Abstract
Background and Objective
An increasing body of evidence suggests that the use of probiotic bacteria is a promising intervention approach for the treatment of inflammatory diseases with polymicrobial etiology. The objective of this study was to determine whether Lactobacillus brevis CD2 could inhibit periodontal inflammation and bone loss in experimental periodontitis.
Materials and Methods
Periodontitis was induced by placing a silk ligature around the second maxillary molar of mice treated with L. brevis CD2 (8×105 CFU in 1-mm2 lyopatch) or placebo, which were placed between the gingiva and the buccal mucosa in the vicinity of the ligated teeth. The mice were euthanized after 5 days and bone loss was measured morphometrically, gingival expression of proinflammatory cytokines was determined by quantitative real-time PCR, and CFU counts of periodontitis-associated bacteria were determined after aerobic and anaerobic culture. To determine the role of arginine deiminase released by L. brevis CD2, soluble extracts with or without formamidine (arginine deiminase inhibitor) were tested in in vitro cellular activation assays.
Results
Mice topically treated with L. brevis CD2 displayed significantly decreased bone loss and lower expression of TNF, IL-1β, IL-6, and IL-17A as compared to placebo-treated mice. Moreover, L. brevis CD2-treated mice displayed lower counts of anaerobic bacteria but higher counts of aerobic bacteria than placebo-treated mice. In in vitro assays, the anti-inflammatory effects of soluble L. brevis CD2 extracts were heavily dependent on the presence of functional arginine deiminase, an enzyme that can inhibit nitric oxide synthesis.
Conclusion
These data provide proof-of-concept that the probiotic L. brevis CD2 can inhibit periodontitis through modulatory effects on the host response and the periodontal microbiota.
Keywords: Lactobacillus brevis CD2, probiotics, arginine deiminase, inflammation, periodontitis
Introduction
Periodontitis is a prevalent chronic inflammatory disease that affects the tooth-supporting tissues and, moreover, can exert an adverse impact on systemic health (1–4). Conventional periodontal treatment is often not sufficient by itself to control destructive inflammation and many patients develop recurrent disease (5). This necessitates the development of novel and effective therapeutic strategies, adjunctive to clinical periodontal treatment. The use of probiotics is one of several approaches being considered for the treatment of periodontitis (6).
According to the World Health Organization, probiotic bacteria are live microorganisms that can confer health benefits to the host when consumed in adequate amounts. Several reports suggest that lactic acid bacteria (LAB), or their products, have beneficial effects on human health, as these probiotics can protect against or mitigate gastrointestinal inflammation, aphthous oral ulceration, and radiation- and chemotherapy-induced mucositis (7–10). The mechanisms of action of LAB and other probiotics are poorly understood although they appear to involve host immunomodulation and remodeling of the structure and function of microbial communities (11,12). On the basis of animal and human studies, the use of probiotics is emerging as a potential adjunctive therapy in periodontitis although the underlying mechanisms remain poorly defined (13–18). The probiotic Lactobacillus brevis CD2 was previously shown to inhibit gingival inflammation in humans (15,16), although whether it can also inhibit periodontal bone loss is yet to be determined. The CD2 strain of L. brevis (a Gram-positive rod-shaped species of LAB) produces high levels of arginine deiminase, an enzyme that inhibits the production of nitric oxide by competing with nitric oxide synthase for the same substrate, arginine (19). This property of arginine deiminase is thought to be responsible for the anti-inflammatory effects of L. brevis CD2 (19).
In this study, we investigated whether topical treatment with L. brevis CD2 can inhibit inflammatory periodontal bone loss, the hallmark of periodontitis that may lead to tooth loss. For this purpose, we used a very rigorous and highly reproducible model of periodontal bone loss, the ligature-induced periodontitis model in mice (20,21). This murine model has been used to identify novel mechanisms and pathways involved in periodontal pathogenesis and associated systemic diseases (22–24).
Materials and methods
Ligature-induced periodontitis and L. brevis CD2 intervention
All animal procedures described in this study were approved by the Institutional Animal Care and Use Committee, in compliance with established federal and state policies. Six-week-old C57BL/6 male mice (The Jackson Laboratory) were treated three times daily with L. brevis CD2 (8.2 × 105 bacteria in 1 mm2 lyopatch) or placebo (control lyopatch without bacteria), both kindly provided by VSL Pharmaceuticals, Inc. In each mouse, the experimental or control lyopatch was applied topically between the gingiva and the buccal mucosa corresponding to the tooth to be ligated for induction of periodontitis. Periodontitis was induced one day after initiation of the L. brevis CD2 or placebo treatments by tying a 5-0 silk ligature around the maxillary left second molar (20). The contralateral molar tooth in each mouse was left unligated to serve as baseline control for bone height measurements. The ligatures remained in place in all mice throughout the experimental period. The mice were euthanized 5 days after placement of the ligatures.
Ligature-associated microbiota counts
The sutures were recovered from the mice at day 5 and bacteria were extracted with phosphate-buffered saline (PBS) (20). Serial dilutions of the suture extracts were plated onto blood agar plates for aerobic and anaerobic growth and CFU determination. Results were normalized by dividing CFU by the length (mm) of the corresponding suture (20).
Determination of periodontal bone loss
Periodontal bone heights were measured as the distances from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) using a Nikon SMZ800 microscope (Nikon Instruments) and a 40 × objective. Images of the maxillae were captured using a Nicon Digital Sight DS-U3 camera controller (Nikon Instruments Inc.) and bone heights were measured using NIS-Elements software (Nikon Instruments) (20). Bone measurements were performed on the ligated second molar (3 sites corresponding to mesio-buccal cusp, buccal groove, and disto-buccal cusp) and the affected adjacent regions (sites corresponding to disto-buccal groove and distal cusp of the first molar, and buccal cusp of the third molar). The 6-site total CEJ-ABC distance for the ligated side of each mouse was calculated and subtracted from the 6-site total CEJ-ABC distance of the contralateral unligated side of the same mouse. The results were presented in mm and negative values indicated bone loss relative to unligated sites.
Real-time PCR
Gingival tissue was dissected from around the maxillary molars. Total RNA was extracted using the PerfectPure RNA cell kit (5 Prime, Fisher) and quantified by spectrometry at 260 and 280 nm. The RNA was reverse-transcribed using the High-Capacity cDNA Archive kit (Applied Biosystems) and real-time PCR with cDNA was performed using the ABI 7500 Fast System, according to the manufacturer’s protocol (Applied Biosystems). TaqMan probes, sense primers, and antisense primers for detection and quantification of cytokine genes (and the GAPDH housekeeping gene) by quantitative real-time PCR were purchased from Applied Biosystems. Data were analyzed using the 2−ΔCT method (2^((−1)*(mean cycle number of target gene − mean cycle number of housekeeping gene)) (25).
Preparation of L. brevis CD2 extracts
Lyophilized L. brevis CD2 bacteria (108 CFU per gram) were diluted in 10 ml PBS and sonicated for 30min (10-s sonication period alternating with 10-s pause) using a EpiShear™ Probe Sonicator (Active Motif). The resulting preparation was centrifuged at 7500 g for 20 min at 4°C and the supernatants (soluble bacterial extracts) were stored at −20°C until used.
Cell activation assays
The human monocytic cell line THP-1 was maintained in 25 mM Hepes-buffered RPMI 1640, supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/ml streptomycin, hereafter referred to as ‘medium’. All incubations were carried out at 37°C and 5% CO2 in a humidified atmosphere. To induce their differentiation into macrophage-like cells, THP-1 cells were incubated in 24-well culture plates (105 cells per well) in medium supplemented with 10 ng/ml PMA for 48 h (26). Subsequently, the cells were washed extensively with RPMI 1640 and cultured further in the medium without FCS for 12 h. After the medium was changed to remove cytokines induced by cell adherence, the differentiated THP-1 cells (hereafter referred to as ‘THP-1 macrophages’) were pretreated or not for 1h with soluble L. brevis CD2 extracts (20 μg per 105 THP-1 cells) followed by stimulation for 18h with medium only or with 1 μg/ml Porphyromonas gingivalis LPS, 1 μg/ml Escherichia coli LPS, or 0.1 μg/ml Pam3CSK4, a synthetic lipopeptide that is a TLR2 ligand (both LPS molecules and Pam3CSK4 were purchased from InVivogen). In some experiments, the cells were pretreated with L. brevis CD2 extracts together with 20 mM formamidine (Sigma). At the end of the 18-h stimulation period, culture supernatants were collected for measuring cytokine responses by ELISA (using eBioscience kits) and nitric oxide production. Nitric oxide production was assessed by measuring the amount of NO2− (stable oxidative metabolite of nitric oxide) in the stimulated culture supernatants using an assay kit based on the Griess reaction (Sigma).
Statistical analysis
Data were evaluated by analysis of variance and the Dunnett multiple-comparison test with the InStat program (GraphPad Software). Where appropriate (comparison of two groups only), two-tailed t-tests were performed. P values < 0.05 were considered statistically significant. All experiments were performed at least twice for confirmation.
Results
L. brevis CD2 inhibits ligature-induced periodontitis
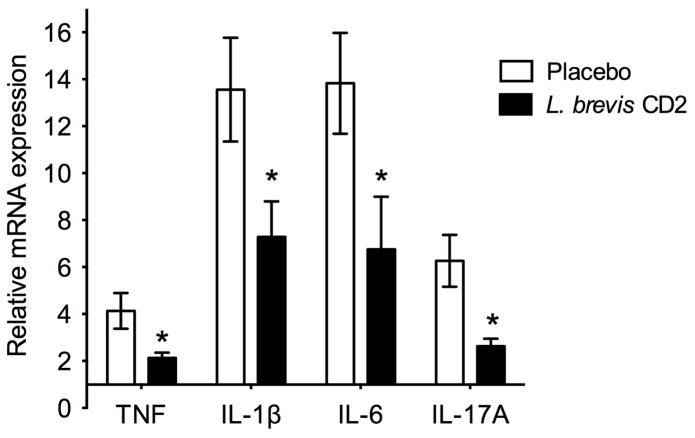
Following the placement of ligatures in mice treated with placebo or L. brevis CD2, periodontal bone loss was induced in both groups at the ligated sites relative to the corresponding contralateral unligated sites (zero baseline) (Fig. 1). Importantly, mice topically treated with L. brevis CD2 displayed 60% less bone loss compared with placebo-treated mice (P < 0.01; Fig. 1A). We also assessed mRNA expression of proinflammatory cytokines in gingiva dissected from the two groups of mice. The expression of a number of cytokines (TNF, IL-1β, IL-6, and IL-17A) that have been implicated in inflammatory periodontal bone loss (27) was readily induced in the ligated sites of placebo-treated mice (Fig. 2). On the other hand, L. brevis CD2-treated mice exhibited significantly decreased expression of all proinflammatory cytokines tested (P < 0.01; Fig. 2). Taken together, these data show that topical treatment with the probiotic L. brevis CD2 inhibits periodontal inflammation and bone loss.
FIGURE 1. L. brevis CD2 inhibits ligature-induced periodontal bone loss.
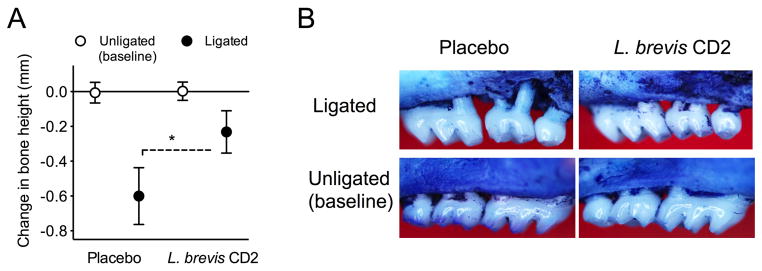
Periodontitis was induced by placing a silk ligature around the second maxillary left molar of mice, which were treated topically with a 1-mm2 lyopatch containing L. brevis CD2 (8.2 × 105 CFU) or with control lyopatch (placebo) placed between the gingiva and the buccal mucosa in the vicinity of the ligated teeth. The contralateral molar teeth were left unligated to serve as baseline controls. The treatments were applied three times daily for 5 days, at which time the mice were euthanized for bone loss measurements in defleshed maxillae. Negative values indicate bone loss in the placebo and L. brevis CD2 groups relative to the corresponding baselines (A) and representative images of maxillae exhibiting bone loss are shown (B). Data are means ± SD and are pooled from two independent experiments with a total of 8–10 mice per group (placebo, n = 10; L. brevis CD2, n = 8). *P < 0.01 between the indicated groups.
FIGURE 2. L. brevis CD2 inhibits mRNA expression of proinflammatory cytokines.
Gingiva were dissected from ligated or unligated sites of mice treated with placebo or L. brevis CD2 and were processed for quantitative real-time PCR to determine mRNA expression of the indicated cytokines. Results were normalized against GAPDH mRNA and expressed as fold change in the transcript levels in the ligated sites relative to those of the corresponding contralateral unligated sites, which were assigned an average value of 1. Data are means ± SD and are pooled from two independent experiments with a total of 8–10 mice per group (placebo, n = 10; L. brevis CD2, n = 8). *P < 0.01 between the indicated groups.
L. brevis CD2 causes changes to the ligature-associated periodontal microbiota
The accelerated bone loss seen in ligature-induced periodontitis is thought to result from massive local bacterial accumulation in the placed sutures; the sutures per se do not cause bone loss (20,21,28). We reasoned that the protective effects of L. brevis CD2 against induction of periodontal inflammation and bone loss (Figs. 1 and 2) could be related, at least in part, to possible influence on the ligature-associated microbiota. Our microbiological analysis of recovered sutures revealed that topical treatment with L. brevis CD2 exerted differential effects on the aerobic and anaerobic microbiotas. Specifically, L. brevis CD2 treatment resulted in significantly higher counts of aerobic bacteria (Fig. 3A) and, conversely, significantly lower numbers of anaerobic bacteria (Fig. 3B), as compared to the placebo-treated control group (P < 0.01). Since periodontitis-associated bacteria are predominantly (if not exclusively) anaerobic (29), this finding suggests that L. brevis CD2 treatment may inhibit periodontitis, at least in part, through modulation of the periodontal microbiota.
FIGURE 3. L. brevis CD2 causes alterations to the periodontal microbiota.
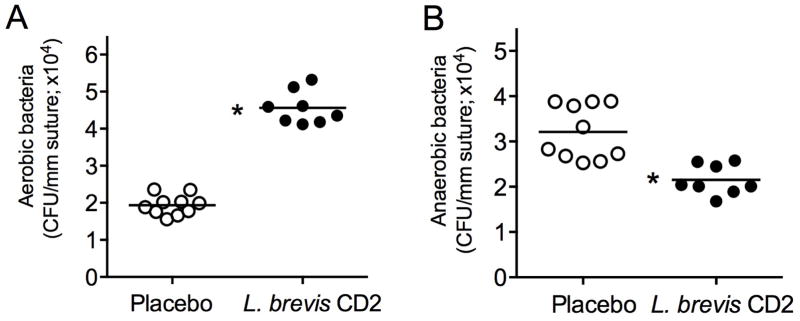
Bacteria were extracted from sutures recovered 5 days after their placement in placebo- or L. brevis CD2-treated mice. Serial dilutions of bacterial suspensions were plated onto blood agar plates for aerobic and anaerobic growth and CFU enumeration. Each symbol represents an individual mouse and small horizontal lines indicate the means. Data are pooled from two independent experiments with a total of 8–10 mice per group, as indicated. *P < 0.01.
L. brevis CD2 extracts inhibit production of inflammatory mediators in arginine deiminase-dependent way
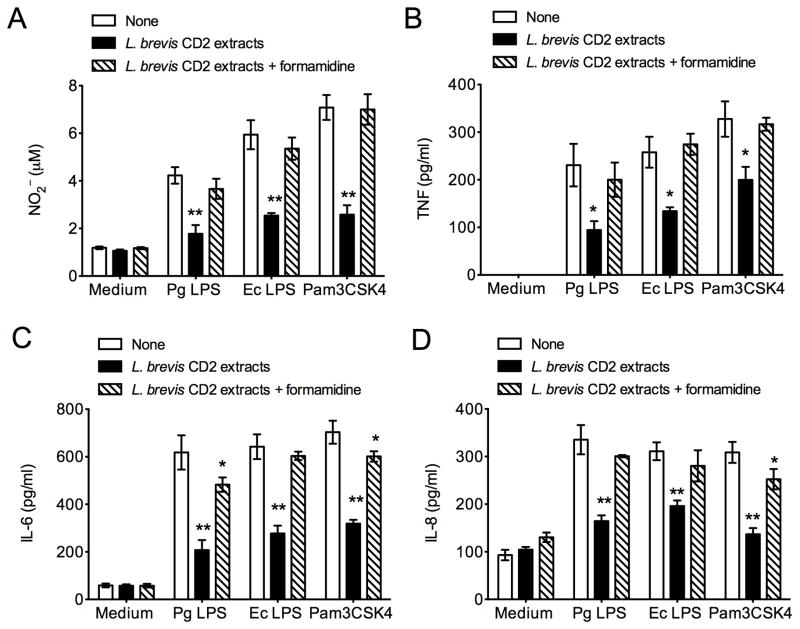
Although L. brevis CD2 can modulate the periodontal microbiota in a way that could potentially inhibit periodontitis, another protective mechanism could involve direct anti-inflammatory effects. As the anti-inflammatory effects cannot be readily dissociated from anti-microbial effects in vivo, we determined whether L. brevis CD2 extracts could inhibit inflammatory responses in THP-1 macrophages stimulated with various inflammatory stimuli. All stimuli used, bacterial lipopolysaccharide (LPS) from Porphyromonas gingivalis or Escherichia coli or the Pam3CSK4 lipopeptide, induced the production of nitric oxide, as shown by the detection of its stable metabolite NO2− in the culture supernatants (Fig. 4A). Moreover, all stimuli induced the production of the proinflammatory cytokines TNF, IL-6, and IL-8 (Fig. 4 B–D). Importantly, the production of all inflammatory mediators in activated THP-1 macrophages was significantly (P < 0.05) inhibited in the presence of soluble L. brevis CD2 extracts (Fig. 4). To determine whether arginine deiminase present in the L. brevis CD2 extracts mediates anti-inflammatory effects, groups of activated cells were treated with both L. brevis CD2 extracts and formamidine, a specific inhibitor of arginine deiminase (30). The presence of formamidine caused a complete or near complete reversal of the anti-inflammatory effects of L. brevis CD2 extracts (Figs. 4 and 5), indicating that arginine deiminase is the major anti-inflammatory agent in the extracts. The capacity of nitric oxide to exert pro-inflammatory effects (31) suggests that the L. brevis CD2-mediated inhibition of proinflammatory cytokine responses could be attributed to the decreased production of nitric oxide.
FIGURE 4. L. brevis CD2 extracts inhibit production of inflammatory mediators in arginine deiminase-dependent way.
THP-1 macrophages were pretreated for 1h with soluble L. brevis CD2 extracts (20 μg per 105 THP-1 cells) with or without formamidine (20 mM), a specific inhibitor of arginine deiminase, followed by 18-h stimulation with medium only or with P. gingivalis LPS (Pg LPS; 1 μg/ml), E. coli LPS (Ec LPS; 1 μg/ml), or the TLR2 ligand Pam3CSK4 lipopeptide (0.1 μg/ml). Subsequently, culture supernatants were collected for measuring the indicated cytokine responses by ELISA and the production of NO2− by the Griess method. Data are means ± SD (n = 3 sets of cells). *P < 0.05 and **P < 0.01 compared with no pretreatment (‘None’).
Discussion
In the present study, treatment of mice with L. brevis CD2 resulted in inhibition of periodontal inflammation and bone loss and modulated the oral microbiota in a way that favored the growth of aerobic bacteria at the expense of anaerobic bacteria that are strongly associated with periodontitis. Our data suggest that this probiotic preparation could have therapeutic potential in human periodontitis, possibly through both anti-inflammatory and antimicrobial effects.
Arginine deiminase metabolizes arginine to citrulline and ammonia and therefore can compete with inducible nitric oxide synthase (iNOS) for arginine. By this mechanism, arginine deiminase can inhibit nitric oxide production (19). Studies in human periodontal patients and animal models suggest that iNOS-derived nitric oxide plays a role in periodontal inflammation and bone loss (32–35). In fact, nitric oxide levels are increased in the saliva of patients with chronic periodontitis and are correlated with elevated periodontal clinical parameters (36). In addition to inducing inflammatory mediators, inappropriately high levels of nitric oxide could potentially facilitate the infiltration of inflammatory cells to the periodontal tissue by inducing pathological vascular permeability. Arginine deiminase released by L. brevis CD2 in vivo is therefore likely to have contributed to the anti-inflammatory and anti-bone resorptive effects seen in the periodontal tissue of mice treated topically with L. brevis CD2. This notion was substantiated in in vitro experiments with THP-1 macrophages. Indeed, the capacity of soluble L. brevis CD2 extracts to inhibit inflammatory responses in THP-1 cells activated by TLR2 or TLR4 ligands was heavily dependent on functional arginine deiminase.
The observed decline in the counts of anaerobic bacteria associated with ligature-induced periodontitis could, in part, be due to the decreased inflammation caused by L. brevis CD2. Indeed, many anaerobic bacteria associated with periodontitis utilize tissue breakdown products (e.g., degraded proteins/peptides and hemin, a source of essential iron) for their growth (22,37), which could thus be affected under conditions of decreased inflammation. Additionally, whether L. brevis CD2 extracts have a direct inhibitory effect on periodontal anaerobic bacteria (and, conversely, a stimulatory effect on aerobic bacteria) cannot be ruled out. In this regard, LAB isolated from the human oral cavity have been shown to suppress the in vitro growth of bacteria associated with human periodontitis, specifically P. gingivalis, Prevotella intermedia, and Aggregatibacter (Actinobacillus) actinomycetemcomitans (38). P. gingivalis has been characterized as a keystone pathogen that can promote the virulence of the entire biofilm community (39), P. intermedia strains have been strongly associated with both chronic and aggressive periodontitis (40), whereas A. actinomycetemcomitans is the major organism implicated in localized aggressive periodontitis (41). In another study, oral administration of LAB in human volunteers caused a significant reduction in the counts of Tannerella forsythia in subgingival plaque, although reductions in the counts of other periodontitis-associated bacteria, including P. gingivalis and Treponema denticola, did not reach statistical significance (18). These three bacteria comprise the red complex of select periopathogens (42), although it is now increasingly acknowledged that periodontitis is caused synergistically by dysbiotic polymicrobial communities that include these important bacteria (43). Such dysbiotic polymicrobial communities also contain pathobionts, that is, commensals that can become pathogenic when tissue homeostasis is disrupted (44). The placement of ligature around teeth, as also performed in this study, disrupts host tissue homeostasis and leads to the emergence of pathobionts that amplify inflammatory bone loss (28).
In contrast to the decline of anaerobic bacterial counts, we observed a rise of aerobic bacteria in mice treated with L. brevis CD2. As alluded to above, inflammation favors the growth of anaerobic bacterial species that not only can endure the host inflammatory response but can also thrive by utilizing tissue-breakdown products as nutrients, whereas aerobic bacteria are likely outcompeted under these conditions (44). Conversely, therefore, the inhibition of inflammation by L. brevis CD2 would allow favorable conditions for the aerobes to flourish. Certain species have been shown to be associated with human periodontal health and are considered ‘beneficial’ by exerting anti-inflammatory effects or stimulating host immunity (45,46). However, the murine oral microbiota has not been adequately investigated and it is thus uncertain whether the murine aerobic microbiota contains such beneficial bacteria.
It should be noted that probiotic LAB, including L. brevis CD2, antagonize also bacteria that are not strictly anaerobic, such as the cariogenic organism Streptococcus mutans, thereby being useful also for the treatment of dental caries (47–49). Lactobacilli and other probiotics colonize human mucosal tissues only transiently (50). To maintain a protective effect against periodontitis or dental caries, frequent administrations (e.g., in the form of daily dietary supplements) may be necessary. Overall, probiotics have an excellent safety record but they should be used with caution in oral or other diseases, as they are not without potential risks in certain patient groups, such as immunocompromised individuals and premature neonates (50).
In summary, our data are consistent with the notion that LAB are useful probiotics for promoting oral health (14–18,38) and provide proof-of-concept that L. brevis CD2 can inhibit inflammatory bone loss. Whether this probiotic preparation can find application as an adjunctive therapy in human periodontitis is yet to be investigated in future clinical trials.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Dental and Craniofacial Research (grant DE017138 to GH) and by VSL Pharmaceuticals, Inc.
References
- 1.Genco RJ, Van Dyke TE. Prevention: Reducing the risk of CVD in patients with periodontitis. Nat Rev Cardiol. 2010;7:479–480. doi: 10.1038/nrcardio.2010.120. [DOI] [PubMed] [Google Scholar]
- 2.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 3.Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 4.Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 5.Armitage GC. Classifying periodontal diseases--a long-standing dilemma. Periodontol 2000. 2002;30:9–23. doi: 10.1034/j.1600-0757.2002.03002.x. [DOI] [PubMed] [Google Scholar]
- 6.Bizzini B, Pizzo G, Scapagnini G, Nuzzo D, Vasto S. Probiotics and oral health. Curr Pharm Des. 2012;18:5522–5531. doi: 10.2174/138161212803307473. [DOI] [PubMed] [Google Scholar]
- 7.Niscola P, Tendas A, Scaramucci L, Giovannini M, Trinchieri V, De Fabritiis P. Aphthous oral ulceration and its successful management by Lactobacillus brevis CD2 extract in an adult haemophilic patient. Haemophilia. 2012;18:e78–79. doi: 10.1111/j.1365-2516.2012.02757.x. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A, Rath GK, Chaudhary SP, Thakar A, Mohanti BK, Bahadur S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: a randomized double-blind placebo-controlled study. Eur J Cancer. 2012;48:875–881. doi: 10.1016/j.ejca.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Ganguli K, Walker WA. Probiotics in the prevention of necrotizing enterocolitis. J Clin Gastroenterol. 2011;45 (Suppl):S133–138. doi: 10.1097/MCG.0b013e318228b799. [DOI] [PubMed] [Google Scholar]
- 10.Dylag K, Hubalewska-Mazgaj M, Surmiak M, Szmyd J, Brzozowski T. Probiotics in the mechanism of protection against gut inflammation and therapy of gastrointestinal disorders. Curr Pharm Des. 2013 doi: 10.2174/13816128113199990422. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6:39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dongarra ML, Rizzello V, Muccio L, et al. Mucosal immunology and probiotics. Curr Allergy Asthma Rep. 2013;13:19–26. doi: 10.1007/s11882-012-0313-0. [DOI] [PubMed] [Google Scholar]
- 13.Messora MR, LFFO, Foureaux RC, et al. Probiotic therapy reduces periodontal tissue destruction and improves the intestinal morphology in rats with ligature-induced periodontitis. J Periodontol. 2013;84 doi: 10.1902/jop.2013.120644. [DOI] [PubMed] [Google Scholar]
- 14.Teughels W, Durukan A, Ozcelik O, Pauwels M, Quirynen M, Haytac MC. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol. 2013;40 doi: 10.1111/jcpe.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ierardo G, Bossu M, Tarantino D, Trinchieri V, Sfasciotti GL, Polimeni A. The arginine-deiminase enzymatic system on gingivitis: preliminary pediatric study. Ann Stomatol (Roma) 2010;1:8–13. [PMC free article] [PubMed] [Google Scholar]
- 16.Riccia DN, Bizzini F, Perilli MG, et al. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis. 2007;13:376–385. doi: 10.1111/j.1601-0825.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 17.Vivekananda MR, Vandana KL, Bhat KG. Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: a preliminary randomized clinical trial. J Oral Microbiol. 2010;2 doi: 10.3402/jom.v3402i3400.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayanagi G, Kimura M, Nakaya S, et al. Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: a double-blinded, placebo-controlled, randomized clinical trial. J Clin Periodontol. 2009;36:506–513. doi: 10.1111/j.1600-051X.2009.01392.x. [DOI] [PubMed] [Google Scholar]
- 19.Di Marzio L, Russo FP, D’Alo S, et al. Apoptotic effects of selected strains of lactic acid bacteria on a human T leukemia cell line are associated with bacterial arginine deiminase and/or sphingomyelinase activities. Nutr Cancer. 2001;40:185–196. doi: 10.1207/S15327914NC402_16. [DOI] [PubMed] [Google Scholar]
- 20.Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 2013;394:49–54. doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskan MA, Jotwani R, Abe T, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe T, Hosur KB, Hajishengallis E, et al. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol. 2012;189:5442–5448. doi: 10.4049/jimmunol.1202339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q, Leeman SE, Amar S. Signaling mechanisms in the restoration of impaired immune function due to diet-induced obesity. Proc Natl Acad Sci U S A. 2011;108:2867–2872. doi: 10.1073/pnas.1019270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajishengallis G, Martin M, Sojar HT, et al. Dependence of bacterial protein adhesins on toll-like receptors for proinflammatory cytokine induction. Clin Diagn Lab Immunol. 2002;9:403–411. doi: 10.1128/CDLI.9.2.403-411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiao Y, Darzi Y, Tawaratsumida K, et al. Induction of bone loss by pathobiont-mediated nod1 signaling in the oral cavity. Cell Host Microbe. 2013;13:595–601. doi: 10.1016/j.chom.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 30.Weickmann JL, Himmel ME, Squire PG, Fahrney DE. Arginine deiminase from Mycoplasma arthritidis. Properties of the enzyme from log phase cultures. J Biol Chem. 1978;253:6010–6015. [PubMed] [Google Scholar]
- 31.Hunter RP. Nitric oxide, inducible nitric oxide synthase and inflammation in veterinary medicine. Anim Health Res Rev. 2002;3:119–133. [PubMed] [Google Scholar]
- 32.Herrera BS, Martins-Porto R, Maia-Dantas A, et al. iNOS-derived nitric oxide stimulates osteoclast activity and alveolar bone loss in ligature-induced periodontitis in rats. J Periodontol. 2011;82:1608–1615. doi: 10.1902/jop.2011.100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan Z, Guzeldemir E, Toygar HU, Bal N, Bulut S. Nitric oxide synthase in gingival tissues of patients with chronic periodontitis and with and without diabetes. J Periodontol. 2010;81:109–120. doi: 10.1902/jop.2009.090454. [DOI] [PubMed] [Google Scholar]
- 34.Leitao RF, Ribeiro RA, Chaves HV, Rocha FA, Lima V, Brito GA. Nitric oxide synthase inhibition prevents alveolar bone resorption in experimental periodontitis in rats. J Periodontol. 2005;76:956–963. doi: 10.1902/jop.2005.76.6.956. [DOI] [PubMed] [Google Scholar]
- 35.Kendall HK, Haase HR, Li H, Xiao Y, Bartold PM. Nitric oxide synthase type-II is synthesized by human gingival tissue and cultured human gingival fibroblasts. J Periodontal Res. 2000;35:194–200. doi: 10.1034/j.1600-0765.2000.035004194.x. [DOI] [PubMed] [Google Scholar]
- 36.Reher VG, Zenobio EG, Costa FO, Reher P, Soares RV. Nitric oxide levels in saliva increase with severity of chronic periodontitis. J Oral Sci. 2007;49:271–276. doi: 10.2334/josnusd.49.271. [DOI] [PubMed] [Google Scholar]
- 37.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koll-Klais P, Mandar R, Leibur E, Marcotte H, Hammarstrom L, Mikelsaar M. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol. 2005;20:354–361. doi: 10.1111/j.1399-302X.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 39.Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potempa M, Potempa J, Kantyka T, et al. Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3. PLoS Pathog. 2009;5:e1000316. doi: 10.1371/journal.ppat.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fine DH, Markowitz K, Furgang D, et al. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J Clin Microbiol. 2007;45:3859–3869. doi: 10.1128/JCM.00653-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 43.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: The Polymicrobial Synergy and Dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2013 doi: 10.1016/j.it.2013.09.001. Epub ahead of print: http://dx.doi.org/10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed]
- 45.Colombo AP, Bennet S, Cotton SL, et al. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J Periodontol. 2012;83:1279–1287. doi: 10.1902/jop.2012.110566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berezow AB, Darveau RP. Microbial shift and periodontitis. Periodontol 2000. 2011;55:36–47. doi: 10.1111/j.1600-0757.2010.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nase L, Hatakka K, Savilahti E, et al. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 2001;35:412–420. doi: 10.1159/000047484. [DOI] [PubMed] [Google Scholar]
- 48.Caglar E, Kuscu OO, Cildir SK, Kuvvetli SS, Sandalli N. A probiotic lozenge administered medical device and its effect on salivary mutans streptococci and lactobacilli. Int J Paediatr Dent. 2008;18:35–39. doi: 10.1111/j.1365-263X.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- 49.Campus G, Cocco F, Carta G, et al. Effect of a daily dose of Lactobacillus brevis CD2 lozenges in high caries risk schoolchildren. Clin Oral Investig. 2013 doi: 10.1007/s00784-00013-00980-00789. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256–1264. doi: 10.1093/ajcn/83.6.1256. [DOI] [PubMed] [Google Scholar]