Abstract
Chemotherapy-induced mucositis (CIM) is a major does limiting side-effect of chemoagents such as 5-fluorouracil (5-FU). Molecules involved in this disease process are still not fully understood. We proposed that the homeostatically regulated genes during CIM may participate in the disease. A cluster of such genes were previously identified by expression gene-array from the mouse jejunum in 5-FU-induced mucositis model. Here, we report that CXCL4 is such a homeostatically regulated gene and serves as a new target for the antibody treatment of CIM. CXCL4 and its receptor CXCR3 were confirmed at both the gene and protein levels to be homeostatically regulated during 5-FU-induced mucositis. Using of CXCL4 neutralizing monoclonal antibody (CXCL4mab) decreased the incidence, severity, and duration of the chemotherapy-induced diarrhea, the major symptom of CIM, in a 5-FU mouse CIM model. Mechanistically, CXCL4mab reduced the apoptosis of the crypt epithelia by suppression of the 5-FU-induced expression of p53 and Bax through its receptor CXCR3. The downstream signaling pathway of CXCL4 in activation of the epithelial apoptosis was identified in an intestinal epithelial cell line (IEC-6). CXCL4 activated the phosphorylation of p38 MAPK, which mediated the stimulated expression of p53 and Bax, and resulted in the ultimate activation of Caspase-8, -9, and -3. Taken together, activation of CXCL4 expression by 5-FU in mice participates in 5-FU-induced intestinal mucositis through upregulation of p53 via activation of p38-MAPK, and CXCL4mab is potentially beneficial in preventing CIM in the intestinal tract.
Keywords: CXCL4, chemotherapy, intestinal mucositis, apoptosis, p53, p38
Introduction
The fast-renewing intestinal epithelium is vulnerable to the cytotoxicity of chemotherapy. Chemotherapy-induced mucositis (CIM) is a dose-limiting side effect of many chemoagents. It has been pathologically described as an intestinal inflammation characterized by an early event of epithelia apoptosis.1-3 The apoptosis is localized mostly in the mucosa, particularly in proliferating cells at the base of the intestinal crypts.4-7 It is clear that the tumor suppressor protein 53 (p53) is one of the important determinants of 5-FU-induced apoptosis in vivo.5 However, the genetic signal mediating the 5-FU upregulation of p53 is still unclear.
Platelet factor 4 (PF4/CXCL4), a heparin-binding protein, is a chemokine isolated from platelets. Different from other chemokines, CXCL4 is involved in the control of many biological processes including cellular apoptosis, survival, differentiation, and proliferation.8 CXCL4 supports the survival of hematopoietic stem cells and progenitor cells,9 inhibits the proliferation of endothelial cells and fibroblasts,10 and suppresses the development and maturation of cells of the megakaryopoietic lineage.11 Its expression is markedly upregulated in diseases, such as inflammatory bowel disease (IBD) and cerebral malaria.12 It has been proposed as a biomarker of early tumor growth in different tumor types, such as human liposarcoma, mammary adenocarcinoma, and osteosarcoma.13 More recently, CXCL4 has been shown to represent an important mediator of intestinal damage in a mouse model of mesenteric ischemia/reperfusion injury,14 which is supported by localization of its receptor CXCR3 to the intestinal epithelia.15 However, its role in CIM is unknown.
CIM is a self-healing disease in that the tissue mass of the recovered intestine is unchanged. We propose that the homeostatically-regulated gene-expression during the CIM is informative for the disease processes. Identification of these genes and characterization of their roles may lead to the discovery of the genome governing the onset, progress, and healing of the disease. We refer to this as intestinal regeneromics; the technology involved in the study of it is referred to as Prometheus Technology. Thus, we took a regeneromics approach to perform a genome-wide gene expression array to screen for homeostatically-regulated genes in a 5-FU induced mucositis mouse model.
Here, we show that CXCL4 is such an informative gene in CIM. It directly induced apoptosis of intestinal epithelial cells (IEC) after its expression was activated by 5-FU. We showed that neutralization of CXCL4 by its monoclonal antibody (CXCL4mab) significantly reduced the incidence, severity, and duration of chemotherapy-induced diarrhea (CID), the common symptom of CIM, in a 5-FU mouse model. CXCL4mab inhibited 5-FU-induced apoptosis by suppression of p53 and Bcl-2-associated X protein (Bax) induction through the CXCL4 receptor CXCR3. Furthermore, CXCL4 was demonstrated to induce IEC apoptosis directly, which was mediated by the upregulation of p53 and Bax through the activation of p38 MAPK in an IEC cell line model. Thus, 5-FU-induced IEC apoptosis in the mucositis is, at least, partially due to its activation of CXCL4, which serves as a new target for the antibody treatment of CIM.
Results
Expression of CXCL4 and CXCR3 are homeostatically-regulated in 5-FU-induced mouse intestinal mucositis
CIM is a self-healing disease that can occur after the cessation of chemotherapy. The homeostasis of intestinal regeneration in CIM, the full recovery of the tissue in mass and function, is a hallmark of normal tissue regeneration after the injury. We propose that the homeostatically-regulated transcriptome carries information about the homeostasis of intestinal regeneration. Genome-wide gene expression array has identified the transcriptome of CIM, CXCL4, as being one of them.
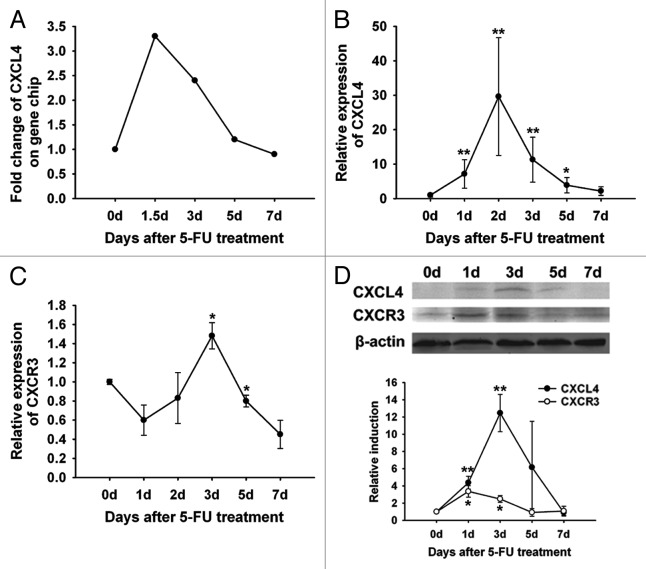
mRNA expression in the jejunum of 5-FU-treated mice was profiled using a high-density oligonucleotide microarray.16 The expression of a chemokine cxcl4 was identified to be increased during the damage phase (0–3 d) and returned back to the normal level during the regeneration phase (3–7 d) in mice treated with 5-FU (Fig. 1A). The homeostatically-regulated cxcl4 expression in the expression array was confirmed by real-time RT-PCR analysis of the jejunum (Fig. 1B). The expression of its receptor cxcr3 was also transiently increased in the jejunum (Fig. 1C). The early elevation of the protein levels of CXCL4 and CXCR3 returned to the baseline after 3 d following 5-FU-treatment (Fig. 1D). Taken together, the homeostatically-regulated expression of CXCL4 and its receptor CXCR3 in CIM suggest their participation in the pathogenesis and/or regeneration of the intestine after chemotherapy.
Figure 1. Expression of CXCL4 and CXCR3 are homeostatically-regulated in the 5-FU-induced mouse intestinal mucositis. BALB/c mice were treated with 5-FU (250 mg/kg) and sacrificed at 1.5, 3, 5, and 7 d or at 1, 2, 3, 5, and 7 d after the 5-FU treatment. Controls were sacrificed on day 0. The expression of CXCL4 and CXCR3 are shown. (A) The signal intensity of cxcl4 mRNA by gene expression array is presented. (B and C) The expression of cxcl4 and cxcr3 by real-time RT-PCR are presented relative to their baseline at day 0. (D) The CXCL4 and CXCR3 protein expression by western blot are presented as their relative induction over the untreated mice at day 0 after normalization to their respective β-actin loading controls. Data are presented as mean ± SE (n = 3 mice per group). *P < 0.05, **P < 0.01 vs. day 0 controls.
Blockade of CXCL4 protects mouse intestine from chemotoxicity
The homeostatically-regulated chemokine CXCL4 in the intestine strongly suggests its role in CIM. A strategy of neutralizing monoclonal antibody (mAb) was used to block CXCL4 to evaluate its role in CIM. After immunization with recombinant human CXCL4, the rat spleen lymphocyte was fused with the mouse myeloma to form hybridoma. A single hybridoma clone was screened out using the recombinant murine CXCL4, and the mAb was named CXCL4mab. It was produced and purified from the ascites of the nude mice. Its purity, specificity, bioactivity, and affinity were determined, and the antibody gene regions encoding the variable heavy chain (VH) and variable light chain (VL) were sequenced (manuscript in preparation).
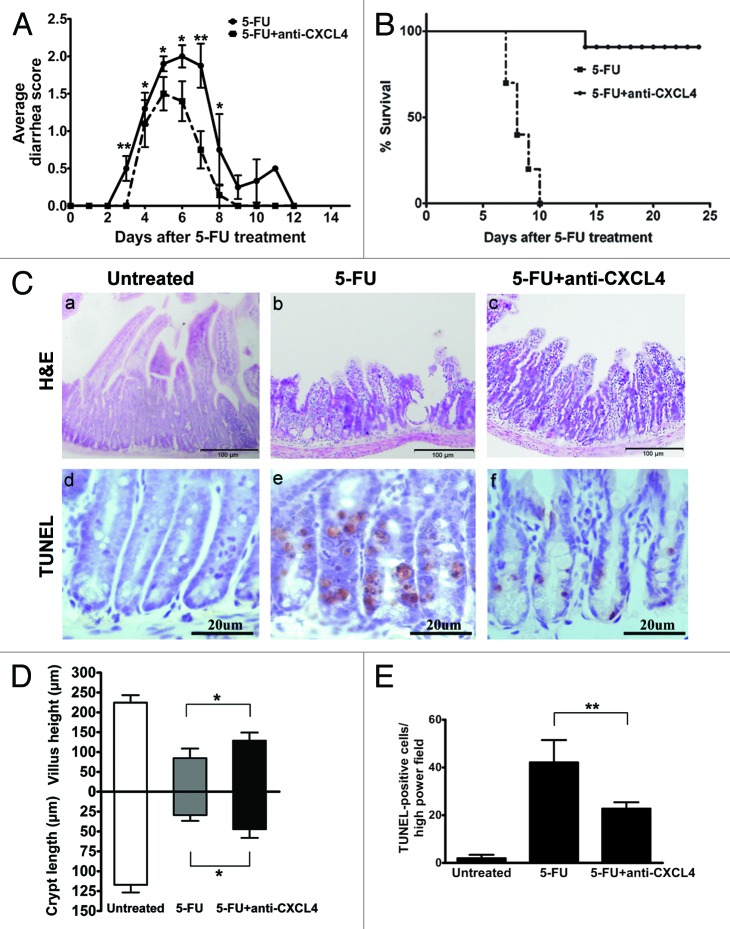
CXCL4mab was given systemically 2 h before 5-FU to neutralize the anticipated elevation of CXCL4. The CXCL4mab significantly reduced the incidence and severity of diarrhea in the mouse 5-FU-induced mucositis model. The diarrhea-free mice after the antibody treatment increased from 12.5% to 47.9%, and the most severe diarrhea (score 3) was abolished (Table 1, P < 0.01). The duration of diarrhea was also shortened from 12 to 8 d (Fig. 2A). The protective effect of the antibody was observed from day 3 to 8 (Fig. 2A, P < 0.05). Significantly, CXC4mab reduced the acute lethal toxicity of 5-FU, which dropped from 100% to 10% (Fig. 2B, P < 0.01). On day 3 after 5-FU, the intestinal histology in the control group was massively damaged compared with the antibody group (Fig. 2C, a–c). The villus length and crypt depth in the control were significantly shortened compared with those in the antibody group (Fig. 2C, g, P < 0.05). The antibody-reduced intestinal damage in CIM points to the role of CXCL4 in the initiation phase of CIM, the induction of intestinal apoptosis.
Table 1. Anti-CXCL4 mAb reduces the severity of 5-FU-induced diarrhea in mice.
| Group | Diarrhea (%) | P | |||
|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | Score 3 | ||
| 5-FU | 12.4 | 31.3 | 50.0 | 6.3 | 0.003 |
| 5-FU + anti-CXCL4 | 47.9 | 20.8 | 31.3 | 0.00 | |
Mice were treated with anti-CXCL4 mAb (1 mg/kg) or equal volume saline 2 h before 5-FU (250 mg/kg) administration. Diarrhea was scored from day 3 to 7 after 5-FU. Average diarrhea score is reported as mean ± SD. n = 10 mice per group.
Figure 2. Anti-CXCL4 mAb attenuates the severity of the 5-FU-induced murine intestinal mucositis. (A) Anti-CXCL4 mAb reduced the severity and duration of diarrhea in CIM. Mice were injected with anti-CXCL4 mAb 2 h before 5-FU (250 mg/kg). Diarrhea was scored daily. n = 10 mice per group. (B) Anti-CXCL4 mAb reduced the lethal toxicity of 5-FU. Mice were treated with anti-CXCL4 mAb (1 mg/kg) or equal volume saline 2 h before 5-FU (400 mg/kg) administration. Animal survival for 25 d are presented as Kaplan–Meier survival curves and analyzed by a long-rank test. n = 10 mice per group. (C) Histological and apoptotic presentation of the jejunum. H&E (a–c) and TUNEL staining (d–f) are shown. Anti-CXCL4 mAb was injected 2 h before 5-FU. Mice were sacrificed on day 1 for apoptosis and day 3 for morphometry after 5-FU injection (300 mg/kg). Untreated, normal mice; 5-FU, mice treated with 5-FU only; and 5-FU and anti-CXCL4 mAb, mice treated with 5-FU and anti-CXCL4 mAb, respectively. Average villus length and crypt depth of the mice are shown (g). Twenty villi and crypts were counted per mouse. n = 3 mice per group. A quantitative presentation of the TUNEL-positive cells is shown (h). The average number of TUNEL-positive cells in ten fields per mouse was determined under microscope (400×). n = 3 mice per group. Data are presented as mean ± SD *P < 0.05, **P < 0.01 vs. 5-FU-treated mice.
Chemotherapy is known to induce apoptosis in the intestinal mucosa, particularly in the proliferating cells of the crypt. The number of apoptotic cells was markedly increased in the intestinal crypt from 6 h, and reached the maximum by 24 h after the administration of 5-FU in mice; whereas, it was significantly reduced in the CXCL4mab-treated group by as much as 50% (Fig. 2C, d–f and h, P < 0.01). The results suggest that CXCL4 mediates at least part of the crypt apoptotic effect of 5-FU. CXCL4mab by blocking the apoptotic effect of CXCL4 alleviates 5-FU-induced mucositis.
CXCL4mab suppresses the induction of p53 and Bax through CXCR3 to reduce the apoptosis of intestinal epithelia by 5-FU
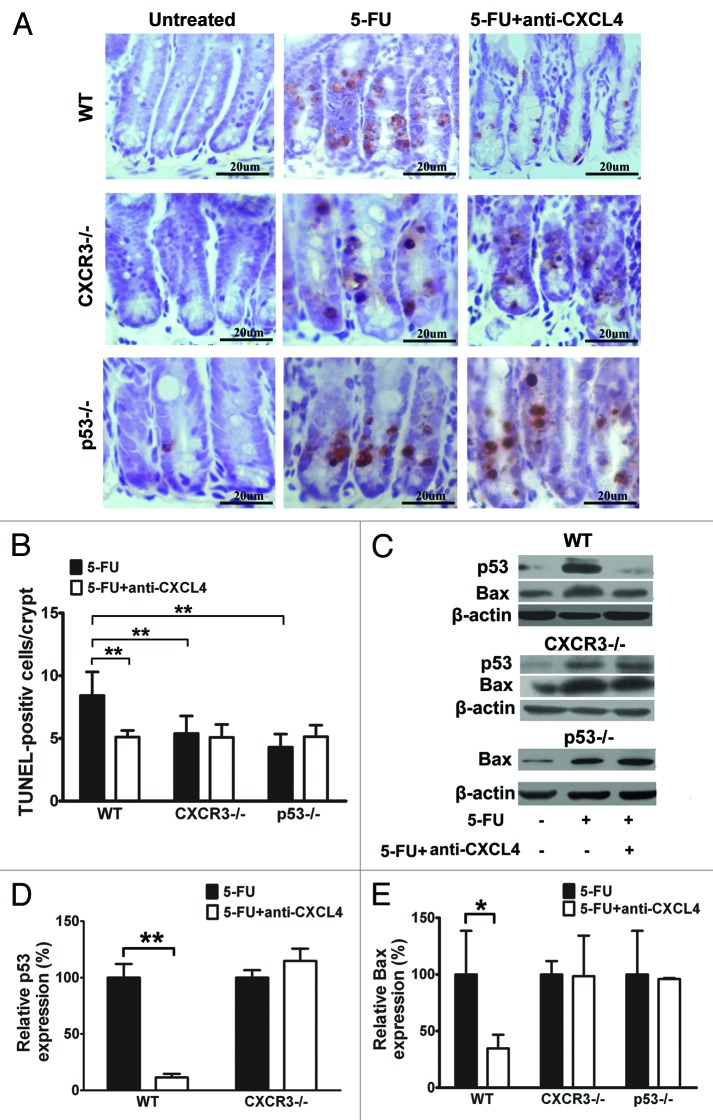
CXCR3 is reported as a functional receptor for CXCL4,17 and is expressed in both human and mouse intestinal epithelia.15 The 5-FU-induced apoptosis of crypt epithelia is dependent upon induction of the p53 tumor suppressor.5 Our finding that CXCL4mab blocks 5-FU-induced crypt apoptosis correlates well with the induction of CXCL4 by 5-FU. To understand the molecular mechanism of the antibody action, cxcr3 and p53 knockout (cxcr3−/−, p53−/−) mice were compared with the same genetic background wild-type (WT) mice C57BL/6. The mice were treated with CXCL4mab 2 h before 5-FU, and examined for their intestinal apoptosis by TUNEL staining 1 d after 5-FU-treatment. The antibody significantly reduced the number of apoptotic crypt epithelia in the WT mice, but not in the cxcr3−/− and p53−/− mice (Fig. 3A and B). Interestingly, the number of apoptotic cells decreased in both knockout mice compared with the WT (P < 0.01). The results suggest that 5-FU-induced apoptosis is partially dependent upon p53, and also newly-identified CXCR3. Thus, CXCL4mab is able to reduce the 5-FU-induced intestinal apoptosis that is mediated by CXCR3 and p53.
Figure 3. Anti-CXCL4 mAb suppresses the induction of p53 and Bax through CXCR3 to reduce the apoptosis of intestinal epithelia by 5-FU. (A) Representative photomicrographs of apoptotic cells in the jejunum from WT, cxcr3−/−, and p53−/− mice. The mice were treated with 1 mg/kg anti-CXCL4 mAb 2 h before 5-FU (300 mg/kg) and sacrificed at 24 h post-5FU for the TUNEL staining of the apoptotic cells. Figure 2C (d–f) and (A) are identical pictures from the same experiment. (B) Quantitative presentation of the TUNEL-positive cells per crypt is shown in (A). Ten crypts per microscope field and 10 fields per mouse were counted for TUNEL-positive cells. (C) Photographs of the western blot analysis of the jejunum tissues from the mice described in (A) with antibodies against p53 and Bax. (D and E) Quantitative presentation of p53 and Bax is shown in (C). The p53 and Bax levels in the antibody treatment group (5-FU+anti-CXCL4 mAb) are expressed as a percent of their respective controls (5-FU). Data are presented as mean ± SD, n = 3 mice per group. *P < 0.05, **P < 0.01 vs. control group.
Crypt epithelia being protected by CXCL4mab from 5-FU damage requires cxcr3 and p53 genetically. Its biochemical mechanism was investigated in a similar experiment design as described above for the genetic study. The cellular levels of p53 and Bax in the jejunum were detected by western blot in WT, cxcr3, and p53 knockout mice after 5-FU treatment. They were compared between the mice treated with or without CXCL4mab for each genotype mice. 5-FU treatment induced the expression of p53 and Bax in all three genotype mice (Fig. 3C). Clearly, the antibody prevented the induction of p53 by 5-FU in the WT mice, which was abolished completely in the cxcr3−/− mice (Fig. 3C and D); it also reduced the induction of Bax by 5-FU in the WT mice, but not in the cxcr3 and p53 knockout mice (Fig. 3C and E). Thus, the 5-FU toxicity to crypt epithelia is at least partially due to its induction of the pro-apoptotic genes p53 and Bax. CXCL4mab protects the epithelia through prevention of p53 and Bax induction by 5-FU, which requires the cxcr3 locus. In addition, the data also suggest that CXCR3 is upstream of p53 in the apoptosis pathway affected by CXCL4mab.
CXCL4 induces apoptosis of intestinal epithelial cell line IEC-6
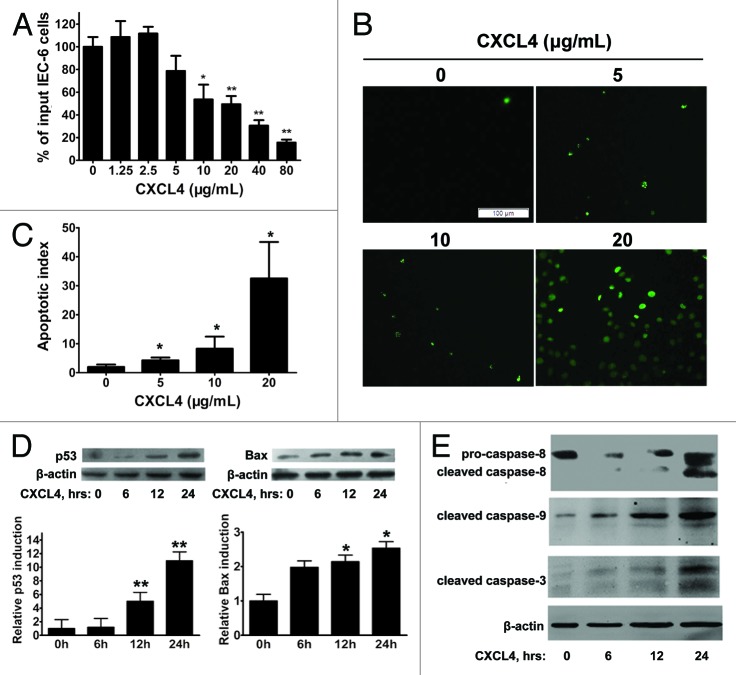
CXCL4mab protects the crypt epithelia from 5-FU-induced apoptosis through preventing the induction of pro-apoptotic genes p53 and Bax. This strongly suggests that the chemokine CXCL4 induced by 5-FU plays the critical role in the initiation of the p53-dependent apoptosis of the epithelia. A rat immortal crypt epithelia cell line, IEC-6, was cultured to test the direct effect of CXCL4. The recombinant human CXCL4 (rhCXCL4) significantly inhibited cell growth, which correlated well with its induction of cellular apoptosis, and both effects of the growth arrest and apoptosis were shown to be dose-dependent (Fig. 4A–C).
Figure 4. CXCL4 induces apoptosis of an intestinal epithelial cell line IEC-6. (A) The cell proliferation of IEC-6 as a percent of input cells in the cell culture. The IEC-6 cell line cultured with the indicated amount of rhCXCL4 was measured in the MTT assay. (B) Representative photomicrographs of apoptotic IEC-6 cells by TUNEL staining. IEC-6 cells were treated with 0, 5, 10, and 20 μg/mL rhCXCL4 for 24 h. (C) The apoptotic index is defined as the average number of TUNEL-positive cells under 5 continuous microscopic fields (200×). (D) Western blot analysis of p53 and Bax. IEC-6 cells were treated with rhCXCL4 (10 μg/mL) for the indicated times. The relative induction of p53 and Bax over the untreated cells is shown after normalization to their respective β-actin loading controls. (E) Western blot analysis of capases. IEC-6 cells were treated with rhCXCL4 (10 μg/mL) for the indicated time. The anti-capase-8 antibody recognizes the full-length (pro-caspase-8) and the cleaved form of caspase-8. Anti-capase-3 and -9 antibodies can only react with the cleaved forms of caspases-3 and -9. The active forms of the capase-3, -8, and -9 were detected. β-actin was used as the loading control. The data represent mean ± SD from three independent experiments. *P < 0.05, **P < 0.01 vs. control group.
The apparent direct apoptotic effect of CXCL4 on the epithelia was further supported by its induction of p53 and Bax. rhCXCL4 elevating the cellular levels of pro-apoptotic gene products in the IEC-6 cells peaked at 24 h after exposure to the chemokine (Fig. 4D). Finally, activation of caspase-3, -8, and -9 were assessed for the IEC-6 cells cultured with rhCXCL4. The activated forms, the cleaved protein fragments, of the three caspases were clearly detected after the incubation of the cells with rhCXCL4 (Fig. 4E). The data suggest that rhCXCL4 induces apoptosis of the epithelia by activation of both extrinsic and intrinsic apoptosis pathways.
CXCL4-induced expression of p53 and Bax is mediated through activation of p38 MAPK
Signal transduction via p38-mitogen-activated protein kinase (MAPK) activation is essential for CXCR3-induced cell death in endothelial cells.18 Additionally, p38-MAPK plays a prominent role in the stabilization of p53, which further regulates p53-mediated apoptosis in response to external stimuli.19
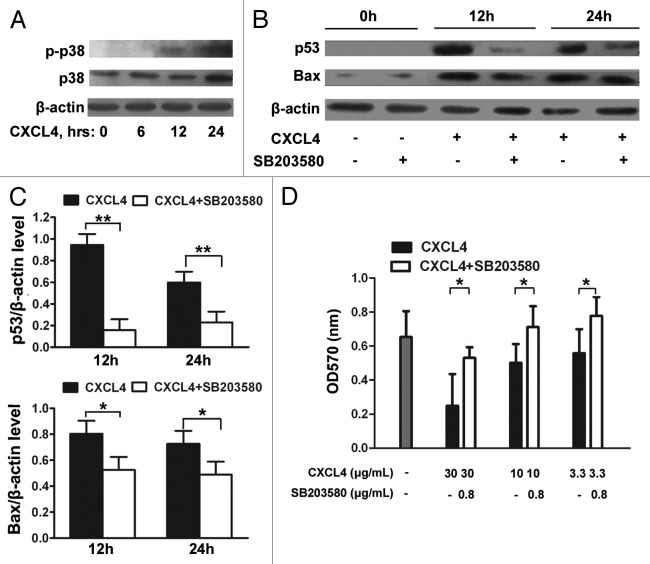
Here, the IEC-6 cell was further examined to confirm the upregulation of p53 and Bax by CXCL4 mediating the apoptosis, and to identify the signals upstream of p53. The activation of p38-MAPK was demonstrated by the detection of the phosphorylated form of p38-MAPK in the rhCXCL4-cultured IEC-6 cells (Fig. 5A). The role of p38-MAPK activation in CXCL4-induced p53 and Bax expression was investigated by the treatment of the cells with SB203580, a specific chemical inhibitor of p38-MAPK. The inhibitor significantly impaired CXCL4-induced expression of p53 and Bax after being cultured for 12 and 24 h (Fig. 5B and C). Functionally, CXCL4-induced apoptosis of IEC-6 cells was blocked by the inhibitor, which rescued cell survival in a dose-dependent manner in the MTT assay (Fig. 5D). Taken together, the results demonstrate that CXCL4 activates p38-MAPK to increase the cellular levels of p53 and Bax, which leads to cellular apoptosis in an intestinal epithelial cell line.
Figure 5. CXCL4-induced expression of p53 and Bax is mediated through activation of p38-MAPK. (A) Western blot analysis of p38-MAPK. IEC-6 cells were treated with rhCXCL4 (10 μg/mL) for the indicated time, and analyzed by western blot using antibodies against phosphor-p38 and p38-MAPK. (B) Western blot analysis of p53 and Bax. The IEC-6 cells were incubated with rhCXCL4 (10 μg/mL) with or without 0.8 μg/mL SB203580, and analyzed by western blot using antibodies against p53, Bax, and β-actin as the loading control. The ratios of p53/β-actin and Bax/β-actin are shown (C). (D) The cell proliferation of IEC-6. IEC-6 cells were treated with the indicated concentrations of rhCXCL4 and SB203580 for 48 h, and analyzed by MTT assay. (C and D) Experiments were repeated three times individually, and the data are presented as mean ± SD *P < 0.05, **P < 0.01 vs. CXCL4-treated group.
Discussion
Previously, to elucidate the relationship between 5-FU distribution and 5-FU-induced apoptosis, microautoradiography was performed on murine intestinal crypts exposed to the [14C]-5-FU. The histologic location of the apoptotic cells in the crypt did not correlate with that of the labeled 5-FU.7 A possible explanation of the result is that 5-FU-induced crypt apoptosis is an indirect effect of 5-FU. Here, we first provide the link between 5-FU and apoptosis. Expression of CXCL4 and its receptor CXCR3 were both induced by 5-FU locally in murine intestine (Fig. 1), which may serve that linkage. CXCL4 has been demonstrated to be expressed by intestinal epithelial cells (IEC) of villi, and upregulated in mesenteric ischemia–reperfusion [IR] injury.14 CXCL4 knockout mice and platelet transfusion studies have demonstrated that CXCL4 is an important mediator of intestinal damage in murine IR injury.14 CXCR3 has also been shown to be expressed in intestinal epithelia, and its expression is elevated in active celiac disease, but returns to the baseline following the removal of the gluten diet.15 Thus, it is possible that the local induction of CXCL4 and its functional receptor CXCR3 mediate reversible intestinal damage in extreme injury/disease conditions.
Multiple genetic loci and signaling pathways may be responsible for 5-FU-induced apoptosis of IEC. In the p53 knockout mice, there is a significant reduction of 5-FU-induced apoptosis of IEC, which demonstrates that the intestinal toxicity of 5-FU is genetically determined by the p53 locus rather than by direct drug action.5,20 In addition to p53-dependent IEC apoptosis, we have also observed that the apoptosis was CXCR3-dependent in the murine 5-FU model (Fig. 3). The antibody-treatment decreased the levels of p53 and Bax, suggesting that CXCL4 is responsible for the observed upregulation of p53 and Bax in vivo (Fig. 3). The loss of responsiveness to the antibody in the cxcr3−/− and p53−/− mice demonstrate that CXCL4-induced apoptosis is mediated by CXCR3 and p53 (Fig. 3). The in vitro IEC cell line experiment provided direct support that CXCL4 directly induced the expression of p53 and Bax, resulting in the apoptosis of IEC (Fig. 4). The expression of Bax has been reported to be accompanied by 5-FU-induced IEC apoptosis.7 To our knowledge, there are no reports in the literature describing the role of CXCL4 in mediating p53-dependent IEC apoptosis.
However, it is noteworthy that there was p53 and CXCR3-independent apoptosis in 5-FU-induced apoptosis of IEC (Fig. 3).5 This is a part of the IEC apoptosis that was not affected by CXCL4mab, cxcr3−/−, or p53−/−. p53 and Bax upregulation were present in the cxcr3−/− mice, and Bax upregulation was also observed in the p53−/− mice, though at much reduced levels than those in the WT mice (Fig. 3). This may be explained by other pathways, such as IL-121 and 5-HT.22 Additional studies exploring multiple apoptotic pathways of CXCL4, IL-1, and 5-HT in murine 5-FU mucositis are currently being conducted in our laboratory.
The chemokines CXCL4, CXCL9, CXCL10, and CXCL11 share the same receptor CXCR3, mediating their chemotactic effect on the activated T lymphocytes.23 CXCR3 is coupled to a G-protein signaling pathway with its downstream protein p38-MAPK.23-26 It has been reported that CXCL10 can trigger apoptosis in several normal cellular models, such as acinar cells,27 neurons,28 microvascular endothelial cells,29 and recently blood T lymphocytes.26 However, the apoptosis pathway of CXCR3 is not yet identified. Here, we report that CXCL4 using the p38-MAPK pathway activates the expression of p53 and Bax, resulting in the apoptosis of IEC (Fig. 5). Thus, to our knowledge, in addition to CXCL10, CXCL4 for the first time is added to the CXCR3 agonist list that can induce apoptosis of a new cell type, IEC. Importantly, the newly-identified apoptotic role of CXCL4 is in the context of the molecular pathology of 5-FU-induced mucositis; therefore, it has potential clinical significance in oncology.
In addition to normal cells, CXCR3 agonists have been reported to induce tumor cell apoptosis. CXCL4 induces cell apoptosis by inhibition of STAT3 via upregulation of SOCS3 expression in multiple myeloma. Interestingly, the receptor mediating CXCL4-induced tumor cell apoptosis is LRP1, not CXCR3.30 Another CXCR3 agonist, CXCL10, also induces HeLa cell apoptosis through a p53-dependent pathway by suppression of the human papillomavirus expression.31 We have tested CXCL4 in the culture of mouse and human colon-rectal cancer cell lines. Unlike the normal intestinal epithelial cell line, where it induced apoptosis (Fig. 4), it had no effect on the survival, growth, and apoptosis of murine and human colon-rectal cancer cell lines (Fig. S1). We have further demonstrated that CXC4mab did not affect tumor growth in a tumor transplantation model of murine colon cancer, CT26-WT (Fig. S2). Thus, the current data support the use of CXCL4mab in treating 5-FU-induced intestinal mucositis in at least colon-rectal cancer. Other types of cancers and their respective chemotherapy regimens will be investigated to determine the scope of indications for the antibody treatment.
CXCL4 was shown to activate both caspase-8, the extrinsic apoptosis pathway,32,33 and caspase-9, the intrinsic mitochondrial apoptosis pathway.34 Activation of caspase-3, downstream of caspase-8 and -9, was also detected in rhCXCL4-treated IEC-6 cells. Activation of the two apoptosis pathways by CXCL4 may be explained by its activation of the p53 expression. It is known that p53 induces apoptosis by multiple mechanisms including upregulation of pro-apoptotic genes of both intrinsic and extrinsic pathways, such as Apaf-1, Bax, and Fas at the transcriptional level, direct promotion of cytochrome-C release from the mitochondria, and Fas relocation to the cell membrane.35
In conclusion, our results add new insight into the mechanisms of IEC apoptosis induced by 5-FU (Fig. 6). CXCL4 is identified to mediate 5-FU-induced intestinal apoptosis. It induces apoptosis by enhancing the expression of p53 and Bax via activation of the p38-MAPK signaling pathway. Blocking CXCL4 protects against 5-FU-induced mucositis by reducing intestinal apoptosis. Therefore, therapeutic approaches that neutralize CXCL4, the newly identified target of CIM, may represent a novel strategy for treating chemotherapy-induced intestinal mucositis.
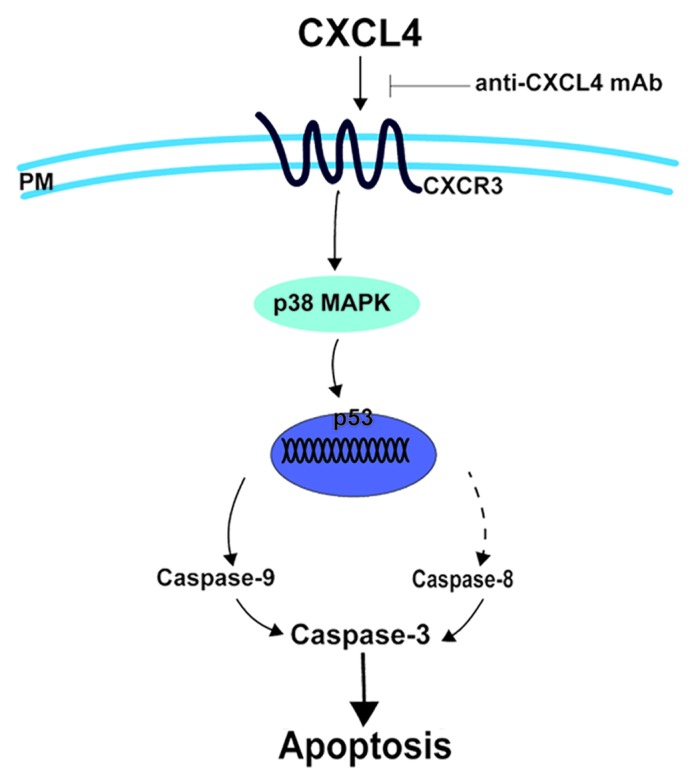
Figure 6. CXCL4 mediates 5-FU-induced apoptosis of the intestinal epithelia. 5-FU induces the local expression of CXCL4, which initiates the intestinal apoptosis pathway. By binding to its CXCR3 receptor, CXCL4 activates p38-MAPK to increase the level of p53, which leads to caspase activation and, ultimately, apoptosis. Anti-CXCL4 mAb effectively blocks the apoptotic role of CXCL4 in 5-FU-induced mucositis, PM: plasma membrane.
Materials and Methods
Mice and reagents
Pathogen-free, male BALB/c and C57BL/6 mice (8–10 wk old, SLACCAS) were maintained in air-filtered units at 23 ± 5 °C with 50 ± 15% relative humidity throughout the experimental period. The cxcr3 (cxcr3−/−) knockout mice (kindly provided by Dr Bao Lu, Harvard Medical School) and the p53 (p53−/−) knockout mice (MARC) generated on the C57BL/6 background were provided with sterile water and rodent chow. Animal experiments were performed with the authorization of the Animal Care and Use Committee of the School of Pharmacy of Shanghai Jiaotong University. 5-FU was purchased from Shanghai Xudong Haipu Pharmaceutical Co., Ltd. Hoechst 33342 (B2261) and propidium iodide (PI, P4170) were purchased from Sigma Chemical. Antibodies against p53 (2524), Bax (2772), p38 (9212), the phosphorylated p38 MAPK (4511), and the cleaved-forms of caspase-3 (9664) and -9 (9507) were from Cell Signaling. Antibodies against CXCL4 (sc-50300), CXCR3 (sc-9902), and β-actin (sc-47778) were from Santa Cruz Biotechnology. Antibody against caspase-8 was from Bioworld Technology (BS1387). Other reagents were from Sigma Chemical. CXCL4 monoclonal antibody (CXCL4mab) and recombinant human CXCL4 (rhCXCL4) were produced internally, and their endotoxin levels were tested using the limulus amebocyte assay (Chinese Horseshoe Crab Reagent Manufactory, Ltd., G052000).
Real-time RT-PCR analysis of cxcl4 and cxcr3
Total RNA was extracted from the small intestine using the Trizol reagent (Invitrogen, 15596-018), and reverse transcription (RT) reactions were performed with the total RNA (2 μg) according to the ExScript RT reagent kit (Takara Bio Inc., RR037A). The product was amplified in a reaction volume of 20 μL containing 9.2 μL RT product, 10 μL SYBR Premix Ex TaqII (2×), and 20 pmol of each primer. PCRs were performed for 40 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s in a 7900HT Fast Real Time RT-PCR System (ABI). All data were normalized to β-actin mRNA levels, and the fold change for each mRNA was calculated using the delta Ct method. Primer sequences for cxcl4, cxcr3, and β-actin are shown as below:
cxcl4: forward primer: 5′-TGAGAGCGAA CATCCCAACA A, reverse primer: 5′- TGTCCCCACG ATCTTCATCT T;
cxcr3: forward primer: 5′-CAGCCTGAAC TTTGACAGAA CCT, reverse primer: 5′-GCAGCCCCAG CAAGAAGA;
β-actin: forward primer: 5′-CTTCCTTAAT GTCACGCACG ATTTC, reverse primer: 5′-GTGGGGCGGC CCAGGCACCA.
Mouse model of 5-FU-induced intestinal mucositis
Intestinal mucositis was induced in BALB/c mice by a single dose of 5-FU (250 mg/kg) intraperitoneally on day 0. Disease severity was assessed daily by recording diarrhea scores on days 0 to 10. Each mouse was examined twice daily. The severity of the diarrhea was scored using the following scale: 0, normal (normal stool or absent); 1, slight (slightly wet and soft stool); 2, moderate (wet and unformed stool with moderate perianal staining of the coat); and 3, severe (watery stool with severe perianal staining of the coat).36,37 The average diarrhea score was used to evaluate the severity of the diarrhea.
For the survival experiment, C57BL/6J mice were administered with either CXCL4mab (1 mg/kg) or saline subcutaneously starting 2 h prior to 5-FU-injection. A single dose of 5-FU (400 mg/kg) was injected on day 0. The mice were examined three times per day for 25 d, and their health status was recorded.
Histology and morphometry
For the assessment of 5-FU-induced intestinal mucositis, the mouse intestinal tract from the pyloric sphincter to the rectum was dissected out, flushed with isotonic saline, and the wet weights of the small intestine were measured on day 3. Segments of the mid-jejunum were harvested and fixed in formaldehyde, embedded in paraffin, stained with hematoxylin and eosin (H&E), and subjected to blinded histological assessment. The villus height and crypt length were measured microscopically.
Cell culture
IEC-6, a rat intestinal epithelial cell line, was purchased from ATCC. IEC-6 cells were cultured in DMEM (Invitrogen, 12800-017) with 5% fetal bovine serum (FBS) and 5 μg/mL insulin. Cells were used at the 17th to 22nd passage for all experiments.
Immunoblotting
For the preparation of western-blot samples, the murine intestine was rapidly ground in liquid nitrogen, and the cultured IEC-6 cells were harvested. The tissue powder or the isolated IEC-6 cells was reconstituted in the ice-cold RIPA buffer with protease and phosphatase inhibitors. Supernatants were recovered after centrifugation, and determined for their protein content using the BCA method (Beyotime Biotechnology, P0010). The sample of 50 mg protein was subjected to sodium dodecyl sulfate-PAGE (SDS-PAGE), and blotted following standard methods with the first and the corresponding second antibodies. The membranes were washed and developed using the ECL-detection system (Beyotime Biotechnology, P0018).
Assessment of apoptosis
Cellular apoptosis was quantified for the intestine and the IEC-6 cell samples using the deoxyuridine triphosphate nick-end labeling (TUNEL) assay. Briefly, tissues were fixed in 4% buffered formaldehyde, embedded in paraffin, and 4 mm-thick sections were attached to glass slides. The tissue sections were stained using the TUNEL agents according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, G002-2). The TUNEL-positive cells were counted under microscope at the magnification of 400 times (400×). Ten crypts per microscope field and 10 fields per mouse were counted for TUNEL-positive cells. Apoptosis in IEC-6 cells were quantified using the TUNEL assay according to the manufacturer’s protocol (Roche, 11684795910). Briefly, the cells were stained and examined directly using fluorescence microscopy. For the quantification of apoptosis, a total of at least 3000 cells were counted for each treatment group.
Statistical analysis
The data are presented as mean ± SD. The parametric and non-parametric data from the two groups are analyzed for significance using the two-tailed Student t test and the Mann–Whitney U test, respectively. A one-way analysis of variance with the Kruskal–Wallis H test is used to compare multiple groups. Results from the survival experiments are analyzed using a log-rank test and are expressed as Kaplan–Meier survival curves. P values less than 0.05 are considered statistically significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author’s Contributions
W.H. and Y.Y. developed the concept for the studies; W.H., Y.Y., and J.G. designed the experiments; Jin G. and J.G. participated in the production of recombinant human CXCL4 protein; X.W. and Y.Z. developed methods to produce the CXCL4 antibody and performed in vivo analysis of CXCL4 function; J.G., Jin G., L.Q., X.W., and Y.Z. performed in vitro assays; H.Y. performed the tumor experiments of CXCL4mab; and W.H., Y.Y., and J.G. wrote the manuscript.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant 81373467/H3108, 81173113/H3109, and 81273573/H3108).
Glossary
Abbreviations:
- 5-FU
5-fluouracil
- Bax
Bcl-2-associated X protein
- CID
chemotherapy-induced diarrhea
- CIM
chemotherapy-induced intestinal mucositis
- CXCL4mab
anti-CXCL4 monoclonal antibody
- IBD
inflammatory bowel disease
- IECs
intestinal epithelial cells
- mAb
monoclonal antibody
- p38-MAPK
p38-mitogen-activated protein kinase
- p53
tumor suppressor protein 53
- rhCXCL4
recombinant human CXCL4
- WT
wild-type
References
- 1.Daniele B, Secondulfo M, De Vivo R, Pignata S, De Magistris L, Delrio P, Palaia R, Barletta E, Tambaro R, Carratù R. Effect of chemotherapy with 5-fluorouracil on intestinal permeability and absorption in patients with advanced colorectal cancer. J Clin Gastroenterol. 2001;32:228–30. doi: 10.1097/00004836-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Duncan M, Grant G. Oral and intestinal mucositis - causes and possible treatments. Aliment Pharmacol Ther. 2003;18:853–74. doi: 10.1046/j.1365-2036.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 3.Bowen JM, Gibson RJ, Cummins AG, Keefe DM. Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support Care Cancer. 2006;14:713–31. doi: 10.1007/s00520-005-0004-7. [DOI] [PubMed] [Google Scholar]
- 4.Anilkumar TV, Sarraf CE, Hunt T, Alison MR. The nature of cytotoxic drug-induced cell death in murine intestinal crypts. Br J Cancer. 1992;65:552–8. doi: 10.1038/bjc.1992.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pritchard DM, Potten CS, Hickman JA. The relationships between p53-dependent apoptosis, inhibition of proliferation, and 5-fluorouracil-induced histopathology in murine intestinal epithelia. Cancer Res. 1998;58:5453–65. [PubMed] [Google Scholar]
- 6.Keefe DM, Brealey J, Goland GJ, Cummins AG. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut. 2000;47:632–7. doi: 10.1136/gut.47.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inomata A, Horii I, Suzuki K. 5-Fluorouracil-induced intestinal toxicity: what determines the severity of damage to murine intestinal crypt epithelia? Toxicol Lett. 2002;133:231–40. doi: 10.1016/S0378-4274(02)00204-7. [DOI] [PubMed] [Google Scholar]
- 8.Kasper B, Petersen F. Molecular pathways of platelet factor 4/CXCL4 signaling. Eur J Cell Biol. 2011;90:521–6. doi: 10.1016/j.ejcb.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Han ZC, Lu M, Li J, Defard M, Boval B, Schlegel N, Caen JP. Platelet factor 4 and other CXC chemokines support the survival of normal hematopoietic cells and reduce the chemosensitivity of cells to cytotoxic agents. Blood. 1997;89:2328–35. [PubMed] [Google Scholar]
- 10.Watson JB, Getzler SB, Mosher DF. Platelet factor 4 modulates the mitogenic activity of basic fibroblast growth factor. J Clin Invest. 1994;94:261–8. doi: 10.1172/JCI117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han ZC, Bellucci S, Walz A, Baggiolini M, Caen JP. Negative regulation of human megakaryocytopoiesis by human platelet factor 4 (PF4) and connective tissue-activating peptide (CTAP-III) Int J Cell Cloning. 1990;8:253–9. doi: 10.1002/stem.5530080409. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava K, Cockburn IA, Swaim A, Thompson LE, Tripathi A, Fletcher CA, Shirk EM, Sun H, Kowalska MA, Fox-Talbot K, et al. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe. 2008;4:179–87. doi: 10.1016/j.chom.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cervi D, Yip TT, Bhattacharya N, Podust VN, Peterson J, Abou-Slaybi A, Naumov GN, Bender E, Almog N, Italiano JE, Jr., et al. Platelet-associated PF-4 as a biomarker of early tumor growth. Blood. 2008;111:1201–7. doi: 10.1182/blood-2007-04-084798. [DOI] [PubMed] [Google Scholar]
- 14.Lapchak PH, Ioannou A, Rani P, Lieberman LA, Yoshiya K, Kannan L, Dalle Lucca JJ, Kowalska MA, Tsokos GC. The role of platelet factor 4 in local and remote tissue damage in a mouse model of mesenteric ischemia/reperfusion injury. PLoS One. 2012;7:e39934. doi: 10.1371/journal.pone.0039934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204, e3. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han X, Wu Z, Di J, Pan Y, Zhang H, Du Y, Cheng Z, Jin Z, Wang Z, Zheng Q, et al. CXCL9 attenuated chemotherapy-induced intestinal mucositis by inhibiting proliferation and reducing apoptosis. Biomed Pharmacother. 2011;65:547–54. doi: 10.1016/j.biopha.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–49. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green LA, Petrusca D, Rajashekhar G, Gianaris T, Schweitzer KS, Wang L, Justice MJ, Petrache I, Clauss M. Cigarette smoke-induced CXCR3 receptor up-regulation mediates endothelial apoptosis. Am J Respir Cell Mol Biol. 2012;47:807–14. doi: 10.1165/rcmb.2012-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, Fornace AJ., Jr. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999;18:6845–54. doi: 10.1093/emboj/18.23.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritchard DM, Watson AJ, Potten CS, Jackman AL, Hickman JA. Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil: evidence for the involvement of RNA perturbation. Proc Natl Acad Sci U S A. 1997;94:1795–9. doi: 10.1073/pnas.94.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu ZQ, Han XD, Wang Y, Yuan KL, Jin ZM, Di JZ, Yan J, Pan Y, Zhang P, Huang XY, et al. Interleukin-1 receptor antagonist reduced apoptosis and attenuated intestinal mucositis in a 5-fluorouracil chemotherapy model in mice. Cancer Chemother Pharmacol. 2011;68:87–96. doi: 10.1007/s00280-010-1451-5. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda M, Kato S, Yamanaka N, Iimori M, Matsumoto K, Utsumi D, Kitahara Y, Amagase K, Horie S, Takeuchi K. 5-HT₃ receptor antagonists ameliorate 5-fluorouracil-induced intestinal mucositis by suppression of apoptosis in murine intestinal crypt cells. Br J Pharmacol. 2013;168:1388–400. doi: 10.1111/bph.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korniejewska A, McKnight AJ, Johnson Z, Watson ML, Ward SG. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology. 2011;132:503–15. doi: 10.1111/j.1365-2567.2010.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahabuddin S, Ji R, Wang P, Brailoiu E, Dun N, Yang Y, Aksoy MO, Kelsen SG. CXCR3 chemokine receptor-induced chemotaxis in human airway epithelial cells: role of p38 MAPK and PI3K signaling pathways. Am J Physiol Cell Physiol. 2006;291:C34–9. doi: 10.1152/ajpcell.00441.2005. [DOI] [PubMed] [Google Scholar]
- 25.Petrai I, Rombouts K, Lasagni L, Annunziato F, Cosmi L, Romanelli RG, Sagrinati C, Mazzinghi B, Pinzani M, Romagnani S, et al. Activation of p38(MAPK) mediates the angiostatic effect of the chemokine receptor CXCR3-B. Int J Biochem Cell Biol. 2008;40:1764–74. doi: 10.1016/j.biocel.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Sidahmed AM, León AJ, Bosinger SE, Banner D, Danesh A, Cameron MJ, Kelvin DJ. CXCL10 contributes to p38-mediated apoptosis in primary T lymphocytes in vitro. Cytokine. 2012;59:433–41. doi: 10.1016/j.cyto.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh L, Arora SK, Bakshi DK, Majumdar S, Wig JD. Potential role of CXCL10 in the induction of cell injury and mitochondrial dysfunction. Int J Exp Pathol. 2010;91:210–23. doi: 10.1111/j.1365-2613.2009.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sui Y, Potula R, Dhillon N, Pinson D, Li S, Nath A, Anderson C, Turchan J, Kolson D, Narayan O, et al. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;164:1557–66. doi: 10.1016/S0002-9440(10)63714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman ED, Weinreich DM, Carroll NM, Burness ML, Feldman AL, Turner E, Xu H, Alexander HR., Jr. Interferon gamma-inducible protein 10 selectively inhibits proliferation and induces apoptosis in endothelial cells. Ann Surg Oncol. 2006;13:125–33. doi: 10.1245/ASO.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 30.Liang P, Cheng SH, Cheng CK, Lau KM, Lin SY, Chow EY, Chan NP, Ip RK, Wong RS, Ng MH. Platelet factor 4 induces cell apoptosis by inhibition of STAT3 via up-regulation of SOCS3 expression in multiple myeloma. Haematologica. 2013;98:288–95. doi: 10.3324/haematol.2012.065607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang HM, Yuan J, Cheung P, Chau D, Wong BW, McManus BM, Yang D. Gamma interferon-inducible protein 10 induces HeLa cell apoptosis through a p53-dependent pathway initiated by suppression of human papillomavirus type 18 E6 and E7 expression. Mol Cell Biol. 2005;25:6247–58. doi: 10.1128/MCB.25.14.6247-6258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 33.Lacour S, Hammann A, Wotawa A, Corcos L, Solary E, Dimanche-Boitrel MT. Anticancer agents sensitize tumor cells to tumor necrosis factor-related apoptosis-inducing ligand-mediated caspase-8 activation and apoptosis. Cancer Res. 2001;61:1645–51. [PubMed] [Google Scholar]
- 34.Nuñez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–45. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 35.Pietsch EC, Sykes SM, McMahon SB, Murphy ME. The p53 family and programmed cell death. Oncogene. 2008;27:6507–21. doi: 10.1038/onc.2008.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrell CL, Bready JV, Rex KL, Chen JN, DiPalma CR, Whitcomb KL, Yin S, Hill DC, Wiemann B, Starnes CO, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998;58:933–9. [PubMed] [Google Scholar]
- 37.Gibson RJ, Bowen JM, Inglis MR, Cummins AG, Keefe DM. Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol. 2003;18:1095–100. doi: 10.1046/j.1440-1746.2003.03136.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.