77 µmol) and 0.2 mg/mouse (0.55 µmol). Given the substantial effects on tumor angiogenesis noted from the in vivo experiments, we asked whether clodronate could affect production of angiogenic factors by different cell types. Using ELISA, we assessed the production of angiogenic cytokines by tumor cells, endothelial cells, and macrophages in the presence or absence of clodronate. In SKOV3 cells, cytokine secretion did not change significantly; however, both endothelial cell (RF24) and macrophage (THP1) secretion of pro-angiogenic cytokines decreased significantly after treatment with clodronate (Fig. 2C). Endothelial cells exhibited a 52.1% decrease in IL6 secretion (P < 0.001) and a 61.9% decrease in IL8 secretion (P < 0.001). Macrophages showed a 24.0% decrease in FGF-2 secretion (P < 0.05) with no significant change in TNFα or VEGF secretion. Decreased secretion of pro-angiogenic cytokines by multiple components of the tumor microenvironment suggests that clodronate has an indirect, upstream inhibitory effect on tumor angiogenesis.
Figure 2. Clodronate treatment inhibits cytokine production by endothelial cells and macrophages but has no significant effect on tumor cytokine secretion or cell proliferation. (A) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay shows the response of endothelial cells (RF24) to increasing doses of clodronate at 180 h. Cells showed an IC50 of 3.725 mM (95% CI: 3.540–3.909 mM) determined by interpolation of data. (B) MTT cell viability assay shows the response of response of tumor cells (SKOV3) to increasing doses of clodronate at 72 h. Cells showed an IC50 of 3.941 mM (95% CI: 3.488–4.393 mM) as determined by interpolation of data. (C) Quantification of EGF, FGF2, TGFα, IFNγ, IL6, IL8, IL10, TNFα, and VEGF secretion (pg/mL) by in vitro endothelial (RF24) tumor (SKOV3) cells and activated macrophages (THP-1) treated with media (control) or filtered clodronate salt (2 mM) shows significant decrease in cytokine secretion of endothelial cells in the clodronate treatment group relative to the control group, but shows mixed results in cytokine secretion of activated macrophages and no significant change in cytokine secretion of tumor cells between the clodronate treatment and control groups. Supernatant was collected 48 h after treatment administration for endothelial and tumor cells, and 12 h after treatment administration for activated macrophages. n = 3 samples collected per group, measured in duplicates by ELISA.*P < 0.05, ****P < 0.0001 and NS > 0.05, not significant, as determined by the Student t test. Data are shown as means ± SEM.
Clodronate impairs endothelial tube formation
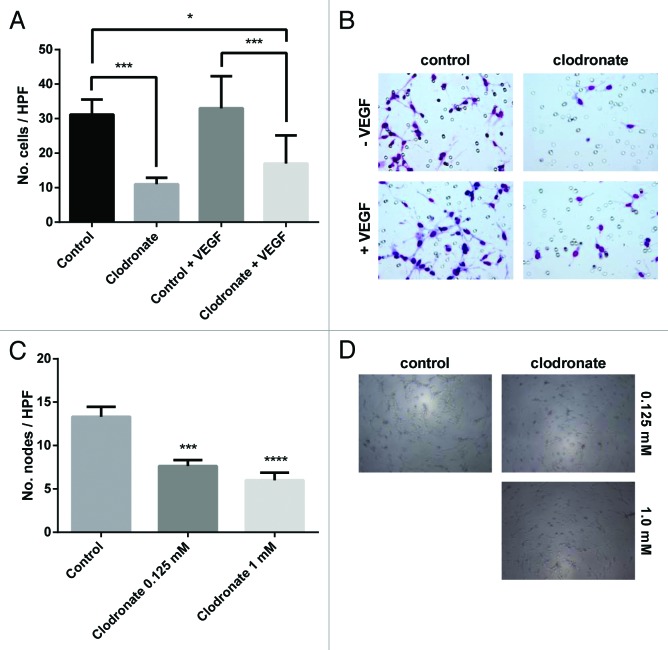
To address whether clodronate could directly affect endothelial cells, we assessed endothelial cell migration and tube formation in 3D gels. Treatment with clodronate resulted in a 64.7% decrease in endothelial cell migration relative to controls (P < 0.001). After VEGF stimulation, normal migratory capacity recovered only partially (endothelial cell migration inhibition with a 45.5% decrease in number of cells which migrated into the insert relative to control; Fig. 3A and B). Additionally, clodronate treatment significantly impaired tube formation in vitro, with a 42.6% decrease in the number of nodes formed relative to the control group (Fig. 3C and D).
Figure 3. Clodronate impairs capillary recruitment and formation. (A) Quantification of endothelial cell (RF24) migration after treatment with 2 mM clodronate-encapsulated liposomes or equally diluted control liposomes ± VEGF (20 ng/mL) indicates clodronate treatment significantly decreases endothelial cell migration compared with the control group. n = 2 inserts per group, 5 pictures per insert. *P < 0.02, ***P < 0.0007 as determined by the Student t test. Data are shown as means ± SEM. (B) Representative images of migration assay seen in (A) show a decreased number of endothelial cells within the insert of the clodronate treatment group relative to the control group. Images were taken after prior treatment with 2 mM clodronate-encapsulated liposomes, equally diluted liposomes (control), control plus 20 ng/mL VEGF, or 2 mM clodronate-encapsulated liposomes plus 20 ng/mL VEGF. Images were captured at 200× magnification. (C) Endothelial vessel formation was quantified by counting the number of nodes (points at which where three or more elongated cells meet) formed on the gel matrix after prior treatment with 2 mM, 0.125 mM clodronate-encapsulated liposomes or equally diluted control liposomes (to 0.125 mM, control). n = 3 wells per group, 5 pictures per well. ***P < 0.0003, ****P < 0.0001 (compared with media) as determined by the Student t test. Data are shown as means ± SEM. (D) Representative images of 3D tube formation seen in (C) on a gel matrix correlating to capillary formation show decreased vessel formation in the clodronate treatment group relative to the control group.. Images were taken after treatment with 0.125 mM or 2 mM clodronate-encapsulated liposomes or equally dilute liposomes (to 0.125 mM, control). Images were taken at 100× magnification.
Discussion
The key findings from our study are that clodronate treatment impairs tumor angiogenesis in an orthotopic model of ovarian cancer through direct and indirect effects. These findings provide insight into the anti-angiogenic and tumor-limiting capabilities of clodronate and support further investigation of clodronate as a therapeutic adjuvant to current chemotherapeutic agents in patients with ovarian cancer.
Our study shows that clodronate inhibits the secretion of cytokines by both endothelial cells and macrophages. Macrophages have been shown to produce growth factors stimulating endothelial cell proliferation and directing capillary formation. Endothelial cells support the differentiation of inactivated macrophages into the repair phenotype through direct contact and the secretion of CSF1.8 Liposomal clodronate-mediated depletion of TAMs has been shown previously to inhibit tumor growth in teratocarcinoma mouse models and increase radiosensitivity in melanoma mouse models.7,9 We found that depletion of TAMs also decreases tumor vascularity, leading to decreased tumor growth.10
Clodronate, a non-aminobisphosphonate, can block adenosine triphosphate (ATP)-binding sites, resulting in the interruption of ATP/energy usage of cells. As a result, clodronate can influence a variety of pathways and trigger apoptosis.9 Clodronate’s ability to disrupt the cell’s source of energy may explain the effect on endothelial cell function and not on tumor cell function as seen in this study. Endothelial cell movement, migration and capillary formation rely on interaction between ATP and actin filaments, which results in a change in the cell’s shape.11 Clodronate has been previously shown to disrupt this ATP-actin interaction in a canine model of malignant histiocytosis.12 The blockage of ATP-binding sites by clodronate has also been implicated in a decreased risk for osteonecrosis of the jaw as compared with the aminobisphosphonates, zoledronic acid and pamidronate. This adverse event is most often encountered in multiple myeloma or metastatic breast cancer patients being treated with monthly intravenous infusions of an aminobisphosphonate, and very few reports have been published worldwide involving clodronate, despite its widespread use for 20 y.13
Other studies of clodronate’s potential use in cancer treatment have had mixed success.14-17 There are conflicting data regarding the ability of clodronate to affect survival rates in early-stage breast cancer.14 However, a recent meta-analysis concluded that clodronate as adjuvant therapy in breast cancer is beneficial, producing an increased 5-y overall survival rate.15 Emerging data in prostate cancer trials show improved survival with the use of clodronate in men with metastatic, androgen-sensitive prostate cancer and delays in time to first bone metastasis in high-risk prostate cancer patients.16,17 Our results favor the introduction of clodronate as a potential anti-angiogenic approach for human ovarian cancer, and may have beneficial effects with the current standard-of-care chemotherapy drugs (e.g., platinum and taxanes).18
Materials and Methods
Cell lines and culture conditions
SKOV3 cells were obtained from the American Type Culture Collection and grown in 5% CO2 at 37 °C. These cells were maintained in RPMI 1640 medium supplemented with 15% fetal bovine serum (FBS) and 0.1% gentamicin sulfate (Gemini Bioproducts, 400-107). The immortalized human endothelial cell line RF24 was maintained in modified Eagle’s medium (MEM) supplemented with 10% FBS, 1% sodium pyruvate, 1% MEM vitamins, 1% nonessential amino acids, 1% l-glutamine, and 1% penicillin/streptomycin. The human monocyte cell line THP-1 was maintained in RPMI 1640 supplemented with 10% FBS, 0.1% gentamicin sulfate, and 0.05 mM 2-mercaptoethanol. THP-1 cells were differentiated into macrophages by the addition of 1 µg/mL 12-O-Tetradecanoylphorbol-13-acetate to cells every 24 h over 3 d. Differentiated macrophages were then stimulated by the addition of 1 µg/mL lipopolysaccharide and incubated for 2 h.
In vitro clodronate treatment
Samples prepared for enzyme-linked immunosorbent assay (ELISA) were treated with 2 mM clodronate salt ([dichloro-phosphono-methyl]phosphonic acid) or culture medium (control). Samples prepared for migration assays were treated with 2 mM clodronate-encapsulated liposomes or the equivalent diluted free liposomes (control) (Encapsula NanoScience). Samples prepared for 3D tube formation were treated with media (control), 0.125 mM or 2 mM clodronate-encapsulated liposomes, or the equivalent diluted free liposomes for 0.125 mM.
Cell viability assays
RF24 and SKOV3 cells (2 × 104 in 100 μL) were plated in 96-well plates. After 24 h, clodronate was added once to each well in increasing concentrations (0.1, 0.25, 1, 2, 3, 4, 7, and 8 mM). After three doubling times, (180 h for RF24 and 72 h for SKOV3) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to each well. The plate was incubated at 37 °C for 20 min and absorbance was then read at 570 nm (Ceres UV 900C; Bio-Tek Instrument Inc.).
ELISA
Samples were prepared per instructions with Milliplex magnetic beads (EMD Millipore, HCYTOMAG-60K) for various angiogenic cytokines (EGF, FGF-2, TGFα, IFNγ, IL10, IL6, IL8, TNFα, and VEGF) and run through a MAGPIX instrument (Luminex). Instrument software was used to analyze standard dilutions and calculate sample concentrations of individual cytokines in pg/mL per well. Cytokine concentrations were then compared within the same cell line and time point per treatment or lack of treatment.
3D tube formation assay
RF24 cells were treated with media (control), 0.125 mM or 2 mM clodronate-encapsulated liposomes, or the equivalent diluted free liposomes for 0.125 mM prior to seeding for 12 h. A total of 3 × 104 RF24 cells were seeded onto 200 μL Matrigel (BD Biosciences, 356237) in a 48-well plate and incubated in 5% CO2/95% air at 37 °C for 10 h. Images were taken using an inverted camera at 200× magnification, and cells forming nodes were counted in five fields (upper left and right, center, and lower left and right).
Migration assay
Cell culture inserts (BD Nanosciences) for 24-well plates were coated with 0.1% gelatin and air-dried. Migration was assessed in four groups: control (empty liposomes), control plus VEGF, clodronate, and clodronate plus VEGF. RF24 cells were treated with 2 mM clodronate-encapsulated liposomes or the equivalent diluted free liposomes (control) prior to seeding for 12 h. Treatment and control groups of RF24 cells (1 × 105) suspended in 100 μL serum-free MEM were added into the upper chamber and complete media for RF24 cells containing 10% FBS (500 μL) or 10% FBS with VEGF (20 ng/mL in 500 μL). The chambers were incubated at 5% CO2/95% air and 37 °C for 6 h. After incubation, cells in inserts were fixed and stained. Inserts were then removed and placed on slides for counting by light microscopy. Images were taken at 200× magnification, and cells were counted in five fields (upper left and right, center, and lower left and right).
Immunohistochemistry
Fresh-frozen sections in optimal cutting temperature (OCT) compound were stained for CD31 and F4/80. Frozen sections were fixed in acetone and acetone-chloroform. After endogenous peroxide blocking and three washes with PBS, the slides were incubated in protein block (5% normal horse serum plus 1% normal goat serum in PBS) for 20 min. After three washes with PBS, the slides were incubated with primary antibody to CD31 (1:800, PharMingen, 550274), F4/80 (1:600, Serotec, MCA497RT), or Ki67 (1:200, BioCare Medical, CRM325) overnight at 4 °C. Matching secondary antibodies were applied for 1 h at room temperature and staining was developed using diaminobenzidine. Hematoxylin was then used to stain nuclei. To calculate the macrophage count and cell proliferation index, five random fields were studied and counted at 200× magnification for each tumor section. To quantify microvessel density, five random fields were studied at 200× magnification for each tumor section, and endothelial cell nodes (where three endothelial cells with nuclei adjoined) or a well-defined capillary lumen were counted as vessels.
In vivo ovarian cancer models
Female C57BL/6 and nude mice were obtained from Taconic. All experiments were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center. Tumor cells from appropriate ovarian cancer mouse models (SKOV3ip1 and ID8-VEGF, 1.0 × 106 cells) were injected intraperitoneally into the mice on day 0. The C57BL/6 mice were used for the ID8-VEGF syngeneic model, while nude mice were used for the SKOV3ip1 orthotopic model. Mice were randomized to treatment or control groups, where treatment began on day 7 and continued weekly until day 28. Control mice received empty liposomes, while the treatment group received clodronate-encapsulated liposomes group (n = 10 mice per group). Treatments were given in 200 μL volumes at 1.0 mg/mouse for ID8-VEGF tumors or 0.2 mg/mouse for SKOV3ip1 tumors. Twenty mice were injected with SKOV3ip1 cells, with ten mice receiving 0.2 mg clodronate-encapsulated liposomes and ten mice receiving empty liposomes; and, 20 mice were injected with ID8-VEGF cells, with ten mice receiving 1.0 mg clodronate-encapsulated liposomes and ten mice receiving empty liposomes. Mice were sacrificed on day 29 and tumors were harvested, counted and weighed.
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software) was used to analyze data. Data are presented throughout the manuscript as means ± the standard error of the mean. Comparisons were made using the Student t test or analysis of variance (ANOVA) with P < 0.05 considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed by the authors.
Authors’ Contributions
Conception and design: N.M. Reusser, A.K. Sood.
Development of methodology: N.B. Jennings, S. Pradeep, R. Rupaimoole, K. Gharpure, A. Nagaraja, Y. Wen, W. Hu, C. Pecot, T. Miyake, J. Huang.
Acquisition of data (provided animals, provided cells, performed experiments, provided facilities, etc.): N.M. Reusser, H.J. Dalton, H.G. Vasquez, K. Gharpure, S. Pradeep, G. Lopez-Berestein, A.K. Sood.
Analysis and interpretation of data (e.q., statistical analysis, computational analysis): N.M. Reusser, H.J. Dalton, H.G. Vasquez, A.K. Sood.
Writing, review, and/or revision of the manuscript: N.M. Reusser, H.J. Dalton, R. Rupaimoole, A.K. Sood.
Administrative, technical, or material support (i.e., reporting or organizing data): H.J. Dalton, N.B. Jennings, S. Pradeep, H.G. Vasquez, C. Pecot, A. Nagaraja, K. Gharpure, R. Rupaimoole.
Study supervision: A.K. Sood.
Acknowledgments
The authors thank D. Hackett and A. Scholtz in the Department of Scientific Publications at MD Anderson Cancer Center for editing our manuscript.
Grant Support
Financial support was provided by the National Institutes of Health (P50 CA098258, CA 109298, P50 CA 083639, CA016672, CA 128797, U54 CA151668), the CPRIT (RP110595), the Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), the US. Department of Defense (OC073399, OC093146), the Chapman Foundation, the Meyer and Ida Gordon Foundation #2, the Betty Anne Asche Murray Distinguished Professorship, and National Cancer Institute institutional Core Grant CA16672. H.J.D. is supported by a T32 Training Grant (T32 CA101642).
Glossary
Abbreviations:
- VEGF
vascular endothelial growth factor
- TAM
tumor-associated macrophage
- ELISA
enzyme-linked immunosorbent assay
- ATP
adenosine triphosphate
References
- 1.Hall M, Gourley C, McNeish I, Ledermann J, Gore M, Jayson G, Perren T, Rustin G, Kaye S. Targeted anti-vascular therapies for ovarian cancer: current evidence. Br J Cancer. 2013;108:250–8. doi: 10.1038/bjc.2012.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Través PG, Luque A, Hortelano S. Macrophages, inflammation, and tumor suppressors: ARF, a new player in the game. Mediators Inflamm. 2012;2012:568783. doi: 10.1155/2012/568783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, Di Serio C, Naldini L, De Palma M, Tozer GM, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011;121:1969–73. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjällman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–81. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baer C, Squadrito ML, Iruela-Arispe ML, De Palma M. Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches. Exp Cell Res. 2013;319:1626–34. doi: 10.1016/j.yexcr.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giger EV, Castagner B, Leroux JC. Biomedical applications of bisphosphonates. J Control Release. 2013;167:175–88. doi: 10.1016/j.jconrel.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Ribatti D, Maruotti N, Nico B, Longo V, Mangieri D, Vacca A, Cantatore FP. Clodronate inhibits angiogenesis in vitro and in vivo. Oncol Rep. 2008;19:1109–12. doi: 10.3892/or.19.5.1109. [DOI] [PubMed] [Google Scholar]
- 11.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–94. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 12.Hafeman SD, Varland D, Dow SW. Bisphosphonates significantly increase the activity of doxorubicin or vincristine against canine malignant histiocytosis cells. Vet Comp Oncol. 2012;10:44–56. doi: 10.1111/j.1476-5829.2011.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diel IJ, Fogelman I, Al-Nawas B, Hoffmeister B, Migliorati C, Gligorov J, Väänänen K, Pylkkänen L, Pecherstorfer M, Aapro MS. Pathophysiology, risk factors and management of bisphosphonate-associated osteonecrosis of the jaw: Is there a diverse relationship of amino- and non-aminobisphosphonates? Crit Rev Oncol Hematol. 2007;64:198–207. doi: 10.1016/j.critrevonc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Winter MC, Coleman RE. Bisphosphonates in the adjuvant treatment of breast cancer. Clin Oncol (R Coll Radiol) 2013;25:135–45. doi: 10.1016/j.clon.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Zheng Y, Zhou Z. Oral adjuvant clodronate therapy could improve overall survival in early breast cancer: results from an updated systematic review and meta-analysis. Eur J Cancer. 2013;49:2086–92. doi: 10.1016/j.ejca.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Saad F, Colombel M. Management of castration-resistant prostate cancer: bisphosphonates and emerging therapies. Expert Rev Anticancer Ther. 2010;10:1991–2002. doi: 10.1586/era.10.191. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues P, Hering FO, Meller A. Adjuvant effect of IV clodronate on the delay of bone metastasis in high-risk prostate cancer patients: a prospective study. Cancer Res Treat. 2011;43:231–5. doi: 10.4143/crt.2011.43.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchetti C, Pisano C, Facchini G, Bruni GS, Magazzino FP, Losito S, Pignata S. First-line treatment of advanced ovarian cancer: current research and perspectives. Expert Rev Anticancer Ther. 2010;10:47–60. doi: 10.1586/era.09.167. [DOI] [PubMed] [Google Scholar]