Abstract
Our prior phase I study of the combination of vascular endothelial growth factor (VEGF) antibody, bevacizumab, and VEGF receptor (VEGFR) inhibitor, sunitinib, in advanced solid tumors identified an encouraging response evaluation. An expansion phase of this study was thus undertaken to obtain further safety data, response assessment and characterization of pharmacodynamic biomarkers in melanoma, renal, and adrenal carcinoma patients.
Patients with metastatic solid tumors received sunitinib (37.5 mg/d, 4 wk on/2 wk off) and bevacizumab (5 mg/kg intravenously every 2 wk). Responses were assessed every 2 cycles. Serum levels of angiogenic molecules were measured using ELISA assays.
Twenty-two patients were enrolled, including 11 melanoma, 5 renal cell carcinoma (RCC), 5 adrenal cancer, and 1 angiosarcoma. Grade 3 or higher adverse events were observed in 15 patients, including hypertension (41%), thrombocytopenia (23%), and fatigue (14%). Three RCC patients, and 1 melanoma patient developed thrombotic microangiopathy (TMA). Partial response (PR) occurred in 21% patients, including melanoma (2), adrenal (1), and renal (1) carcinomas. Overall, 6 patients demonstrated some reduction in their tumor burden. Serum VEGF and several other proangiogenic proteins declined over the first 4 wk of treatment whereas the putative VEGF-resistant protein, prokineticin-2, increased over 10-fold.
Occurrence of TMA related to dual VEGF/VEGFR inhibition can result from systemic or nephron specific injury even in non-renal malignancies. While the combination of sunitinib and bevacizumab was clinically efficacious in renal cell carcinoma and melanoma, the observance of microangiopathy, even in non-RCC patients, is a significant toxicity that precludes further clinical development.
Keywords: angiogenesis, VEGF, VEGFR, sunitinib, bevacizumab
Introduction
The vascular endothelial growth factor (VEGF) and VEGF receptors (VEGFRs) play a pivotal role in tumor growth and metastasis. Emerging data also suggest their involvement in cell differentiation, survival, motility, and tumor invasion.1 Interest in therapeutic implications of anti-angiogenesis originated with the isolation of “tumor angiogenesis factor” and the discovery of its mitogenic effects on endothelium.2,3 Vascular permeability factor was subsequently discovered4,5 and later, identified as VEGF.6,7 In 2003, recombinant monoclonal antibody against circulating VEGF, bevacizumab, demonstrated antiangiogenic clinical efficacy.8 Subsequently, inhibitors of VEGFRs, small molecule tyrosine kinase inhibitors (TKI) were developed that markedly improved treatment of several malignancies. Sunitinib, a TKI inhibitor of VEGFRs 1, 2 and 3, additionally targets c-KIT, FMS-like tyrosine kinase 3, PDGF-α, PDGF-β, and RET signaling pathways.9
Lack of complete regression of tumor vasculature and development of resistance to targeted agents have necessitated quest for alternative anti-angiogenic strategies. These have included newer targeted inhibitors as well as concomitant targeting of VEGF ligands together with VEGF receptors. Sunitinib and bevacizumab inhibit angiogenesis at complementary sites; dual VEGF and VEGFR inhibition has the potential to achieve a more thorough inhibition of the VEGF signaling pathway, which could, in theory, improve anti-tumor efficacy.10,11 The estimated half-lives of bevacizumab and sunitinib with its active metabolite, are 21 d (range of 11–50 d) and 1–3 d respectively. Angiogenic adaptation during combined treatment with bevacizumab and sunitinib occurring in the first 4 wk reflects the effect of combined VEGF and VEGFR inhibition. Between weeks 4 and 6, during the standard treatment break (“off-period”) of sunitinib, rebound changes in the VEGF receptors and associated cytokines are expected, with continued suppression of ligands engaged directly by VEGF. Combined treatment with bevacizumab and sunitinib has previously been evaluated in phase I studies of patients with advanced renal cell carcinoma and other solid tumors.11,12 In addition to metastatic renal cell carcinoma (mRCC), initial studies suggested that this combination might have activity in melanoma and adrenal cortical carcinoma.13 Also noted in these studies, however, was the occurrence of thrombotic microangiopathy (TMA), at least in RCC patients with prior nephrectomies. Taken together with other published reports of TMA in RCC patients,12,14 the combination of sunitinib and bevacizumab may be associated with microangiopathy at both low as well as high doses in patients with mRCC.
We initiated an expansion of our phase I study of bevacizumab and sunitinib, primarily to obtain further safety data in non-RCC tumor types. Secondary goals were to assess objective tumor responses, and to characterize dynamics of angiogenic proteins that might offer insights into mechanisms of tumor response and resistance.
Results
Patient characteristics
Twenty-two patients were enrolled in the expansion cohort, including ten males and 12 females. The median age at enrollment was 58 y. Tumor types included: melanoma (11), adrenal cancer (5), RCC (5), and angiosarcoma (1). Prior systemic treatment included chemotherapy (41%) and immunotherapy (27%). All 5 RCC patients, 3 of the 5 ACC patients and 2 of the 5 melanoma patients had received no prior treatment. Common sites of metastatic disease included lymph nodes (64%), lung/pleura (45%), and liver (32%).
Exposure and safety
A median of 2 cycles per patient were completed (range, 0–8). Upon learning of the joint National Cancer Institute and the Case Comprehensive Cancer Center decision to stop further accrual of RCC patients due to risk of microangiopathy,14 one RCC patient decided to withdraw from the study on day 12. One ACC patient was hospitalized during cycle 1 for severe abdominal pain related to underlying bulky tumor mass. She developed worsening respiratory failure (from suspected pneumonia and/or undocumented pulmonary embolism) that eventually resulted in death. A second ACC patient developed chest pain, ST elevations, and cardiogenic shock during cycle 1. An autopsy identified acute myocardial infarction involving the basal interventricular septum and left lateral ventricular wall, with normal coronary lumens. This cardiovascular toxicity was felt by the investigators to be at least possibly related to treatment.
Asymptomatic grade 4 thrombocytopenia occurred in two patients. Three RCC patients who had previously undergone nephrectomy, developed TMA, reported in part previously.14 One melanoma patient (without nephrectomy) developed low haptoglobin (grade1), thrombocytopenia (grade 3), acute renal failure (grade 2), and schistocytes, consistent with a diagnosis of hemolytic uremic syndrome (a subtype of TMA). This occurred during cycle 2. Discontinuation of treatment led to reversal of TMA in all patients. With the fourth occurrence of TMA, particularly its occurrence in a non-RCC patient, a decision was made to close the trial prior to completion of planned enrollment.
Common all-grade toxicities included fatigue (77%), hypertension (73%), thrombocytopenia (68%), nausea (59%), and oral mucositis (55%) (Table 1). Less commonly, anorexia, dysgeusia, and leucopenia were noted. Adverse effects ≥grade 3 occurred in 15 patients (68%); these included hypertension (41%), thrombocytopenia (23%), and fatigue (14%). Seven patients developed hemorrhagic events, all grade 1 or 2.
Table 1. Toxicity data: Grade ≥3 toxicities and toxicities of special interest.
| Grade | ≥3 | All grade | ≥3 | All grade |
|---|---|---|---|---|
| Toxicity | n | n | % | % |
| Fatigue | 3 | 17 | 13.64 | 77.27 |
| Hypertension | 9 | 16 | 40.91 | 72.73 |
| Thrombocytopenia | 5 | 15 | 22.73 | 68.18 |
| Nausea | 1 | 13 | 4.55 | 59.09 |
| Oral mucositis | 2 | 12 | 9.09 | 54.55 |
| Anemia | 1 | 8 | 4.55 | 36.36 |
| Lymphopenia | 1 | 8 | 4.55 | 36.36 |
| Diarrhea | 1 | 7 | 4.55 | 31.82 |
| Neutropenia | 2 | 6 | 9.09 | 27.27 |
| Palmar–plantar erythrodysesthesia syndrome | 1 | 5 | 4.55 | 22.73 |
| ALT elevation | 1 | 4 | 4.55 | 18.18 |
| Thrombotic microangiopathy | 1 | 4 | 4.55 | 18.18 |
| Pain | 1 | 4 | 4.55 | 18.18 |
| Hypophosphatemia | 1 | 4 | 4.55 | 18.18 |
| Haptoglobin reduction | 0 | 4 | 0.00 | 18.18 |
| Vomiting | 1 | 3 | 4.55 | 13.64 |
| Abdominal pain | 1 | 2 | 4.55 | 9.09 |
Sunitinib was dose-reduced in three patients, and held in at least one cycle in 13 patients. The most common reason was hypertension. Other reasons included fatigue, bleeding, thrombocytopenia, mucositis, acute renal failure, neutropenia, diarrhea, and to facilitate wound healing. Bevacizumab was withheld in one or more cycle(s) in 11 patients. The most common reason for suspension was hypertension; another significant indication was TMA as described above. One patient decided to come off-study after completing cycle 1 due to adverse effects (fatigue), while another came off study after completing 6 cycles due to a combination of adverse effects (diarrhea, stomatitis) and progressive disease on staging.
Therapeutic activity
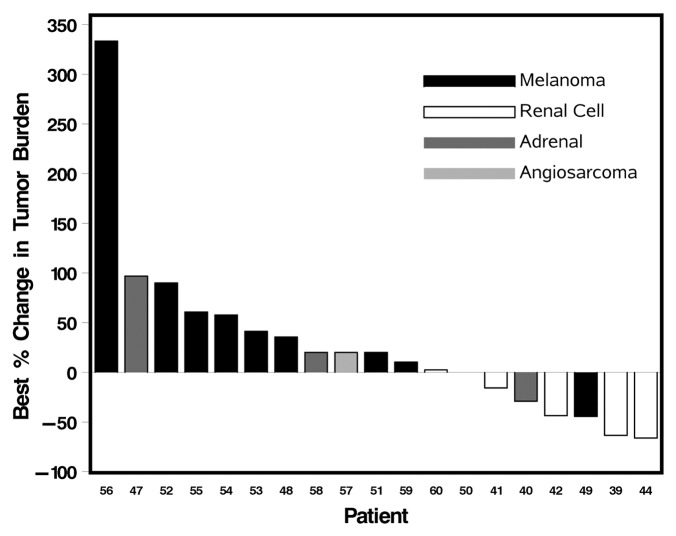
Tumor response was evaluable in the 19 patients who completed 6 wk or more of treatment. Partial responses (PR) resulted in 21% (4/19) patients including one melanoma and three RCC patients. The melanoma patient, who had been refractory to chemotherapy and immunotherapy, developed a durable response to therapy lasting 7 mo, with a progression free survival (PFS) of 10.2 mo. The duration of responses for the three RCC patients with PR varied from 3 to 36 mo. Stable disease (SD) occurred in 16% (3/19) patients. One ACC patient with SD had end of study scans performed at 6 wk when consent was withdrawn secondary to fatigue. This patient demonstrated stable disease for 6 mo after this brief exposure to therapy without need for additional treatment. The other two patients with SD included a melanoma patient (SD lasting for 2 mo), an RCC patient, with SD for 21 mo. Progressive disease occurred in 63% (12/19) patients. Overall, 6 patients had measurable reduction in tumor burden, varying from 21% to 66%. Figure 1 summarizes the best therapeutic responses. Overall, the median PFS was 2.4 mo, with a range from 0.4 to 10.2 mo.
Figure 1. Waterfall plot of best responses. Patients with new lesions were empirically assigned a 20% increase in target tumor measurement for accurate reflection of best response.
Angiogenic biomarkers
Serum VEGF levels declined over the first 4 wk of treatment with this combination, but more markedly at the end of six weeks of bevacizumab (P = 0.03) (Table 2). CECs increased during the combined administration in the first 4 wk interval, from a median of 6.5/mL to 17.75/mL (P = 0.03), but returned to baseline when sunitinib was withdrawn. Between weeks 1 and 4, serum levels of the extracellular domain of the cytokine VEGF receptors (sVEGFR-2 and sVEGF-3) declined (both P = 0.0002); these changes were sustained during the two weeks without sunitinib administration (Table 2). Also consistent with dual inhibition of angiogenic drivers, were declines in sTie-2 (P = 0.0002), Ang-2 (P = 0.0002), endoglin (P = 0.01), and matrix metalloprotease 9 (MMP9) (P = 0.01). On the other hand, CXCL10 (P = 0.01) and vascular cell adhesion protein VCAM-1 (P = 0.006) increased during the first 4 wk.
Table 2. Markers of angiogenesis in patients treated with dual VEGF/VEGFR inhibition.
| Median level baseline (n = 13)a | Median level week 4 (n = 13)a | Median level week 6 (n = 9)a | P valueb baseline vs week 4 | P valueb baseline vs week 6 | P valueb week 4 vs week 6 | |
|---|---|---|---|---|---|---|
| CEC (cells/mL) | 6.5 | 17.75c | 6.12d | 0.03 | 0.85 | 0.64 |
| VEGF (pg/mL) | 360.67 | 217.19 | 167.66 | 0.07 | 0.03 | 0.004 |
| VEGFR1 (pg/mL) | 109.32 | 149.33 | 117.35 | 1.0 | 0.30 | 0.50 |
| VEGFR2 (pg/mL) | 1856.1 | 1264 | 1393.1 | 0.0002 | 0.004 | 0.03 |
| VEGFR3 (ng/mL) | 45.04 | 15.38 | 23.66 | 0.0002 | 0.004 | 0.004 |
| Endoglin (ng/mL) | 3.69 | 3.23 | 3.34 | 0.01 | 0.004 | 0.82 |
| TIE-2 (ng/mL) | 20.77 | 15.48 | 17.42 | 0.0002 | 0.004 | 0.03 |
| VCAM-1 (ng/mL) | 576.6 | 932.18 | 932.18 | 0.006 | 0.04 | 0.73 |
| Angiopoeitin-2 (pg/mL) | 2841.4 | 1537.2 | 1843.8 | 0.0002 | 0.004 | 0.04 |
| MMP-9 (ng/mL) | 609.3 | 312.8 | 363.6 | 0.01 | 0.03 | 0.25 |
| CXCL-10 (pg/mL) | 124.2 | 347.41 | 154.51 | 0.01 | 0.20 | 0.04 |
| Prokineticin-1 (ng/mL) | 0.615 | 0.646 | 0.54 | 0.22 | 1.0 | 0.57 |
| Prokineticin-2 (pg/mL) | 0.35 | 3.95 | 0.83 | 0.01 | 0.11 | 0.43 |
| S100A9 (ng/mL) | 3.26 | 2.55 | 2.8 | 0.64 | 0.10 | 0.73 |
a Unless otherwise noted; bWilcoxon signed rank test; cn = 11; dn = 10.
The pro-angiogenic factor prokineticin-2 also increased >10× over the first 4 wk (P = 0.01), a change that was not sustained after the end of the phase of dual inhibition. Prokineticin-2 levels were undetectable at baseline in 3 melanoma patients. Two of these patients, who had progressive disease at 10 wk, had significant upregulation of prokineticin-2 (week 4 levels 3.95 ng/mL for both). A third melanoma patient, who had a partial response, expressed only a minor increase to 0.1 ng/mL that reverted to undetectable levels at week 6. Interestingly, this melanoma patient also had significantly lower CXCL-10 levels at all time-points in cycle 1, as well as higher baseline MMP-9 (nearly twice the group median).
Discussion
Angiostatic responses have been evaluated in RCC and melanoma in both pre-clinical and clinical studies. In addition to the rationale for study in RCC, vascularity has been correlated with clinical outcome and survival in melanoma.15-17 Furthermore, because of their aggressiveness, murine melanomas were used in early studies of VEGF to define the role of angiogenesis in the metastatic cascade.5,18-22 While the combination of sunitinib and bevacizumab was clinically efficacious in renal cell carcinoma and melanoma, the observance of microangiopathy, even in non-RCC patients, was a significant toxicity that led to early closure of this study and probably precludes further clinical development of this combination. TMA is characterized by development of occlusive microvascular thrombi, microangiopathic hemolytic anemia, consumptive thrombocytopenia, and organ ischemia.23,24 TMA in RCC may be pathophysiologically linked to podocyte specific VEGF disruption in the glomerular microvasculature of the solitary kidney, since many of these patients have previously undergone nephrectomy.25 However, the novel finding of TMA related to dual VEGF/VEGFR inhibition in a melanoma patient suggests that microangiopathy can result from either systemic or nephron specific endothelial injury even in patients with adequate glomerular reserve and in non-renal malignancies.
Clinical exploration of alternate angiogenic molecules to overcome resistance has been a growing focus of pharmacodynamic and translational studies of anti-antiangiogenics. Interest in the evaluation of angiogenic changes in VEGF and alternate pathways is 2-fold. While mechanistic roles of alternate angiogenic proteins might identify novel therapeutic targets, unraveling their correlation with response and resistance could lead to development of predictive biomarkers. Vascularity and vascular endothelial growth factor (VEGF) are adverse prognostic factors in melanoma.15-17,26 Tissue analyses in RCC patients undergoing neo-adjuvant treatment with sunitinib identified suppression of VEGFR-1 and VEGFR-2 gene expression.27 Ang-2 is a cytokine in the tumor microenvironment that binds to TIE-2, an endothelial cell receptor tyrosine kinase of the Tie family, and affects endothelial cell survival and proliferation.28 MMPs are ligands for integrins expressed on the surface of endothelial cells (EC); they have an established role in EC migration and invasion, both of which are essential for vessel sprouting.29,30 Another family of alternate angiogenic molecules is prokineticins. Prokineticin-1 and 2 promote tissue-specific angiogenesis and hematopoietic cell mobilization.31,32 Elevated levels of VEGF, isoforms of VEGFR, Ang-2, Tie-2, and MMP9 have all been associated with outcomes in melanoma.33-43
The observed shifts in angiogenic proteins in this study offer insights into angiostatic responses. The most profound effect of dual VEGF/VEGFR inhibition was observed in prokineticin-2 that increased over 10-fold. That this upregulation was significantly higher during the phase of dual VEGF/VEGFR inhibition, suggests a mechanistic link via VEGF receptors. Prokineticin-2, which can be upregulated by G-CSF, has also been shown to mediate resistance to bevacizumab in murine models.44-47 This functional change in these patients, while potentially of importance, will need further validation. Another interesting trend was the dynamics of the Ang-2 and Tie-2 pathway. Serum Ang-2 levels are higher in patients with metastatic melanoma when compared with early stage disease.37,48 Antibodies targeting Ang-2, are currently being evaluated in advanced solid tumors in phase I and II studies.49,50 Third, MMP-9 changes mirrored those of VEGF. VEGF/VEGFR2 interaction can downregulate MMP-9 expression at the transcriptional level, thereby inhibiting cellular migration.51 Our results provide further evidence that MMP-9 is interlinked with the VEGF pathway. Overall, these results demonstrate significant trends in alternate angiogenic proteins that provide hypothesis generating data for future studies.
Circulating endothelial cells (CECs) are impacted by disruption of tumor vasculature, and play an important role in the tumor microenvironment. CECs have been shown to be elevated at baseline in cancer patients when compared with healthy controls.52-55 In mRCC, CECs initially increase with treatment, and subsequently declined toward baseline.56 In uveal melanoma patients, adjuvant treatment with interferon-α and demonstrated adequate organ function as defined by aspartate/alanine transaminase ≤2.5× upper limit of normal (ULN), bilirubin ≤1.5× ULN, absolute neutrophil count (ANC) ≥1500/μL, platelets ≥100 000/μL, hemoglobin ≥10.0 g/dL, calcium ≤12.0 mg/dL, creatinine ≤1.5× ULN, and urine protein creatinine (UPC) ratio as determined by urinalysis <0.5 (for UPC ratio >0.5, 24-h urine protein level should have been <1000 mg for patient enrollment).
In summary, combined treatment with bevacizumab and sunitinib in patients with melanoma and renal cell carcinoma has clinical activity but limiting angiopathic toxicity precluding further clinical development. Angiostatic responses that were characterized identified significant downregulation of the pro-angiogenic proteins VEGF, sVEGFR2, sVEGFR3, endoglin, sTIE2, angiopoietin 2, and MMP-9 and upregulation of the proangiogenic prokineticin-2, sVCAM-1, and CXCL10. Further research will be required to elucidate the mechanistic roles of these and other non VEGF proteins in mediating resistance to antiangiogenic agents.
Patients and Methods
This study was approved by the Institutional Review Board of the Case Comprehensive Cancer Center and was registered at http://clinicaltrials.gov (NCT00357318). Patients aged 18 or older with histologically proven, metastatic/unresectable solid tumors not amenable to curative surgical or radiation therapy were enrolled in the expansion arm of our phase I study. Patients with squamous cell histology or any histology in close proximity to a major blood vessel were excluded. Patients were enrolled if they had a performance status of ECOG 0 or 1, reported resolution of acute toxic effects of prior therapy, radiotherapy, or surgical procedure; and demonstrated adequate organ function as defined by aspartate/alanine transaminase ≤2.5× upper limit of normal (ULN), bilirubin ≤1.5× ULN, absolute neutrophil count (ANC) ≥1500/μL, platelets ≥100 000/μL, hemoglobin ≥10.0 g/dL, calcium ≤12.0 mg/dL, creatinine ≤1.5× ULN, and urine protein creatinine (UPC) ratio as determined by urinalysis <0.5 (for UPC ratio >0.5, 24-h urine protein level should have been <1000 mg for patient enrollment).
Patients who had previously received bevacizumab or sunitinib were excluded from the study. Patients were also excluded if they had received prior systemic therapy or radiation therapy within 4 wk of starting treatment on protocol. Furthermore, patients with the following conditions were excluded: bleeding diathesis or coagulopathy, history of or known brain metastases, spinal cord compression, or carcinomatous meningitis, new evidence of brain or leptomeningeal disease on screening CT or MRI scan unless without progression on MRI or CT for 3 mo, ongoing cardiac dysrhythmias of NCI CTCAE grade ≥2, atrial fibrillation of any grade, prolongation of the QTc interval to >450 ms for males or >470 ms for females, history of serious ventricular arrhythmia (VT or VF ≥3 beats in a row), conditions classified as NYHA III or IV, patients on full-dose anticoagulants (however patients receiving low-dose anticoagulation therapy were eligible), hypertension uncontrolled by medications to <140/90 mmHg, history of abdominal fistula, gastrointestinal perforation, or intra-abdominal abscess within the previous 28 d, serious, non-healing wound, ulcer, or bone fracture, hypersensitivity of Chinese hamster ovary cell products or other recombinant human antibodies, human immunodeficiency virus (HIV), or acquired immunodeficiency syndrome (AIDS)-related illness, current treatment on another clinical trial, and pregnancy or breastfeeding, or any of the following within the preceding 12 mo: myocardial infarction, severe/unstable angina, severe peripheral vascular disease (claudication), or procedure on peripheral vasculature, coronary/peripheral artery bypass, graft, New York Heart Association (NYHA) grade II or greater congestive heart failure, cerebrovascular accident or transient ischemic attack, clinically significant bleeding, deep venous thrombosis, or pulmonary embolism.
Study design and treatment
Patients received 37.5 mg sunitinib orally once a day from weeks 1–4 in addition to bevacizumab 5 mg/kg intravenously on days 1, 15, and 29 of each 6 wk (42 d) treatment cycle (Fig. 2). This dose schedule corresponded with level +1 of the original phase I trial. Doses were based on patient’s actual body weight. Treatment was continued till patients developed any of the following: progressive disease per RECIST criteria, unacceptable adverse effects, intercurrent illness that precluded further administration of therapy, or patient decision to withdraw consent. Adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events version 4.0. Dose limiting toxicities (DLTs) were defined as: any grade 4 toxicity (except lymphopenia or increased uric acid), any grade 3 cardiac event (except hypertension) or grade 3 venous thrombosis, hypertension unable to be controlled to <160/90 with oral medications within 4 wk, any grade arterial thromboembolic event, any grade 3 non-cardiac toxicity that failed to resolve to ≤ grade 1 within 6 wk (except proteinuria, lymphopenia, hypophosphatemia, and asymptomatic hyperamylasemia/hyperlipasemia) and/or proteinuria >3.5 g/24 h.
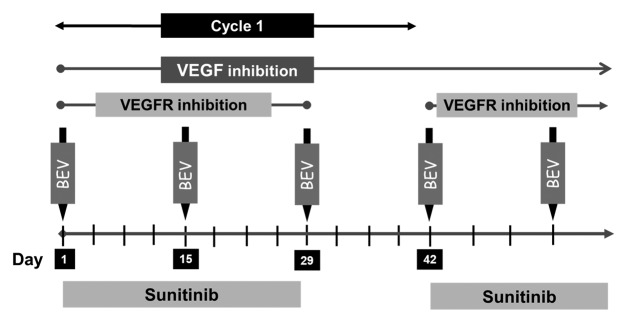
Figure 2. Study schema: Patients received sunitinib 37.5 mg PO daily from weeks 1–4 and bevacizumab (Bev) 5 mg/kg intravenously on days 1, 15, and 29 of each 6-wk cycle.
Dose modification
Sunitinib dose was reduced in patients experiencing non-dose limiting toxicities, based on individual patient tolerance. Sunitinib dose was delayed if the elevation of ALT/AST was greater than 5 times the ULN, or bilirubin was more than 3 times the ULN. Sunitinib could be re-administered when levels of ALT/AST and bilirubin declined to ≤5× and ≤3 ULN. There were no reductions in bevacizumab dose, rather the dose was held in case of adverse events, and was restarted at the same dose upon resolution on non-dose limiting toxicities.
Study assessment
Patients with measurable disease were assessed by RECIST criteria version 1.0 at baseline and day 28 of even numbered cycles.
Correlative studies
Circulating endothelial cells (CECs) and soluble angiogenic proteins were characterized in 10 melanoma patients and 3 additional patients (one each mRCC, angiosarcoma, and ACC) during cycle 1. Blood samples were collected from these 13 patients at baseline and week 4, and from 9 patients at week 6. Peripheral blood samples were drawn in one 10 mL CellSave tube, and one 10 mL serum tube. The serum samples were stored for batch analyses of regulatory circulating angiogenic proteins. Angiogenic proteins were quantified in serum samples at baseline, week 4, and week 6, using solid phase ELISA assays employing a quantitative sandwich immunoassay (all antibodies were obtained from R&D Systems except prokineticin 1, prokineticin 2, and S100A9 that were from Antibodies on Line). The CellTracks® AutoPrep® System and the CellSpotter® Analyzer II System (Veridex, LLC) were used to enumerate circulating endothelial cells (CECs), using immunomagnetic separation.57,59 Briefly, 4 mL of blood was used to enrich CD 146 positive cells using immunomagnetic separation. Subsequently, nuclear dye 4,6-diamidino-2-phenylindole (DAPI), and fluorochrome-conjugated monoclonal antibodies-phycoerythrin-conjugated CD105, and allophycocyanin-conjugated CD45, were added. Using image cytometry, CECs were defined as CD146+, DAPI+, CD105+, and CD45 negative elements. Results were expressed as number of CECs per mL of blood.
Statistical design and data analysis
Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Correlative study data were analyzed using the Wilcoxon signed rank test and P values < 0.05 were considered significant. Data presented are medians. CECs were enumerated as cells per mL blood, while angiogenic proteins were quantified per mL of serum. Progression-free survival was summarized using the Kaplan–Meier method; and was measured from the start of treatment to the date of documented progression, or the date off-treatment for patients who discontinued therapy early for adverse events.
Disclosure of Potential Conflicts of Interest
Dr Rini has consulting and research funding from Pfizer.
Acknowledgments
We are appreciative to Ronald Grane and Barbara Jacobs for the careful and dedicated conduct of the pharmacodynamic assays.
Financial Support
CTEP Grant U01-CA062502 and a philanthropic gift to Taussig Cancer Institute from Maria and Carlos Tejada.
References
- 1.Epstein RJ. VEGF signaling inhibitors: more pro-apoptotic than anti-angiogenic. Cancer Metastasis Rev. 2007;26:443–52. doi: 10.1007/s10555-007-9071-1. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;133:275–88. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175:409–16. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 5.Claffey KP, Brown LF, del Aguila LF, Tognazzi K, Yeo KT, Manseau EJ, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996;56:172–81. [PubMed] [Google Scholar]
- 6.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–12. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 7.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 8.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rini BI. Sunitinib. Expert Opin Pharmacother. 2007;8:2359–69. doi: 10.1517/14656566.8.14.2359. [DOI] [PubMed] [Google Scholar]
- 10.Moreno Garcia V, Basu B, Molife LR, Kaye SB. Combining antiangiogenics to overcome resistance: rationale and clinical experience. Clin Cancer Res. 2012;18:3750–61. doi: 10.1158/1078-0432.CCR-11-1275. [DOI] [PubMed] [Google Scholar]
- 11.Rini BI, Garcia JA, Cooney MM, Elson P, Tyler A, Beatty K, Bokar J, Mekhail T, Bukowski RM, Budd GT, et al. A phase I study of sunitinib plus bevacizumab in advanced solid tumors. Clin Cancer Res. 2009;15:6277–83. doi: 10.1158/1078-0432.CCR-09-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman DR, Baum MS, Ginsberg MS, Hassoun H, Flombaum CD, Velasco S, Fischer P, Ronnen E, Ishill N, Patil S, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–9. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zangari M, Yaccoby S, Pappas L, Cavallo F, Kumar NS, Ranganathan S, Suva LJ, Gruenwald JM, Kern S, Zhan F, et al. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients. Haematologica. 2011;96:333–6. doi: 10.3324/haematol.2010.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rini BI, Garcia JA, Cooney MM, Elson P, Tyler A, Beatty K, Bokar J, Ivy P, Chen HX, Dowlati A, et al. Toxicity of sunitinib plus bevacizumab in renal cell carcinoma. J Clin Oncol. 2010;28:e284–5, author reply e286-7. doi: 10.1200/JCO.2009.27.1759. [DOI] [PubMed] [Google Scholar]
- 15.Vlaykova T, Laurila P, Muhonen T, Hahka-Kemppinen M, Jekunen A, Alitalo K, Pyrhönen S. Prognostic value of tumour vascularity in metastatic melanoma and association of blood vessel density with vascular endothelial growth factor expression. Melanoma Res. 1999;9:59–68. doi: 10.1097/00008390-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Döme B, Paku S, Somlai B, Tímár J. Vascularization of cutaneous melanoma involves vessel co-option and has clinical significance. J Pathol. 2002;197:355–62. doi: 10.1002/path.1124. [DOI] [PubMed] [Google Scholar]
- 17.Kashani-Sabet M, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR., 3rd Tumor vascularity in the prognostic assessment of primary cutaneous melanoma. J Clin Oncol. 2002;20:1826–31. doi: 10.1200/JCO.2002.07.082. [DOI] [PubMed] [Google Scholar]
- 18.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–80. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 19.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 20.Jain RK. Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin Oncol. 2002;29(Suppl 16):3–9. doi: 10.1053/sonc.2002.37265. [DOI] [PubMed] [Google Scholar]
- 21.Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. Influence of the microenvironment on melanoma cell fate determination and phenotype. Cancer Res. 2006;66:7833–6. doi: 10.1158/0008-5472.CAN-06-0731. [DOI] [PubMed] [Google Scholar]
- 22.Pötgens AJ, Lubsen NH, van Altena MC, Schoenmakers JG, Ruiter DJ, de Waal RM. Vascular permeability factor expression influences tumor angiogenesis in human melanoma lines xenografted to nude mice. Am J Pathol. 1995;146:197–209. [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai HM. The molecular biology of thrombotic microangiopathy. Kidney Int. 2006;70:16–23. doi: 10.1038/sj.ki.5001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng XL, Sadler JE. Pathogenesis of thrombotic microangiopathies. Annu Rev Pathol. 2008;3:249–77. doi: 10.1146/annurev.pathmechdis.3.121806.154311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–36. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaki KA, Basu B, Corrie P. The role of angiogenesis inhibitors in the management of melanoma. Curr Top Med Chem. 2012;12:32–49. doi: 10.2174/156802612798919240. [DOI] [PubMed] [Google Scholar]
- 27.Griffioen AW, Mans LA, de Graaf AM, Nowak-Sliwinska P, de Hoog CL, de Jong TA, Vyth-Dreese FA, van Beijnum JR, Bex A, Jonasch E. Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res. 2012;18:3961–71. doi: 10.1158/1078-0432.CCR-12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cascone T, Heymach JV. Targeting the angiopoietin/Tie2 pathway: cutting tumor vessels with a double-edged sword? J Clin Oncol. 2012;30:441–4. doi: 10.1200/JCO.2011.38.7621. [DOI] [PubMed] [Google Scholar]
- 29.Basu B, Biswas S, Wrigley J, Sirohi B, Corrie P. Angiogenesis in cutaneous malignant melanoma and potential therapeutic strategies. Expert Rev Anticancer Ther. 2009;9:1583–98. doi: 10.1586/era.09.135. [DOI] [PubMed] [Google Scholar]
- 30.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–85. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monnier J, Samson M. Prokineticins in angiogenesis and cancer. Cancer Lett. 2010;296:144–9. doi: 10.1016/j.canlet.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Shojaei F, Wu X, Zhong C, Yu L, Liang X-H, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–31. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 33.Brennecke S, Deichmann M, Naeher H, Kurzen H. Decline in angiogenic factors, such as interleukin-8, indicates response to chemotherapy of metastatic melanoma. Melanoma Res. 2005;15:515–22. doi: 10.1097/00008390-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Chua R, Setzer S, Govindarajan B, Sexton D, Cohen C, Arbiser JL. Maspin expression, angiogenesis, prognostic parameters, and outcome in malignant melanoma. J Am Acad Dermatol. 2009;60:758–66. doi: 10.1016/j.jaad.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Dréau D, Foster M, Hogg M, Swiggett J, Holder WD, White RL. Angiogenic and immune parameters during recombinant interferon-alpha2b adjuvant treatment in patients with melanoma. Oncol Res. 2000;12:241–51. doi: 10.3727/096504001108747738. [DOI] [PubMed] [Google Scholar]
- 36.Yurkovetsky ZR, Kirkwood JM, Edington HD, Marrangoni AM, Velikokhatnaya L, Winans MT, Gorelik E, Lokshin AE. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13:2422–8. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
- 37.Helfrich I, Edler L, Sucker A, Thomas M, Christian S, Schadendorf D, Augustin HG. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin Cancer Res. 2009;15:1384–92. doi: 10.1158/1078-0432.CCR-08-1615. [DOI] [PubMed] [Google Scholar]
- 38.Nikkola J, Vihinen P, Vuoristo MS, Kellokumpu-Lehtinen P, Kähäri VM, Pyrhönen S. High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clin Cancer Res. 2005;11:5158–66. doi: 10.1158/1078-0432.CCR-04-2478. [DOI] [PubMed] [Google Scholar]
- 39.Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Circulating levels of vascular endothelial growth factor (VEGF), matrix metalloproteinase-3 (MMP-3), and BCL-2 in malignant melanoma. Med Oncol. 2008;25:431–6. doi: 10.1007/s12032-008-9058-y. [DOI] [PubMed] [Google Scholar]
- 40.Ascierto PA, Leonardi E, Ottaiano A, Napolitano M, Scala S, Castello G. Prognostic value of serum VEGF in melanoma patients: a pilot study. Anticancer Res. 2004;24:4255–8. [PubMed] [Google Scholar]
- 41.Osella-Abate S, Quaglino P, Savoia P, Leporati C, Comessatti A, Bernengo MG. VEGF-165 serum levels and tyrosinase expression in melanoma patients: correlation with the clinical course. Melanoma Res. 2002;12:325–34. doi: 10.1097/00008390-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Straume O, Akslen LA. Importance of vascular phenotype by basic fibroblast growth factor, and influence of the angiogenic factors basic fibroblast growth factor/fibroblast growth factor receptor-1 and ephrin-A1/EphA2 on melanoma progression. Am J Pathol. 2002;160:1009–19. doi: 10.1016/S0002-9440(10)64922-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–83. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 44.Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A. 2009;106:6742–7. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shojaei F, Ferrara N. Refractoriness to antivascular endothelial growth factor treatment: role of myeloid cells. Cancer Res. 2008;68:5501–4. doi: 10.1158/0008-5472.CAN-08-0925. [DOI] [PubMed] [Google Scholar]
- 46.Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A. 2008;105:2640–5. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–31. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 48.Gardizi M, Kurschat C, Riese A, Hahn M, Krieg T, Mauch C, Kurschat P. A decreased ratio between serum levels of the antagonistic angiopoietins 1 and 2 indicates tumour progression of malignant melanoma. Arch Dermatol Res. 2012;304:397–400. doi: 10.1007/s00403-012-1228-2. [DOI] [PubMed] [Google Scholar]
- 49.Karlan BY, Oza AM, Richardson GE, Provencher DM, Hansen VL, Buck M, Chambers SK, Ghatage P, Pippitt CH, Jr., Brown JV, 3rd, et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol. 2012;30:362–71. doi: 10.1200/JCO.2010.34.3178. [DOI] [PubMed] [Google Scholar]
- 50.Rosen LSMD, Cohen RB, Gordon MS, Goldman JW, Bear IK, Byrnes B, Perea R, Schoenfeld SL, Gollerkeri A. First-in-human dose-escalation safety and PK trial of a novel intravenous humanized monoclonal CovX body inhibiting angiopoietin 2. J Clin Oncol. 2010;28(Suppl):abstr 2524. [Google Scholar]
- 51.Ugarte-Berzal E, Redondo-Muñoz J, Eroles P, Del Cerro MH, García-Marco JA, Terol MJ, García-Pardo A. VEGF/VEGFR2 interaction down-regulates matrix metalloproteinase-9 via STAT1 activation and inhibits B chronic lymphocytic leukemia cell migration. Blood. 2010;115:846–9. doi: 10.1182/blood-2009-08-239426. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Vakil V, Braunstein M, Smith ELP, Maroney J, Chen L, Dai K, Berenson JR, Hussain MM, Klueppelberg U, et al. Circulating endothelial progenitor cells in multiple myeloma: implications and significance. Blood. 2005;105:3286–94. doi: 10.1182/blood-2004-06-2101. [DOI] [PubMed] [Google Scholar]
- 53.Beerepoot LV, Mehra N, Vermaat JSP, Zonnenberg BA, Gebbink MFGB, Voest EE. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol. 2004;15:139–45. doi: 10.1093/annonc/mdh017. [DOI] [PubMed] [Google Scholar]
- 54.Beerepoot LV, Mehra N, Linschoten F, Jorna AS, Lisman T, Verheul HMW, Voest EE. Circulating endothelial cells in cancer patients do not express tissue factor. Cancer Lett. 2004;213:241–8. doi: 10.1016/j.canlet.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Kim HK, Song KS, Kim HO, Chung J-H, Lee KR, Lee Y-J, Lee DH, Lee ES, Kim HK, Ryu KW, et al. Circulating numbers of endothelial progenitor cells in patients with gastric and breast cancer. Cancer Lett. 2003;198:83–8. doi: 10.1016/S0304-3835(03)00268-4. [DOI] [PubMed] [Google Scholar]
- 56.Gruenwald V, Beutel G, Schuch-Jantsch S, Reuter C, Ivanyi P, Ganser A, Haubitz M. Circulating endothelial cells are an early predictor in renal cell carcinoma for tumor response to sunitinib. BMC Cancer. 2010;10:695. doi: 10.1186/1471-2407-10-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Triozzi PL, Achberger S, Aldrich W, Singh AD, Grane R, Borden EC. The association of blood angioregulatory microRNA levels with circulating endothelial cells and angiogenic proteins in patients receiving dacarbazine and interferon. J Transl Med. 2012;10:241. doi: 10.1186/1479-5876-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang T, Zhuang J, Duan H, Luo Y, Zeng Q, Fan K, Yan H, Lu D, Ye Z, Hao J, et al. CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood. 2012;120:2330–9. doi: 10.1182/blood-2012-01-406108. [DOI] [PubMed] [Google Scholar]
- 59.Woywodt A, Blann AD, Kirsch T, Erdbruegger U, Banzet N, Haubitz M, Dignat-George F. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost. 2006;4:671–7. doi: 10.1111/j.1538-7836.2006.01794.x. [DOI] [PubMed] [Google Scholar]