Abstract
Although concerted efforts have been directed toward eradicating health disparities in the United States, the disease and mortality rates for African American men still are among the highest in the world. We focus here on the role of microRNAs (miRNAs) in the signaling pathways of androgen receptors and growth factors that promote the progression of prostate cancer to more aggressive disease. We explore also how differential expression of miRNAs contributes to aggressive prostate cancer including that of African Americans.
Keywords: African Americans, androgens, growth factors, miRNAs, prostate cancer, receptors
Prostate cancer (PCa) is the most common tumor among men in the United States. Worldwide epidemiological studies have demonstrated that ethnic origin is an important determinant of PCa risk, incidence and disease progression (DeLancey et al. 2008, Jemal et al. 2010). It has been reported that African American men are more likely to develop PCa at an earlier age, which translates to a 60% greater risk of developing PCa, twice the risk of metastatic disease and greater than twice the PCa associated mortality of Caucasian Americans (DeLancey et al. 2008, Wallner et al. 2009). Many factors, including dietary differences, socio-economic environment, lifestyle and access to adequate medical care have been implicated in the aggressiveness of PCa in African Americans (Sanderson et al. 2004, Williams and Powell 2009); however, these variables do not explain the incidence, aggressiveness and mortality associated with PCa among African Americans. It is Important that differential gene expression and molecular features of PCas in African Americans as contributing variables have not been investigated adequately. Over the past several years, several reports have identified molecular factors that may contribute to the aggressiveness of prostatic neoplastic lesions including those in African Americans.
Altered expression of a host of genes is widely believed to underlie tumor development, progression and metastasis. Although differences in genomic features have been identified, the expression of proteins that ultimately causes tumors to develop and progress is regulated at many levels. Furthermore, identification of molecular mechanisms that regulate critical cellular processes, including cell signaling, cell communication, cell cycle control, cell death, hormonal responses and tumor-cell invasion/metastasis, remain elusive. We focus here on the role of multiple signaling pathways associated with the aggressiveness of PCas that are understood to be influenced strongly by post-transcriptional regulation of prostatic neoplasia by microRNAs (miRNAs).
MiRNAs are small non-coding RNAs that can regulate gene expression post-transcriptionally (reviewed by McNally et al. 2013). Hundreds of miRNAs have been identified as regulators of various molecular processes and each miRNA may regulate the expression of many genes. Cancer type-specific miRNAs can function as oncogenes (oncomiRNAs), tumor suppressor genes, and regulators of metastasis (mestastamiRNAs) (Cho 2007, Esquela-Kerscher et al. 2006, Hurst et al. 2009). Few miRNAs have been studied specifically in relation to PCa in African Americans; however, some of the miRNAs and genes that are expressed differentially in PCas of Caucasian Americans likely are important in PCas in African Americans. Also, their actions in African Americans likely are similar to their actions in Caucasian Americans.
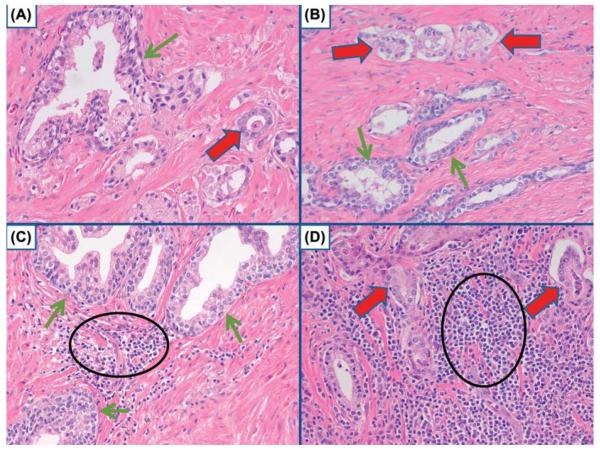
The literature concerning the expression of miRNAs in PCas is mixed; many reports do not identify the same pattern of differential expression of miRNAs between PCas and prostate tissues without cancer (Table 1). This likely is due to several factors that include the lack of homogeneity of the malignant tissues and/or the tissues used as controls as well as the choice of controls (Grizzle et al. 2013, McNally et al. 2013). For example, in many tissues, both malignant and nonmalignant, extensive infiltration by inflammatory cells may occur. Inflammatory cells can produce miRNAs and these may contribute to overall miRNA in the tissues; this could result, for example, in the miRNAs from inflammatory cells being incorrectly attributed to malignant cells, i.e., a false positive result. Uninvolved nonmalignant prostatic glands and stroma also frequently contaminate samples of PCa, or alternatively, neoplastic cells may contaminate control samples of the prostate if controls are obtained from tumor-free regions of prostates with PCa; such factors decrease the clarity of assessment or may cause false positive results. As demonstrated in Fig. 1, infiltrating cancer cells may be mixed with the glands of BPH or PCa. Similarly, uninvolved glands may be mixed with cancer and cancer may be mixed with uninvolved glands.
Table 1.
Examples of miRNAs reported to be inconsistently expressed differentially (different directions)
| MiRNA | References to reports of down-regulation of miRNA in prostate adenocarcinomas (fold change if specified) | References to reports of up-regulation of miRNA in prostate adenocarcinomas (fold change if specified) |
|---|---|---|
| Let-7c | Nadiminty et al. 2012 | Szczyrba et al. 2010 (2.7) |
| Ozen et al. 2008 | ||
| Porkka et al. 2007 | ||
| miRNA-26b | Porkka et al. 2007 1 | Szczyrba et al. 2010 (1.7) |
| miRNA-125b | Szczyrba et al. 2010 (−1.5) | Shi et al. 2007 |
| Ozen et al. 2008 | ||
| miRNA-126 | Carlsson et al. | Szczyrbra et al. 2010 (1.9) |
| miRNA-223 | Szczyrba et al. 2010 | Volinia et al. 2006 |
Only in hormone resistant cases
Fig. 1.
Various mixtures of cell types. A) Benign prostate gland (green arrow) with adjacent PCa (red arrow). B) PCa (red arrows) adjacent to benign prostate glands (green arrows). C) Benign prostate glands (green arrows) with intermixed inflammatory cells, mostly lymphocytes (black circle). D) PCa (red arrows) surrounded by inflammatory cells that primarily are lymphocytes (black circle). Hematoxylin and eosin. x 200.
The results for some miRNAs may vary depending on the preparation of the tissue specimens (e.g., frozen vs. paraffin embedded) or whether data are obtained and reported using only cell lines. The literature indicates that miRNAs in PCa may vary depending on subcategories of PCa, e.g., aggressive vs. non-aggressive, primary vs. metastatic lesions, hormonally responsive PCa vs. hormonally resistant, variations among races and mixtures of these categories (Porkka et al. 2007, Tong et al. 2009, Szczyrba et al. 2010, Watahiki et al. 2011). The molecular features of uninvolved prostate glands also may be influenced by the presence of adjacent tumor (Gaston et al. 2012). Specific molecular subtypes of prostatic adenocarcinomas, e.g., PCas with mutations of p53 and/or with gene fusions such as TMPRESS2:ERG, may produce different patterns of expression of miRNAs. An example of this is miRNA-21, which has been reported by some to be up-regulated in PCa (Volinia et al. 2006, Szczyrba et al. 2010), but not reported by others to be expressed differentially in PCa (Folini et al. 2010). This contradiction may be explained by the reported miRNA-21 suppression of the protein, reversion-inducing cysteine-rich protein with Kazal motifs (RECK), in PCa. While Folini et al. (2010) and Reis et al. (2012) found miRNA-21 to be decreased in the majority of cases of PCa, the latter investigators found that it was selectively overexpressed and RECK, and under-expressed in higher stage (pT3) lesions, especially in metastatic lesions (Watahiki et al. 2011). It is possible also that experimental errors or incorrect interpretations contribute to variable results.
Our review emphasizes the miRNAs that may be subtype dependent, e.g., androgen refractory PCa, as well as miRNAs that have been reported more than once to be involved in PCa and/or to be relevant to PCas in African Americans. We emphasize miRNAs and genes that contribute to aggressive PCa, especially in African Americans, and the role, or putative role, of miRNAs in regulating genes that affect progression and metastasis of PCa.
MiRNAs considered to be regulated differentially in PCas
Several studies have reported that miRNAs are involved in the development and progression of PCas. Specifically, miRNAs are known to be expressed differentially in PCas compared to benign prostatic tissues (Table 2).
Table 2.
Examples of miRNAs reported more consistently to be expressed differentially in prostate adenocarcinomas
| MiRNA | References to consistent reports of down-regulation of miRNA in prostate adenocarcinomas (fold change if specified) | References to consistent reports of up-regulation of miRNA in prostate adenocarcinomas (fold change if specified) |
|---|---|---|
| miRNA-20a | Szczyrba et al. 2010 (1.5) | |
| Volinia et al. 2005 | ||
| miRNA-23b | Tong et al. 2008 (−1.2) | |
| Porkka et al. 2007 1 | ||
| miRNA-25 | Szczyrba et al. 2010 (1.8) | |
| Volinia et al. 2006 | ||
| Ambs et al. 2008 | ||
| miRNA-26a | Carlsson et al. 2011 | |
| Porkka et al. 2007 | ||
| miRNA-27b | Carlsson et al. 2011 | |
| Szczyrba et al. 2010 | ||
| Porkka et al. 2007 1 | ||
| miRNA-29a | Volinia et al. 2005 | |
| Porkka et al. 2007 1 | ||
| Szczyrba et al. 2010 (−2.4) | ||
| Ru et al. 2012 | ||
| Porkka et al. 2007 1 | ||
| Steele et al. 2010 | ||
| miRNA-32 | Volinia et al. 2006 | |
| Ambs et al. 2008 | ||
| miRNA-100 | Tong et al. 2008 (−1.2) | |
| Porkka et al. 2007 1 | ||
| miRNA-106a | Szczyrba et al. 2010 (2.8) | |
| Volinia et al. 2006 | ||
| miRNA-106b | Szczyrba et al. 2010 (2.3) | |
| miRNA-143 | Szczyrba et al. 2010 (−4) | |
| Porkka et al. 2007 | ||
| Carlsson et al. 2011 | ||
| Tong et al. 2008 (−1.3) | ||
| miRNA-145 | Zaman et al. 2010 | |
| Porrka et al. 2007 | ||
| Ozen et al. 2008 | ||
| miRNA-205 | Hulf et al. 2012 | |
| Porkka et al. 2007 1 | ||
| miRNA-221 | Tong et al. 2008 (−1.3) | |
| Porkka et al. 2007 | ||
| miRNA-222 | Tong et al. 2008 (−1.5) | |
| Porkka et al. 2007 |
Only in hormone resistant cases.
When PCa occurs, it is treated first using methods to reduce androgens or the effects of androgens; however, most tumors ultimately become resistant to the withdrawal of androgens. Several miRNAs have been associated with the effects of androgens or modulation of androgen receptors. Examples of such miRNAs are listed in Table 3.
Table 3.
MiRNAs associated with hormonally resistant prostate cancer
| MiRNA | Reference to down-regulation in hormonally resistant prostate cancer | Reference to up-regulation in hormonally resistant prostate cancer |
|---|---|---|
| miRNA-100 | Porkka et al. 2007 | |
| miRNA-124 | Shi et al. 2012 | |
| miRNA-125b | Shi et al. 2007 1 | |
| Porkka et al. 2007 2 | ||
| miRNA-146a | Xu et al. 2012 | |
| Li et al. 2008 | ||
| miRNA-148a | Porkka et al. 2007 | |
| miRNA-198 | Porkka et al. 2007 | |
| miRNA-205 | Porkka et al. 2007 | |
| miRNA-221 | Sun et al. 2009 | |
| miRNA-222 | Sun et al. 2009 | |
| miRNA-345 | Porkka et al. 2007 | |
| miRNA-448 | ||
| miRNA-616 | Ma et al. 2011 |
Cell line
All PCa
MiRNAs appear to play an important role in the progression of PCa to metastatic disease. In a study of miRNAs expressed differentially in a metastatic PCa cell line developed using xenografts, Watahiki et al. (2011) identified 23 miRNAs that were up-regulated more than fivefold in the metastatic line compared to the matching non-metastatic cell line and 24 miRNAs that were down-regulated more than fivefold in the metastatic line. Of the down-regulated miRNAs, miRNA-205, miRNA-503, miRNA-708 and miRNA-2115 that were detected in the non-metastatic cell line, were undetected in the metastatic line. In the metastatic cell line, miRNA-21 was expressed most strongly and miR-148 was expressed most strongly in the non-metastatic cell line. Also, miRNAs from different DNA arms, e.g., 5p and 3p, sometimes showed different expressions between metastatic and non-metastatic subgroups as well as miRNAs with very small differences between their sequences. For example, miRNA-126* was down-regulated in the metastatic cell line (3.4 fold change), but miRNA-126 was increased in the metastatic cell line (14 fold change). Selected results from the study of Watahiki et al. (2011) and other studies are included in Table 4.
Table 4.
Examples of miRNAs expressed differentially in metastatic prostate cancer and also identified by other studies (based primarily on Volinia et al. 2006, Porkka et al. 2007, Tong et al. 2009, Szczyrba et al. 2010, Watahiki et al. 2011, Carlsson et al. 2011)
| MiRNA | Down-regulated in metastatic cell line (fold change if specified) | Previously reported to be differentially expressed in prostate cancer compared to benign prostatic tissue (fold change if specified) | Previously identified as modulating metastasis of prostate cancer | Previously reported as affecting metastasis in a different cancer (e.g., breast cancer) |
|---|---|---|---|---|
| miRNA-16 | (−20) | Down-regulated2 | Yes | Yes |
| miRNA-24 | (−8) | (−1.5) | Yes | |
| miRNA-29a | (−30) | (−2) | ||
| miRNA-34a | (−3) | Down-regulated2 | Yes | Yes |
| miRNA-126* | (−3) | Down-regulated2 | ||
| miRNA-145 | (−3) | (−4) | Yes | Yes |
| miRNA-195 | (−5) | Down-regulated2 | ||
| miRNA-203 | (−1.5) | Down-regulated2 | Yes | Yes |
| miRNA-205 | (NA)1 | Down-regulated2 | Yes | Yes |
| miRNA-221 | Down-regulated2 | (−1.3)(−2) | Yes* | ** |
| miRNA-425 | (−6) | Down-regulated2 |
| miRNA | Up-regulated in metastatic cell line (fold change if specified) | Previously reported to be differentially expressed in prostate cancer compared to benign prostatic tissue (fold change if specified) | Previously identified as modulating metastasis of prostate cancer | Previously reported as affecting metastasis in a different cancer (e.g., breast cancer) |
|---|---|---|---|---|
| Let7i | (2) | Up-regulated2 | Yes | |
| miRNA-21 | (1.7) | Variable reports | Yes | |
| miRNA-106a | (3.3) | (2.8) | Yes |
Cannot be calculated because it was not detectible in metastatic tumors
Extent of differences in expression
Correlated with TMPRESS2:ERG fusion (Gordanpour et al. 2011)
Up-regulated in aggressive breast cancer and hepatocellular carcinoma (Shah and Calin 2011, Fu et al. 2010)
Several of the miRNAs associated with differential expression in metastatic PCa have been associated with genes involved in metastasis. For example, miRNA-205 reduces the protein expression of ZEB1, which is involved in epithelial to mesenchymal transition (EMT), and p63 acts by stimulating miRNA-205 to inhibit ZEB1 and therefore EMT (Tucci et al. 2012). Similarly, miRNA-203 targets and inhibits ZEB2 and other miRNAs involved in metastasis including Runx2, a major participant in bone metastases, and survivin, poly-comb repressor, Bmi and SMAD4 (Saini et al. 2011).
MiRNAs expressed differentially in African American prostate tumors
Calin and Croce (2006) reported that miRNA-1b-1, miRNA-26a and miRNA-30c-1, miRNA-219, and miRNA-301 are expressed differentially in PCas from African Americans compared to those from Caucasian Americans (Calin and Croce 2006). We extended this study using cultures of the novel African American PCa cell lines, RC77N/E, RC77T/E and MDA-2Pca-2b. The expression of miRNA-26a in these cell lines was compared to its expression in the Caucasian American derived cell lines, PrEC, RC-92a and PC-3 (Theodore et al. 2010a,b). Comparing African American to Caucasian American derived cell lines derived from tumors of the same grade and stage, we found a 2.3 fold increase of miRNA26a in nonmalignant cell lines, a 13.4 fold increase in malignant cell lines and 2.4 fold increase in metastatic cell lines. PCa cell lines from African Americans showed the greatest expression of miRNA-26a among all cell lines tested. Our unpublished data indicate that after only 10 min, miRNA-26a is responsive to EGF stimulation and depletion of miRNA-26a induces G2/M arrest and subsequent activation of caspase 3/7. Thus, the increased incidence of PCa associated with the African American race may be associated with regulation of genes associated with apoptosis. For example, Bcl-2, an anti-apoptotic protein and a miRNA-26a target, has altered expression in African American PCa (Guo et al. 2000).
The expression of miRNA-151 in African American men after radical prostatectomy with undetectable postoperative prostate specific antigen has been compared with miRNA-151 expression in African Americans who had radiation or androgen ablation therapy followed by rising prostate specific antigen levels. MiR-151 was increased and the NKX3-1 gene, which it regulates, decreased in 67% of African American patients with rising PSA who were treated with radiation or androgen ablation compared to only 17% of African American patients without increasing PSA who were treated with radical prostatectomy (Barnabas et al. 2011). Similarly, miRNA151 expression has been reported to be correlated with the aggressive characteristics of hepatocellular carcinoma cell lines (Ding et al. 2010). Thus, miRNA-151 eventually might be useful for identifying aggressive PCa in African American men.
MiRNAs, androgens, androgen receptors and race
PCa growth depends on testosterone and its active metabolite, 5α-dihydrotestosterone (DHT). Levels of testosterone and DHT differ among different ethnic groups; both male and female African Americans show greater levels of both hormones from birth through adolescence than Caucasian Americans (Abdelrahaman et al. 2005, Joseph et al. 2002, Winters et al. 2001).
The enzyme, 5-α-reductase type 2, which converts testosterone into DHT, may cause elevated levels of DHT in African Americans, because the activity of the gene, SRD5A2, which codes for this enzyme, is elevated in African Americans (Morissette et al. 1996, Litman et al. 2006, Thomas et al. 2008). Also, genetic variations of the SRD5A2 gene in African American men, such as TA repeat alleles and mis-sense mutations (A49T variant), cause increased activity of 5-α-reductase (Giwercman et al. 2005), which is correlated with increased risk of PCa (Kubricht et al. 1999, Li et al. 2011). In addition, over-expression of 5-α-reductase type 1 causes increased sensitivity to androgens in PCa cells (Thomas et al. 2009).
African American men have fewer G and GGC repeats in the androgen receptor gene than Caucasian American men (Bennett et al. 2002, Irvine et al. 1995, Platz et al. 2000, Sartor et al. 1999); this is correlated with a more active androgen receptor and increased sensitivity to circulating androgens (Beilin et al. 2000, Chamberlain et al. 1994). Increased androgenic activity has been demonstrated in both benign and malignant prostatic tissue in African American men and may explain why African Americans with PCa have lower biochemical failure rates after androgen deprivation therapy (androgen deprivation therapy) than Caucasian Americans (Gaston et al. 2003). It is interesting that African Americans with metastatic PCa whose tumors have become androgen resistant (characteristic of the phenotype that develops under conditions of low androgen receptor levels) exhibit poorer responses to chemotherapy, poorer therapeutic outcomes, and poorer quality of life than comparable Caucasian American patients (Thatai et al. 2004).
As a transcription factor, androgen receptor regulates positively and negatively the expression of hundreds of both coding and noncoding RNA targets, including miRNA-125b, which is up-regulated by androgens. MiRNA-125b suppresses Bak1 and induces androgen-independent growth of LNCaP and the subline, cda1 LNCaP (Shi et al. 2007). Similarly, it has been reported that miRNAs are involved in the androgen receptor signaling pathway. Ribas et al. (2009) demonstrated that androgen receptor binds to the promoter of miRNA-21, which in turn can stimulate both hormone-dependent and -independent PCa growth. Forced expression of miRNA-21 enhanced PCa growth in vivo and enabled androgen-dependent cancer cell lines to overcome castration-mediated growth arrest (Ribas et al. 2009). MiRNA-21 has been reported to be associated with high stage and metastatic PCas (Reis et al. 2012, Li et al. 2012) and serum levels of miRNA-21 have been reported to be elevated in patients with metastatic hormone-refractory PCa (Li et al. 2011, Zhang et al. 2011, Agaoglu et al. 2011). Agaoglu et al. (2011) also reported higher levels of miRNA-221 in the blood of patients with PCa. Although it has not been determined whether there is racially associated expression of miRNA-21, this miRNA could provide valuable insight into the progression of aggressive PCas.
Östling et al. (2011) reported that miRNA-9, miRNA-34a, miRNA-34c, miRNA-135b, miRNA-185, miRNA-297, miRNA-299-3p, miRNA-371-3p, miRNA-421, miRNA-449a, miRNA-449b, miRNA-634, and miRNA-654-5p can bind directly to the 6kB extended arm of the 3′ UTR of androgen receptor. The majority of these miRNAs regulate androgen receptor primarily through the extended arm region, and miRNA-34a and miRNA-34c correlate negatively with androgen receptor expression levels in clinical cases of PCa (Östling et al. 2011). MiRNA-34a also was demonstrated to be a key regulator of the CD44 positive putative stem cell population in PCas (Östling et al. 2011, Liu et al. 2011). Given the important role of cancer stem cells (CSC) in development of PCa, miRNA-34a could be a novel therapeutic target for PCa (Fujita et al. 2008).
The expression and activity of androgen receptor plays not only a central role in development of PCa, but also is important for the development of castration-resistant or hormonally resistant PCa (HRPC). The let 7 family of miRNA's have been shown to play a pivotal role in PCa. Let-7c is a key suppressor of androgen receptor expression by targeting c-Myc. (Chang et al. 2009). Down-regulation of let-7c in PCa specimens is correlated inversely with androgen receptor expression, while the expression of Lin-28, a repressor of let-7, is correlated positively with androgen receptor expression. Furthermore, suppression of androgen receptor by let-7c causes decreased proliferation of PCa cell lines (Nadiminty et al. 2010) and miRNA-146a behaves similarly by targeting the androgen receptor directly (Lin et al. 2008). The miRNAs that affect or are affected by androgens including let7c, miRNA-34a, miRNA-34c, and miRNA-146a have not been evaluated directly in PCa of African Americans, but given the clear contribution of androgen receptor signaling to aggressive PCa in African American men, more investigation in this area is warranted.
Although once viewed as separate events in the development and progression of PCa, Sun et al. (2012) found a relationship between epithelial to mesenchymal transition (EMT) and androgen-deprivation therapy; this is discussed below.
MiRNAs and growth factor expression
The growth factors and their receptors that have been reported to be involved in PCa include members of the epidermal growth factor (EGF), scatter factor/hepatocyte growth factor (SF/HGF), transforming growth factor beta (TGFβ) and basic fibro-blast growth factor (bFGF) families. These growth factors are responsible for modulating cellular differentiation, migration, proliferation and cell death, e.g., apoptosis. EGFR has been implicated in epithelial cell malignant transformation in most PCa cell lines in which androgen-independent cells, e.g., DU145, express more EGFR than the androgen dependent cell lines (De Miguel et al. 1999, Kim et al. 1999, Turner et al. 1996). Membrane-specific EGFR has been reported to be over-expressed in prostate tumors from African Americans and to be correlated with higher pre-treatment levels of prostate specific antigen and higher stage tumors (Shuch et al. 2004, Douglas et al. 2006). Douglas et al. (2006) also reported four novel mis-sense mutations in exons 19, 20 and 21 of the EGFR tyrosine kinase domain in Koreans and Caucasian Americans, but none of these mutations was found in African Americans (Douglas et al. 2006). It is interesting that in hormonally resistant PCa, miRNA-146a, binds to the 3′-UTR of the mRNA of EGFR and inhibits its downstream signaling, e.g., pERK1/2 and MMP2. The expression of miRNA-146a was decreased in HRPC compared to androgen-dependent PCa (ADPC)(Li et al. 2008, Xu et al. 2012). This correlates with increased expression of EGFR in DU145 cells. Similarly, miRNA-146a down-regulates EGFR in pancreatic cancer (Li et al. 2010). MiRNA-132, which targets heparin-binding epidermal growth factor and TALIN2, is thought to control cellular adhesion and to play a role in suppressing metastases in PCa (Formosa et al. 2012).
In PCa, autocrine signaling by EGFR leads to dysregulation of various signaling cascades that have been shown to be dysregulated in PCa in African Americans. For example, over-expression of EGFR associated Son of Sevenless homolog 1 (SOS1), activator of Ras/MAPK and downstream MMP proteins, have been linked with PCa in African Americans (Hatcher et al. 2009, Shuch et al. 2004, Timofeeva et al. 2009). Recently, we demonstrated that Kaiso, a BTB-POZ methylation binding protein, is up-regulated in late stage prostate tumors, and this is the result of EGFR signaling (Jones et al. 2012). In addition to EGFR, TGFα, HER2 and HER3 also are expressed in PCa (Myers et al. 1994, 1995). MiRNA-331-3p binds to the 3′ UTR of the mRNA for HER2, inhibits the expression of HER2 and indirectly reduces androgen receptor signaling by decreasing HER2 (Epis et al. 2009).
Androgen deprivation therapy has been shown to enhance EMT in LnCaP cells and to promote chemoresistance by ZEB1, which mediates androgen deprivation-induced EMT by a bi-directional, negative feedback loop including miRNA-200. Expression of ZEB1 and ZEB2, which facilitate development of EMT, are regulated negatively by five members of the miRNA-200 family (miRNA-141, miRNA-200a, miRNA-200b, miRNA-200c, and miRNA-429), which are highly homologous. These miRNAs have been reported to bind directly to the 3′ UTR of ZEB1 and to cause degradation of its mRNA, which results in up-regulation of E-cadherin in cell lines of several cancers (Burk et al. 2008, Wellner et al. 2009, Gregory et al. 2011).
Several reports have shown that EGFR and platelet-derived growth factor receptor (PDGF-R) influence the expression of the miRNA-200 family. By their regulation of the EMT process, the miRNA-200 family affects sensitivity to therapy focused on EGFR and its signal transduction pathway in cell lines of several cancers including those of the bladder, breast, lung and pancreas. In general, there are both autocrine and paracrine signaling networks that involve TGFβ and the miRNA-200 family; a ZEB/MiR-200 balance shifts cells between epithelial and mesenchymal phenotypes (Gregory et al. 2011). The re-expression of miRNA-200s is sufficient to reverse the mesenchymal phenotype to an epithelial profile (Korpal et al. 2008) and to re-establish EGFR dependency in several cell lines of various cancers. ZEB1 also represses the epithelial phenotype in PCa cells and therefore facilitates cellular transendothelial migration (Drake et al. 2009).
Platelet-derived growth factor-D (PDGF-D) has been shown to induce EMT in PC-3 cells and to decrease the expression of members of the miRNA-200 family. Specifically, PDGF-D induces EMT by up-regulation of ZEB1, ZEB2 and Snail by down-regulation of miRNA-200a, miRNA-200b and miRNA-200c. TGFβ increases the expression of ZEBs and DNA methylation of miRNA-200s, while miRNA-200s inhibit ZEBs (Gregory et al. 2011). When miRNA-200b was re-expressed in PC-3 cells, ZEB1, ZEB2, and Snail decreased and this decrease caused a reversal of EMT; EMT has been associated with decreased cellular migration and invasion as well as changes in cancer stem-like cells (Kong et al. 2008, 2009, 2010). In breast cancer, miRNA-221/222 opposes the actions of the miRNA-200s (Howe et al. 2012), so the effects of the miRNA-200s in PCa might be reversed by miRNA-221/222. Confirmation of this in PCa would be useful for understanding how the actions of specific miRNAs can be generalized. The interactions between the miRNA-200 family and ZEB1, ZEB2 and Snail have been reported for several types of tumors, so this seems to be a general feature of EMT regulation of several types of cancer. MiRNA-203 and miRNA-205 also are likely to regulate EMT (Tucci et al. 2012, Vandenabeele et al. 2012, Saini et al. 2011).
In addition to EGFR and PDGF-R, activation of IGFR is involved in modulating EMT. High serum levels of insulin-like growth factor-I (IGF-1) and lower levels of IGF binding protein-3 (IGFBP-3) have been shown to be associated with increased risk of PCa (Chan et al. 2000, Hernandez et al. 2007, Tricoli et al. 1999). In a cohort of 401 patients with PCa and 366 age, sex and ethnicity matched controls, it was found that variations in the 5′-untranslated region of both the IGF-1 and IGFBP-3 genes may have increased IGF serum levels and that the increased levels were correlated with PCa in African Americans (Hernandez et al. 2007, Yates et al. 2005, 2007). As with EGFR, we and others have demonstrated that activation of IGFR-1 is responsible for the loss of cell-cell adhesion, which leads to EMT promotion of metastasis (Graham et al. 2008, Yates et al. 2007) by increased Snail, ZEB1 and ZEB2 (Graham et al. 2008). Interestingly, in metastatic pancreatic cancer cell lines, IGF-R1 is regulated positively by miRNA-100; both miRNA-100 and IGF-R1 are increased in pancreatic cancer cell lines that have the potential to metastasize, but not in pancreatic cancer cell lines that do not have the potential to metastasize (Huang et al. 2013).
MiRNAs and regulation of oncogenes, suppressor genes and other genes that affect PCa
Several miRNAs may affect the growth and progression of PCa by interactions with oncogenes, suppressor genes and genes involved in progression and metastasis. For example, miRNA-221 and miRNA-222 target the mRNA of the suppressor gene, androgen receptor, ARHI (Chen et al. 2011); it is interesting that genistein down-regulates miRNA-221 and miRNA-222 and by this pathway decreases androgen receptor, ARHI. MiRNA-221 and miRNA-222 also down-regulate the androgen-sensitive cell cycle inhibitor, p27kip-1 (Myers et al. 1999, Sun et al. 2009). Similarly, by analogy to breast cancer, miRNA-221 and miRNA-222 might counter the effects of the miRNA-200 family on EMT (Howe et al. 2012). The targets of the miRNA-200 family also include ERRFI-1, which is a novel regulator of EGFR-independent growth. Similarly, the protein, high motility group AT-hockgene 1(HMGA1), is a nuclear binding protein that has oncogenic properties by facilitating chromosomal instability. HMGA1 is increased in high grade and advanced stage PCa. The expression of HMGA1 is inhibited by miRNA-296, which acts with HMGA1 to inhibit proliferation (Wei et al. 2011). It is noteworthy that the expressions of miRNA-1 and miRNA-133a are decreased in PCa and that these inhibit purine nucleoside phosphorylase (PNP), which facilitates proliferation, migration and invasion of the PC3 and DU145 PCa cell lines. Thus, miRNA-1 and miRNA-133a act as suppressor genes.
MiRNA-616 induces androgen independent growth by targeting tissue factor pathway inhibitor 2 (TFPI-2). MiRNA-101 may reduce the growth of PCa by inhibiting the expression of Cox2 by targeting the 3′ untranslated region (3′-UTR) of the mRNA of Cox2 (Hao et al. 2011).
MiRNAs and epigenetic control of PCa
Epigenetic control of PCa involves specific miRNAs that are targets, i.e., epigenetically regulated (Table 5), and/or some miRNAs directly affect enzymes and other molecules involved in epigenetic control (Paone et al. 2011). Examples of miRNAs that modulate epigenetic control in PCa include miRNA-101, which inhibits the oncogenetic protein enhancer of zeste homolog 2 (EZH2) and miRNA-449a, which down-regulates histone deacetylase 1 (Varambally et. al. 2008, Cao et al. 2010, Noonan et al. 2009). Similarly, miRNA-34b down-regulates DNA methyl transferases and histone deacetylases (Majid et al. 2012). Regulation of miRNAs by methylation of CpG islands in their promoters may be quite variable in PCa. For example, only 42% of PCas, which had methylated promoters for miRNA-132 were also correlated with both Gleason scores and stages of the tumors (Formosa et al. 2012).
Table 5.
Actions of miRNA on oncogenes and suppressor genes in prostate cancer
| MiRNA | MiRNAs down-regulated by hypermethylation of their control elements | MiRNAs with hypomethylated control elements |
|---|---|---|
| miRNA-34a | Kong et al. 2012 | |
| miRNA-145 | Suh et al. 2011 | |
| miRNA-124 | Lujambio et al. 2007 | |
| Shi et al. 2012 | ||
| miRNA-196b | Hulf et al. 2011 | |
| miRNA-126 | Saito et al. 2009 | |
| miRNA-200c | Vrba et al. 2010 | |
| miNAR-141 | Vrba et al. 2010 | |
| miRNA-615 | Hulf et al. 2011 |
MiRNA therapy for PCa
Because of the involvement of miRNAs in regulating the androgen receptor, it would be logical to expect that targeting selected miRNAs or up-regulation of specific miRNAs might be important for androgen deprivation therapy. The development of “antagomiRNAs,” which are small oligonucleotides chemically engineered to target small nucleotide regions of specific miRNAs to selectively silence the targeted miRNA, should lead to approaches that make androgen deprivation therapy more effective initially and that extend the response of PCa to androgen deprivation therapy (Krützfeldt et al. 2005). Similarly, the involvement of miRNAs in modulating responses to chemotherapeutic drugs could be important for therapies for PCa.
When PCas fail androgen deprivation therapy, chemotherapy is used primarily for palliative therapy and to extend survival. Thus, it would be interesting to determine whether modulating specific miRNAs might make chemotherapeutic agents more effective and reduce and/or retard the development of chemoresistance to specific drugs. In this regard, the ectopic expression of miRNA-21 has been associated with resistance to docetaxel in PC3 cells (Shi et al. 2010). By contrast, ectopic expression of miRNA-34a decreases the phenotypic expression of the protein, silent mating type information regulation 2/homolog 1 (S. conevisiae) (SIRT1) as well as Bcl-2 and HUR., These changes enhanced the chemosensitivity of PC3 cells to camptothecin (Fajita et al. 2008) and PC3 paclitaxel resistant cells (PC3PR) to paclitaxel (Kojima et al. 2010). Also, chemoresistance may be induced by miRNA stimulation of specific pathways such as hedgehog (Singh et al. 2012). In addition, miRNAs such as miRNA-210 modulate a stress response to therapeutic approaches (Cui et al. 2012). These and similar studies suggest that miRNAs are involved in both chemosensitivity and development of chemoresistance, hence their modulation may improve therapy for PCa.
Several signaling pathways appear to be dysregulated at the post-transcriptional level in PCa. The involvement of miRNAs in molecular regulation of genes and underlying tumor biology could explain the aggressiveness observed in some PCa; however, more insight into the post-transcriptional regulation of the genes involved in the development and progression of PCa is needed before miRNAs can be used for therapeutic targeting and as biomarkers. There are a few reports that regulation of gene expression by miRNAs contributes to aggressive disease in African American patients. More work is needed to explore miRNA mediated gene regulation that affects the African American population disproportionally.
Acknowledgments
This work was funded in part by the G12 RR03059-21A1 (NIH/RCMI), a pilot project on U54 Caucasian American118623 (NIH/NCI), and by DOD Grant W81XWH-10-1-0543.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdelrahaman E, Raghavan S, Baker L, Weinrich M, Winters SJ. Racial difference in circulating sex hormone-binding globulin levels in prepubertal boys. Metabolism. 2005;541:91–96. doi: 10.1016/j.metabol.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Agaoglu FY, Kovancilar M, Dizdar Y, Darendeliler E, Holdenrieder S, Dalay N, Grezer U. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumor Biol. 2011;32:583–588. doi: 10.1007/s13277-011-0154-9. [DOI] [PubMed] [Google Scholar]
- Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, Yfantis HG, Stephens RB, Croce CM. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68:6162–6170. doi: 10.1158/0008-5472.CAN-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabas N, Xu L, Savera A, Hou Z, Barrack ER. Chromosome 8 markers of metastatic prostate cancer in African American men: gain of the MIR151 gene and loss of the NKX3-1 gene. Prostate. 2011;718:857–871. doi: 10.1002/pros.21302. [DOI] [PubMed] [Google Scholar]
- Beilin J, Ball EM, Favaloro JM, Zajac JD. Effect of the androgen receptor CAG repeat polymorphism on transcriptional activity: specificity in prostate and non-prostate cell lines. J. Mol. Endocrinol. 2000;251:85–96. doi: 10.1677/jme.0.0250085. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Price DK, Kim S, Liu D, Jovanovic BD, Nathan D, Johnson ME, Montgomery JS, Cude K, Brockbank JC, Sartor O, Figg WD. Racial variation in CAG repeat lengths within the androgen receptor gene among prostate cancer patients of lower socioeconomic status. J. Clin. Oncol. 2002;2017:3599–3604. doi: 10.1200/JCO.2002.11.085. [DOI] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;96:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;611:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- Cao P, Deng Z, Wan M, Huang W, Cramer SD, Xu J, Lei M, Sui G. MicroRNa-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1α/HIF-1β. Molec. Cancer. 2010;9:108. doi: 10.1186/1476-4598-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J, Davidsson S, Helenius G, Karlsson M, Lubovac Z, Andrén O, Olsson B, Klinga-Levan K. A miRNA expression signature that separates between normal and malignant prostate tissues. Cancer Cell Int. 2011;11:14. doi: 10.1186/1475-2867-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucl. Acids Res. 1994;2215:3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Giovannucci E, Ma J, Pollak M. Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and prostate cancer risk: epidemiological studies. Growth Horm. IGF Res. 2000;10(Suppl A):S32–33. doi: 10.1016/s1096-6374(00)90015-7. [DOI] [PubMed] [Google Scholar]
- Chang TC, Zeitels LR, Hwang HW, chivukula RR, Wentzel EA, Dews M, Jung J, Gao P, Dang CV, Beer MA, Thomas-Tikhonenko A, Mendell JT. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc. Natl. Acad. Sci. USA. 2009;1069:3384–3389. doi: 10.1073/pnas.0808300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zaman MS, Deng G, Majid S, Saini S, Liu J, Tanaka Y, Dahhiya R. MicroRNAs 221/222 and genistein-mediated regulation of ARHI tumor suppressor gene in prostate cancer. Cancer Prev. Res. 2011;4:76–86. doi: 10.1158/1940-6207.CAPR-10-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol. Cancer. 2007;6:60. doi: 10.1186/1476-4598-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Grosso S, Schelter F, Mari B, Krüger A. On the pro-metastatic stress response to cancer therapies: evidence for a positive co-operation between TIMP-1, HIF-1α, and miR-210. Front. Pharmacol. 2012;3:134. doi: 10.3389/fphar.2012.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel P, Royuela, Bethencourt R, Ruiz A, Fraile B, Paniagua R. Immunohistochemical comparative analysis of transforming growth factor alpha, epidermal growth factor, and epidermal growth factor receptor in normal, hyperplastic and neoplastic human prostates. Cytokine. 1999;119:722–727. doi: 10.1006/cyto.1998.0443. [DOI] [PubMed] [Google Scholar]
- DeLancey JO, Thun MJ, Jemal A, Ward EM. Recent trends in black-white disparities in cancer mortality. Cancer Epidemiol. Biomarkers Prev. 2008;1711:2908–2912. doi: 10.1158/1055-9965.EPI-08-0131. [DOI] [PubMed] [Google Scholar]
- Ding J, Huang S, Wu S, Zhao Y, Liang L, Yan M, Ge C, Yao J, Chen T, Wan D, Wang H, Gu J, Yao M, Li J, Tu H, He X. Gain of miR-151 on chromosome 8q24.3 facilitates tumour cell migration and spreading through downregulating RhoGDIA. Nat. Cell Biol. 2010;12:390–399. doi: 10.1038/ncb2039. [DOI] [PubMed] [Google Scholar]
- Douglas DA, Zhong H, Ro JY, Oddoux C, Berger AD, Pincus MR, Satagopan JM, Gerald WL, Scher HI, Lee P, Osman I. Novel mutations of epidermal growth factor receptor in localized prostate cancer. Front. Biosci. 2006;11:2518–2525. doi: 10.2741/1986. [DOI] [PubMed] [Google Scholar]
- Drake JM, Strohbehn G, Bair TB, Moreland JG, Henry MD. ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol. Biol. Cell. 2009;20:2207–2217. doi: 10.1091/mbc.E08-10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epis MR, Giles KM, Barker A, Kendrick TS, Leedman PJ. miR-331-3p regulates ERBB-2 expression and androgen receptor signaling in prostate cancer. J. Biol. Chem. 2009;284:24696–24704. doi: 10.1074/jbc.M109.030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;64:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Foliini M, Gandellini P, Longoni N, Profumo V, Callari M, Pennati M, Colecchia M, Supino R, Veneroni S, Salvioni R, Valdagni R, Daidone MG, Zaffarino N. miR-21: an oncomir on strike in prostate cancer. Molec. Cancer. 2010;9:12. doi: 10.1186/1476-4598-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa A, Lena AM, Markert EK, Cortelli S, Miano R, Mauriello A, Croce N, Vandesompele J, Mestdagh P, Finazzi-Argro E, Levine AJ, Melino G, Bernardidni S, Candi E. DNA methylation silences miR-132 in prostate cancer. Oncogene. 2012;32:127–134. doi: 10.1038/onc.2012.14. [DOI] [PubMed] [Google Scholar]
- Fu X, Wang Q, Chen J, Huang X, Chen X, Cao L, Tan H, Li W, Zhang L, Bi J, Su Q, Chen L. Clinical significance of miR-221 and its inverse correlation with p27Kip1 in hepatocellular carcinoma. Molec. Biol. Rept. 2011;38:3029–3035. doi: 10.1007/s11033-010-9969-5. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Kojima K, Hamada N, Ohhashi R, Akao Y, Nozawa Y, Deguchi T, Ito M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem. Biophys. Res. Commun. 2008;377:114–119. doi: 10.1016/j.bbrc.2008.09.086. [DOI] [PubMed] [Google Scholar]
- Gaston KE, Kim D, Singh S, Ford OH, 3rd, Mohler JL. Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer. J. Urol. 2003;1703:990–993. doi: 10.1097/01.ju.0000079761.56154.e5. [DOI] [PubMed] [Google Scholar]
- Giwercman YL, Abramsson PA, Giwercman A, Gadaleanu V, Ahlgren G. The 5 alpha-reductase type II A49T and V89L high-activity allelic variants are more common in men with prostate cancer compared with the general population. Eur. Urol. 2005;484:679–685. doi: 10.1016/j.eururo.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Gordanpour A, Stanimirovic A, Nam RK, Moreno CS, Sherman C, Sugar L, Seth A. miR-221 is down-regulated in TMPRSS2:ERG fusion-positive prostate cancer. Anticancer Res. 2011;31:403–410. [PMC free article] [PubMed] [Google Scholar]
- Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW, O'Regan RM. Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;687:2479–2488. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- Gregory PA, Bracken CP, Smith E, Berg AG, Wright JA, Roslan S, Morris M, Watt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Kew-Goodall Y, Goodall GJ. An autocrine TGF-β/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Molec. Biol. Cell. 2011;22:1686–1698. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Sigman DB, Borkowski A, Kyprianou N. Racial differences in prostate cancer growth: apoptosis and cell proliferation in Caucasian and African-American patients. Prostate. 2000;422:130–136. doi: 10.1002/(sici)1097-0045(20000201)42:2<130::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hao Y, Gu X, Zhao Y, Greene S, Sha W, Smoot DT, Califano J, Wu TC, Pang X. Enforced expression of miR-101 inhibits prostate cancer cell growth b modulating the COX-2 pathway in vivo. Cancer Prev. Res. 2011;4:1073–83. doi: 10.1158/1940-6207.CAPR-10-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher D, Daniels G, Osman I, Lee P. Molecular mechanisms involving prostate cancer racial disparity. Am. J. Transl. Res. 2009;13:235–248. [PMC free article] [PubMed] [Google Scholar]
- Hernandez W, Grenade C, Santos ER, Bonilla C, Ahaghotu C, Kittles RA. IGF-1 and IGFBP-3 gene variants influence on serum levels and prostate cancer risk in African-Americans. Carcinogenesis. 2007;2810:2154–2159. doi: 10.1093/carcin/bgm190. [DOI] [PubMed] [Google Scholar]
- Howe EN, Cochrane DR, Richer JK. The miR-200 and miR-221/222 microRNA families: opposing effects on epithelial identity. J. Mam. Gland Biol. Neoplasia. 2012;17:65–77. doi: 10.1007/s10911-012-9244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JC, Egger ME, Grizzle WE, McNally LR. MicroRNA-100 regulates IGF1-receptor expression in metastatic pancreatic cancer cells. Biotech & Histochem. 2013 doi: 10.3109/10520295.2012.762460. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulf T, Sibbritt T, Wiklund ED, Bert S, Strbenac D, Statham AL, Clark SJ. Discovery pipeline for epigenetically deregulated miRNAs in cancer integration of primary miRNA transcription. BMC Genom. 2011;12:54. doi: 10.1186/1471-2164-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulf T, Sibbritt T, Wiklund ED, Patterson K, Song JZ, Stirzaker C, Qu W, Nair S, Horvath LG, Armstrong NJ, Kench JG, Sutherland RL, Clark SJ. Epigenetic-induced repression of microRNA-205 is associated with MED1 activation and a poorer prognosis in localized prostate cancer. Oncogene. 2012 Aug 6; doi: 10.1038/onc.2012.300. 2012; doi:10.1038/onc.2012.300. [DOI] [PubMed] [Google Scholar]
- Hurst DR, Edmonds MD, Welch DR. Metastamir: the field of metastasis-regulatory microRNA is spreading. Cancer Res. 2009;6919:7495–7498. doi: 10.1158/0008-5472.CAN-09-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine RA, Yu MC, Ross RK, Coetzee GA. The CAG and GGC microsatellites of the androgen receptor gene are in linkage disequilibrium in men with prostate cancer. Cancer Res. 1995;559:1937–1940. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J. Clin. 2010;605:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Jones J, Wang H, Zhou J, Hardy S, Turner T, Austin D, He Q, Wells A, Grizzle WE, Yates C. Nuclear Kaiso indicates aggressive prostate cancers and promotes migration and invasiveness of prostate cancer cells. Am. J. Pathol. 2012;181:1836–1846. doi: 10.1016/j.ajpath.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph MA, Wei JT, Harlow SD, Cooney KA, Dunn RL, Jaffe CA, Montie JE, Schottenfeld D. Relationship of serum sex-steroid hormones and prostate volume in African American men. Prostate. 2002;534:322–329. doi: 10.1002/pros.10154. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kassis J, Souto JC, Turner T, Wells A. EGF receptor signaling in prostate morphogenesis and tumorigenesis. Histol. Histopathol. 1999;144:1175–1182. doi: 10.14670/HH-14.1175. [DOI] [PubMed] [Google Scholar]
- Kojima K, Fujita Y, Nozawa Y, Deguchi T, Ito M. miR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate. 2010;70:101–1512. doi: 10.1002/pros.21185. [DOI] [PubMed] [Google Scholar]
- Kojima S, Chiyomaru T, Kawakami K, Yoshino H, Enokida H, Nohata N, Fuse M, Ichikawa T, Naya Y, Nakagawa M, Seki N. Tumour suppressors miR-1 and miR-132a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br. J. Cancer. 2012;106:405–413. doi: 10.1038/bjc.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Wang Z, Sarkar SH, Li Y, Banerjee S, Saliganan A, Kim HR, Cher ML, Sarkar FH. Platelet-derived growth factor-D overexpression contributes to epithelial mesenchymal transition of PC3 prostate cancer cells. Stem Cells. 2008;26:1425–1435. doi: 10.1634/stemcells.2007-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HRC, Sarkar FH. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–1721. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Banerjee S, Ahmad A, Liu Y, Wang Z, Sethi S, Sarkar FH. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One. 2010;5:e12445. doi: 10.1371/journal.pone.0012445. doi: 10.1371/journal. pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Heath E, Chen W, Cher M, Powell I, Heilbrun L, Li Y, Ali S, Sethi S, Hassan O, Hwang C, Gupta N, Chiale D, Sakr WA, Menon M, Sarkar FH. Epigenetic silencing of miR-34a in human prostate cancer cells and tumor tissue specimens can be reversed by BR-DIM treatment. Am. J. Transl. Res. 2012;4:14–23. [PMC free article] [PubMed] [Google Scholar]
- Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008;28322:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschi T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with `antagomirs.'. Nature. 2005;438 doi: 10.1038/nature04303. doi:10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- Kubricht WS, III, Williams BJ, Whatley T, Pinckard P, Eastham JA. Serum testosterone levels in African-American and white men undergoing prostate biopsy. Urology. 1999;546:1035–1038. doi: 10.1016/s0090-4295(99)00290-3. [DOI] [PubMed] [Google Scholar]
- Li H, Zhou J, Miki J, Furusato B, Gu Y, Srivastava S, McLeod DG, Vogel JC, Rhim JS. Telomeraseimmortalized non-malignant human prostate epithelial cells retain the properties of multipotent stem cells. Exp. Cell. Res. 2008;3141:92–102. doi: 10.1016/j.yexcr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Li T, Li RS, Li YH, Zong S, Chen YY, Zhang CM, Hu MM, Shen ZJ. miR-21 as an independent biochemical recurrence predictor and potential therapeutic target for prostate cancer. J. Urol. 2012;187:1466–1472. doi: 10.1016/j.juro.2011.11.082. [DOI] [PubMed] [Google Scholar]
- Li X, Huang Y, Fu X, Chen C, Zhang D, Yan L, Xie Y, Mao Y, Li Y. Meta-analysis of three polymorphisms in the steroid-5-alpha-reductase, alpha polypeptide 2 gene (SRD5A2) and risk of prostate cancer. Mutagenesis. 2011;263:371–383. doi: 10.1093/mutage/geq103. [DOI] [PubMed] [Google Scholar]
- Li Y, Vandenboom TG, II, Wang Z, Kong D, Ali S, Philip PA, Sarkar FH. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Chiang A, Chang D. Loss of mir-16a function in hormone-refractory prostate cancer. RNA. 2008;14:417–424. doi: 10.1261/rna.874808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB. Serum androgen levels in black, Hispanic, and white men. J. Clin. Endocrinol. Metab. 2006;9111:4326–4334. doi: 10.1210/jc.2006-0037. [DOI] [PubMed] [Google Scholar]
- Liu C, Kelnar K, Liu B, chen X, Calhoun-Davis T, Li H, Patrawala L, Yah H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. Identification of miR-34a as a potent inhibitor of prostate cancer progenitor cells and metastasis by directly repressing CD44. Nat. Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujambio A, Ropero S, Ballestar E, Fraga MF, Cerrato C, Setien F, Casado S, Suarez-Gauthier A, Sanchez-Cespedes M, Git A, Spiteri I, Das PP, Caldas C, Miska E, Esteller M. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis. 2011;32:772–778. doi: 10.1093/carcin/bgr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Chan YP, Kwan PS, Lee TK, Yan M, Tang KH, Ling MT, Vielkind JR, Guan XY, Chan KW. Micro-RNA-616 induces androgen-independent growth of prostate cancer cells by suppressing expression of tissue factors pathway inhibitor TFPI-1. Cancer Res. 2011;71:583–592. doi: 10.1158/0008-5472.CAN-10-2587. [DOI] [PubMed] [Google Scholar]
- Majid S, Dar AA, Saini S, Shahryari V, Arora S, Zaman MS, Chang I, Yamamura S, Tanaka Y, Chiyomaru T, Deng G, Dahiya R. miRNA-34b inhibits prostate cancer through demethylation, active chromatin modifications, and AKT pathways. Clin. Cancer Res. 2012;19:73–84. doi: 10.1158/1078-0432.CCR-12-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally LR, Manne U, Grizzle W. Post-transcriptional processing of genetic information and its relation to cancer. Biotech. & Histochem. 2013 doi: 10.3109/10520295.2012.730152. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette J, Durocher F, Leblanc JF, Normand T, Labrie F, Simard J. Genetic linkage mapping of the human steroid 5 alpha-reductase type 2 gene (SRD5A2) close to D2S352 on chromosome region 2p23-p22. Cytogenet. Cell Genet. 1996;734:304–307. doi: 10.1159/000134362. [DOI] [PubMed] [Google Scholar]
- Myers RB, Srivastava S, Oelschlager DK, Grizzle WE. Expression of p160 erbB-3 and p185 erbB-2 in Prostatic Intraepithelial Neoplasia and Prostatic Adenocarcinoma. J. Natl. Cancer Inst. 1994;86:1140–1145. doi: 10.1093/jnci/86.15.1140. [DOI] [PubMed] [Google Scholar]
- Myers RB, Lampejo O, Herrera GA, Srivastava S, Oelschlager D, Grizzle WE. TGF a expression is a relatively late event in the progression of prostatic adeno-carcinoma. J. Urol. Pathol. 1995;3:195–204. [Google Scholar]
- Myers RB, Oelschlager DK, Coan PN, Frost AR, Weiss HL, Manne U, Pretlow TG, Grizzle WE. Changes in cyclin dependent kinase inhibitors p21 and p27 during the castration induced regression of the CWR22 model of prostatic adenocarcinoma. J. Urol. 1999;161:945–949. [PubMed] [Google Scholar]
- Nadiminty N, Lou WA, Sun M, Chen J, Yue J, Kung HJ, Evans CP, Zhou Q, Gao AC. Aberrant activation of the androgen receptor by NF-kappaB2/p52 in prostate cancer cells. Cancer Res. 2010;708:3309–3319. doi: 10.1158/0008-5472.CAN-09-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadiminty N, Tummala R, Lou W, Zhu Y, Shi XB, Zou JX, Chen H, Zhang J, Chen X, Luo J, de Vere White RW, Kung HJ, Evans CP, Gao AC. MicroRNA let-7C is downregulated in prostate cancer and suppresses prostate cancer growth. PLoS ONE. 2012;7:e32832. doi: 10.1371/journal.pone.0032832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan EJ, Place RF, Pookoot D, Basak S, Whitson JM, Hirata H, Giardina C, Dahiya R. MiR-449a targets HDAC-1 and induces growth arrest in prostate cancer. Oncogene. 2009;28:1714–1724. doi: 10.1038/onc.2009.19. [DOI] [PubMed] [Google Scholar]
- Östling P, Leivonon SK, Aakula A, Kohonen P, Makela R, Hagman Z, Edsjo A, Kangaspeska S, Edgren H, Nicorici D, Bjartell A, Ceder Y, Perala M, Kallioniemi O. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 2011;715:1956–1967. doi: 10.1158/0008-5472.CAN-10-2421. [DOI] [PubMed] [Google Scholar]
- Ozen M, Creighton CJ, Ozdemir M, Ittmann MZ. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27:1788–93. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- Paone A, Galli R, Fabbri M. MicroRNAs as new characters in the plot between epigenetics and prostate cancer. Front. Genet. 2011;2:62. doi: 10.3389/fgene.2011.00062. doi: 10.3389/fgene.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz EA, Rimm EB, Willett WC, Kantoff PW, Giovannucci E. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. J. Natl. Cancer Inst. 2000;9224:2009–2017. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
- Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- Reis ST, Pontes-Junior J, Antunes AA, Dall'Oglio MF, Dip N, Passerotti CC, Rossini GA, Morais DR, Nesrallah AJ, Piantino C, Srougi M, Leite KR. miR-21 may act as an oncomir by targeting RECK, a matrix metalloproteinase regulator, in prostate cancer. BMC Urol. 2012;12:14. doi: 10.1186/1471-2490-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas J, Ni X, Haffner M, Wentzei EA, Salmasi AH, Chowdbury WH, Kudrolli TA, Yegnasubramanian S, Luo J, Rodriguez R, Mendell JT, Lupold SE. MiR-21: an androgen receptor regulated microRNA which promotes hormone dependent and independent prostate cancer growth. Cancer Res. 2009;69:7165–7169. doi: 10.1158/0008-5472.CAN-09-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru P, Steele R, Newhall P, Phillips NJ, Toth K, Ray RB. Micro-RNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol. Cancer Ther. 2012;11:1166–1173. doi: 10.1158/1535-7163.MCT-12-0100. [DOI] [PubMed] [Google Scholar]
- Saini S, Majid S, Yamamura S, Tabatbai L, Suh SO, Shahryari V, Chen Y, Deng G, Tanaka Y, Dahiya R. Regulatory role of mir-203 in prostate cancer progression and metastasis. Clin. Cancer Res. 2010;17:5287–5298. doi: 10.1158/1078-0432.CCR-10-2619. [DOI] [PubMed] [Google Scholar]
- Saito Y, Friedman JM, Chihara Y, Egger G, Chuang JC, Liang G. Epigenetic therapy upregulates the tumor suppressor microRNA-p126 and its host gene EGFL7 in human cancer cells. Biochem. Biophys. Res. Commun. 2009;379:726–731. doi: 10.1016/j.bbrc.2008.12.098. [DOI] [PubMed] [Google Scholar]
- Sanderson M, Coker AL, Logan P, Zheng W, Fadden MK. Lifestyle and prostate cancer among older African-American and Caucasian men in South Carolina. Cancer Causes Control. 2004;157:647–655. doi: 10.1023/B:CACO.0000036172.63845.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor O, Zheng Q, Eastham JA. Androgen receptor gene CAG repeat length varies in a race-specific fashion in men without prostate cancer. Urology. 1999;532:378–380. doi: 10.1016/s0090-4295(98)00481-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Bilim VN, Ugolkov AV, Yuuki K, Tsukigi M, Motoyama T, Tomita Y. The enhancer of zeste homolog 2 (EZH2), a potential therapeutic target, is regulated by miR101 in renal cancer cells. Biophys. Res. Comm. 2012;422:607–614. doi: 10.1016/j.bbrc.2012.05.035. [DOI] [PubMed] [Google Scholar]
- Shah MY, Calin GA. MicroRNAs miR-221 and miR-222: a new level of regulation in aggressive breast cancer. Genome Med. 2011;3:56. doi: 10.1186/gm272. doi: 10.1186/gm272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GH, Ye DW, Yao WD, Zhang SL, Dai B, Zhang HL, Shen YJ, Zhu Y, Zhu XP, Xiao WJ, Ma CG. Involvement of microRNA-21 in mediating chemoresistance to docetaxel in androgen-independent prostate cancer PC3 cells. Acta Pharmacol. Sin. 2010;31:867–873. doi: 10.1038/aps.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WB, Xue L, Yang J, Ma AH, Zhao J, Xu M, Tepper CG, Evans CP, Kung HJ, deVere White RW. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc. Natl. Scad. Sci. USA. 2007;104:19983–19988. doi: 10.1073/pnas.0706641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XB, Xue L, Ma AH, Tepper CG, Gandour-Edwards R, Kung HJ, Devere White RW. Tumor suppressive miR-124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene. 2012 doi: 10.1038/onc.2012.425. doi: 10.1038/onc.2012.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuch B, Mikhail M, Satagopan J, Lee P, Yee H, Chang C, Cordon-Cardo C, Taneja SS, Osman I. Racial disparity of epidermal growth factor receptor expression in prostate cancer. J. Clin. Oncol. 2004;2223:4725–4729. doi: 10.1200/JCO.2004.06.134. [DOI] [PubMed] [Google Scholar]
- Sikand K, Slaibi JE, Singh R, Slane SD, Shukla GC. MiR 488* inhibits androgen receptor expression in prostate carcinoma cells. Int. J. Cancer. 2011;129:810–819. doi: 10.1002/ijc.25753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Chitkara D, Mehrazin R, Behrman SW, Wake RW, Mahato RI. Chemoresistance in prostate cancer cells is regulated by miRNAs and hedgehog pathway. PLoS One. 2012;7:e40021. doi: 10.1371/journal.pone.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R, Mott JL, Ray RB. MBP-1 upregulates miR-29b, which represses McI-1, collagens, and matrix metalloproteinase-2 in prostate cancer cells. Genes Cancer. 2010;1:381–387. doi: 10.1177/1947601910371978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SO, Chen Y, Zaman MS, Hirata H, Yamamura S, Shahryari V, Liu J, Tabatabai ZL, Kakar S, Deng G, Tanaka Y, Dahiya R. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis. 2011;32:772–778. doi: 10.1093/carcin/bgr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Wang Q, Balk S, Brown M, Lee GSM, Kantoff P. The role of micro-RNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res. 2009;69:3356. doi: 10.1158/0008-5472.CAN-08-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang BE, Leong KG, Yue P, Li L, Jhunjhunwala S, Chen D, Seo K, Modrusan Z, Gao WQ, Settleman J, Johnson L. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. 2012;722:527–536. doi: 10.1158/0008-5472.CAN-11-3004. [DOI] [PubMed] [Google Scholar]
- Szczyrba J, Löprich E, Wach S, Jung V, Unteregger G, Barth S, Grobholz R, Wieland W, Stöhr R, Hartmann A, Wullich B, Grässer F. The microRNA profile of prostate carcinoma obtained by deep sequencing. Mol. Cancer Res. 2010;8:529–538. doi: 10.1158/1541-7786.MCR-09-0443. [DOI] [PubMed] [Google Scholar]
- Thatai LC, Banerjee M, Lai Z, Vaishampayan U. Racial disparity in clinical course and outcome of meta-static androgen-independent prostate cancer. Urology. 2004;644:738–743. doi: 10.1016/j.urology.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Theodore S, Sharp S, Zhou J, Turner T, Li H, Miki J, Ji Y, Patel V, Yates C, Rhim JS. Establishment and characterization of a pair of non-malignant and malignant tumor derived cell lines from an African American prostate cancer patient. Int. J. Oncol. 2010a;376:1477–1482. doi: 10.3892/ijo_00000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore SC, Rhim JS, Turner T, Yates C. MiRNA 26a expression in a novel panel of African American prostate cancer cell lines. Ethn. Dis. 2010b;201(Suppl 1):S1-96–100. [PMC free article] [PubMed] [Google Scholar]
- Thomas LN, Douglas RC, Lazier CB, Too CK, Rittmaster RS, Tindall DJ. Type 1 and type 2 5-alpha-reductase expression in the development and progression of prostate cancer. Eur. Urol. 2008;532:244–252. doi: 10.1016/j.eururo.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Thomas LN, Douglas RC, Rittmaster RS, Too CK. Overexpression of 5 alpha-reductase type 1 increases sensitivity of prostate cancer cells to low concentrations of testosterone. Prostate. 2009;696:595–602. doi: 10.1002/pros.20911. [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Zhang X, Ressom HW, Varghese RS, Kallakury BV, Wang K, Ji Y, Cheema A, Jung M, Brown ML, Rhim JS, Dritschilo A. Enhanced expression of SOS1 is detected in prostate cancer epithelial cells from African-American men. Int. J. Oncol. 2009;354:751–760. [PMC free article] [PubMed] [Google Scholar]
- Tong AW, Fulgham P, Jay C, Chen P, Khalil I, Liu S, Senzer N, Eklund AC, Han J, Nemunaitis J. Micro-RNA profile analysis of human prostate cancers. Cancer Gene Ther. 2009;16:206–216. doi: 10.1038/cgt.2008.77. [DOI] [PubMed] [Google Scholar]
- Tricoli JV, Winter DL, Hanlon AL, Raysor SL, Watkins-Bruner D, Pinover WH, Hanks GE. Racial differences in insulin-like growth factor binding protein-3 in men at increased risk of prostate cancer. Urology. 1999;541:178–182. doi: 10.1016/s0090-4295(99)00129-6. [DOI] [PubMed] [Google Scholar]
- Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, Vousden KH, Muller PA, Dötsch V, Kehrloesser S, Sayan BS, Giaccone G, Lowe SW, Takahashi N, Vandenabell P, Knight RA, Levine AJ, Melino G. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc. Nat. Acad. Sci. USA. 2012;109:15312–15317. doi: 10.1073/pnas.1110977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T, Chen P, Goodly LJ, Wells A. EGF receptor signaling enhances in vivo invasiveness of DU-145 human prostate carcinoma cells. Clin. Exp. Metas. 1996;144:409–418. doi: 10.1007/BF00123400. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Knight RA, Levine AJ, Melino G. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc. Nat. Acad. Sci. USA. 2012;109:15312–15317. doi: 10.1073/pnas.1110977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varambally S, Cao Q, Manu R-S, Shankar S, Wang X, Ateeg B, Laxman B, Cao X, Jing X, Ramnarayanan K, Brenner JC, Yu J, Kim JH, Han B, Tan P, Kumar-Sinha C, Lonigro RJ, Palanisam N, Maher CA, Chinnalyan AM. Genomic loss of microRNA-101 leads to over-expression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volinia S, Calin GA, Chang-Gong L, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo CK, Ferracin M, Prueitt ARL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Nat. Acad. Sci. USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrba L, Jensen TJ, Garbe JC, Heimark RL, Cress AE, Dickinson S, Stampfer MR, Futscher BW. Role for DNA methylation in the regulation of miR-200c and miR-141 expression in normal and cancer cells. PLoS One. 2010;5:e8697. doi: 10.1371/journal.pone.0008697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner LP, Clemens JQ, Sarma AV. Prevalence of and risk factors for prostatitis in African American men: the Flint Men's Health Study. Prostate. 2009;691:24–32. doi: 10.1002/pros.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltering KK, Porkka KP, Jalava SE, Urbanucci A, Kohonen PJ, Latonen LM, Kallioniemi OP, Jenster G, Visakorpi T. Androgen regulation of micro-RNAs in prostate cancer. Prostate. 2011;71:604–614. doi: 10.1002/pros.21276. [DOI] [PubMed] [Google Scholar]
- Watahiki A, Wang Y, Morris J, Dennis K, O'Dwyer HM, Gleave M, Gout PW, Wang Y. MicroRNAs associated with metastatic prostate cancer. PLoS One. 2011;6:e24950. doi: 10.1371/journal.pone.0024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J-J, Wu X, Peng Y, Shi G, Basturk O, Yang X, Daniels G, Osman I, Ouyang J, Hernando E, Pellicer A, Rhim JS, Melamed J, Lee P. Regulation of HMGA1 expression by MicroRNA-296 affects prostate cancer growth and invasion. Clin. Cancer Res. 2011;17:1297–1305. doi: 10.1158/1078-0432.CCR-10-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schuler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell. Biol. 2009;1112:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- Williams H, Powell IJ. Epidemiology, pathology, and genetics of prostate cancer among African Americans compared with other ethnicities. Methods Mol. Biol. 2009;472:439–453. doi: 10.1007/978-1-60327-492-0_21. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Brufsky A, Weissfeld J, Trump DL, Dyky MA, Hadeed V. Testosterone, sex hormone-binding globulin, and body composition in young adult African American and Caucasian men. Metabolism. 2001;5010:1242–1247. doi: 10.1053/meta.2001.26714. [DOI] [PubMed] [Google Scholar]
- Xu B, Wang N, Wang X, Tong N, Shao N, Tao J, Li P, Niu W, Feng N, Zhang L, Hua L, Wang Z, Chen M. MiR-146a suppresses tumor growth and progression by targeting EGFR pathway and in a p-ERK-dependent manner in castration-resistant prostate cancer. Prostate. 2012;72:1171–1178. doi: 10.1002/pros.22466. [DOI] [PubMed] [Google Scholar]
- Yates C, Wells A, Turner T. Luteinizing hormone-releasing hormone analogue reverses the cell adhesion profile of EGFR overexpressing DU-145 human prostate carcinoma subline. Br. J. Cancer. 2005;922:366–375. doi: 10.1038/sj.bjc.6602350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates CC, Shepard CR, Stolz DB, Wells A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br. J. Cancer. 2007;968:1246–1252. doi: 10.1038/sj.bjc.6603700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman MS, Chen Y, Deng G, Shahryari V, Suh SO, Saini S, Majid S, Liu J, Khatri G, Tanaka Y, Dahlya R. The functional significance of microRNA-145 in prostate cancer. Br. J. Cancer. 2010;103:256–264. doi: 10.1038/sj.bjc.6605742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HL, Yang LF, Zhu Y, Yao XD, Zhang SL, Dai B, Zhu YP, Shen YJ, Shi GH, Ye DW. Serum mi-RNA-21: Elevated levels in patients with metastatic hormone-refractory prostate cancer and potential predictive factor for the efficacy of docetaxel-based chemotherapy. Prostate. 2011;71:326–331. doi: 10.1002/pros.21246. [DOI] [PubMed] [Google Scholar]