Abstract
Background
Our objective was to develop a point-based tool to predict conversion from amnestic mild cognitive impairment (MCI) to probable Alzheimer’s disease (AD).
Methods
Subjects were participants in ADNI1. Cox proportional hazards models were used to identify factors associated with development of AD, and a point score was created from predictors in the final model.
Results
The final point score could range from 0 to 9 (mean, 4.8) and included Functional Assessment Questionnaire (2–3 points); MRI middle temporal cortical thinning (1 point); MRI hippocampal subcortical volume (1 point); ADAS-cog (2–3 points); and Clock Test (1 point). Prognostic accuracy was good (Harrell’s c, 0.78; 95% CI: 0.75, 0.81); 3-year conversion rates were 6% (0–3 points), 53% (4–6 points) and 91% (7–9 points).
Conclusions
A point-based risk score combining functional dependence, cerebral MRI measures and neuropsychological test scores provided good accuracy for prediction of conversion from amnestic MCI to AD.
Keywords: Alzheimer’s disease, mild cognitive impairment, prognostic modeling, risk factors
1. INTRODUCTION
Mild cognitive impairment (MCI) was conceptualized more than a decade ago as a transitional stage between normal cognitive aging and Alzheimer’s disease (AD).1 More recently, the criteria have been expanded to include both amnestic and non-amnestic sub-types,2 and individuals with amnestic MCI have been found to be especially likely to develop AD.3 In addition, new diagnostic criteria have proposed using biomarkers to identify individuals with preclinical AD (biomarker evidence with no clinical symptoms)4 and MCI due to AD (biomarker evidence with mild clinical symptoms).5 However, not all individuals with MCI (amnestic or non-amnestic, including those with positive biomarkers) progress to AD, particularly in community-based settings.6–8 Therefore, it is critically important to examine alternative strategies for distinguishing between those with MCI who will develop AD from those who will not so that potential treatments and preventative therapies can be tested in and targeted toward those most likely to benefit.
Numerous recent studies have examined the ability of various neuroimaging techniques and biomarkers to predict conversion from MCI to AD. These have primarily included markers of amyloid beta (Aβ) deposition such as Pittsburgh compound B (PiB) positron emission tomography (PET)9, 10 and cerebrospinal fluid (CSF) Aβ levels11 and markers of neuronal injury such as CSF total and phosphorylated tau,11 fluorodeoxyglucose (FDG) PET,12, 13 and structural magnetic resonance imaging (MRI).14, 15 However, to date, no single biomarker has emerged that predicts conversion with high accuracy.
A recent hypothetical model of the AD neuropathological process posited that Aβ deposition and tau-mediated neuronal injury and dysfunction occur earlier in the disease process, many years prior to the onset of symptoms, whereas structural brain changes, cognitive decline and functional decline occur later in the disease process, closer to development of clinical AD.16 We hypothesized that a multi-domain model that included a combination of MRI, cognitive and functional measures would predict conversion from MCI to AD with good accuracy.
Finally, point-based risk prediction tools have proven to be useful in other settings for stratification of individuals into high- and low-risk groups.17–19 Thus, a point-based risk-stratification tool may be useful in research settings to identify individuals with MCI who are at high risk of conversion. Therefore, the goal of the current study was to develop a multi-domain point-based risk prediction tool to stratify patients with amnestic MCI into those with high versus low risk for conversion to AD.
2. METHODS
2.1 Study population
Subjects were participants in the Alzheimer’s Disease Neuroimaging Initiative 1 (ADNI-1), an ongoing, multicenter study initiated in 2003 to develop clinical, imaging, genetic and biochemical biomarkers for the early detection and tracking of AD.20 Detailed information on ADNI study procedures can be found at http://www.adni-info.org/Scientists/ADNIScientistsHome.aspx. Data are publically available at http://adni.loni.ucla.edu/ and were downloaded for this study on July 31, 2012.
This study focuses on the 382 ADNI participants who were diagnosed with amnestic MCI at baseline and had at least one follow-up visit. Baseline interviews were performed from 10/20/2005 to 10/19/2007. All subjects in ADNI were age 55–90 and had no evidence of cerebrovascular disease (Modified Hachinski Ischaemia Score ≤ 4),21 no evidence of depression (Geriatric Depression Scale < 6),22 stable medications, a study partner, no visual or hearing impairment, good general health, six grades of education or equivalent, English or Spanish language fluency, and no medical contraindications to magnetic resonance imaging (MRI). MCI was defined based on the following criteria: memory complaint verified by study partner, abnormal memory function based on education-adjusted cut-off on the Logical Memory II subscale (delayed paragraph recall) from the Wechsler Memory Scale – Revised (WMS-R),23 Mini-Mental State Examination (MMSE)24 score of 24–30 (inclusive), Clinical Dementia Rating (CDR)25 score of 0.5, and cognitive and functional impairment not severe enough to meet criteria for AD or dementia.
All ADNI subjects or their proxies provided written, informed consent. This project was submitted for review to the UCSF Committee on Human Research (CHR). However, since it involved no contact with human subjects and utilized completely de-identified data, UCSF CHR determined this project did not require review.
2.2 Measures
We first reviewed the literature and identified domains of predictors that were available in the ADNI dataset and either had been associated with conversion from MCI to AD in prior studies or could plausibly be considered as potential predictors of conversion. The domains considered included demographic predictors, medical history predictors, symptoms/vital signs, MRI measures, genetic/blood-based biomarkers and neuropsychological tests, and the specific variables considered within each domain are described in more detail below. We did not consider the domains of positron emission tomography (PET) imaging or cerebrospinal fluid (CSF) biomarkers because these were collected only in subsets of ADNI study participants. In addition, we did not consider the Clinical Dementia Rating (CDR) as a potential predictor because we felt that it was too collinear with the outcome variable of conversion to AD. All potential predictor variables were collected at either the screening or baseline visit.
2.2.1 Demographic predictors
Variables included age, sex, race/ethnicity, marital status, family history of AD, education, and premorbid intelligence based on the American National Adult Reading Test (ANART; range: 0–45, higher scores reflect higher intelligence).26
2.2.2 Medical history predictors
History of medical conditions was determined based on self-report and was classified into categories that included history of depression, stroke, hypertension, other cardiovascular disease (e.g., high cholesterol, coronary artery disease), diabetes, respiratory conditions (e.g., asthma, pneumonia), hematopoetic/lymphatic or malignancy (e.g., anemia, prostate cancer), kidney disease (e.g., kidney stones, renal insufficiency), smoking, head injury, and thyroid conditions (e.g., hypothyroidism, hyperthyroidism).
2.2.3 Symptoms/vital signs
Variables considered in the symptoms/vital signs domain included low energy or insomnia (self-reported, present/absent); abnormal gait (neurologic assessment, present/absent); blood pressure (normal: diastolic < 90 mmHg and systolic <140 mmHg; stage 1 hypertension: diastolic 90–99 or systolic 140–159; stage 2 hypertension: diastolic ≥100 or systolic≥160); and pulse (beats/minute). In addition, body mass index (BMI) was calculated from measured weight and height (kg/m2). Functional dependence was assessed with the 10-item Functional Assessment Questionnaire (FAQ; range: 0–30, higher scores reflect greater functional dependence).27 Neuropsychiatric symptoms were assessed with the 12-item Neuropsychiatric Inventory (NPI; range: 0–36, higher scores indicate more severe neuropsychiatric symptoms).28 Depressive symptoms were assessed with the 15-item GDS (range: 0–15, higher scores reflect greater depressive symptomatology).22
2.2.4 Magnetic resonance imaging (MRI) measures
Cerebral MRI predictor variables were selected from those that have been identified as being predictive of conversion from MCI to AD in prior ADNI studies29, 30 and included hippocampal subcortical volume, entorhinal cortical volume, entorhinal cortical thickness, middle temporal cortical volume, middle temporal cortical thickness, inferior temporal cortical thickness, and inferior parietal cortical thickness. For all MRI measures, the mean value for the left and right hemispheres was used. Only 1.5 Tesla MRI data were considered as 3.0 Tesla data were available on only a subset of subjects. ADNI included extensive MRI quality control procedures to ensure consistency of scanning across the study sites.
2.2.5 Genetic/blood-based biomarkers
Variables included apolipoprotein-E (APOE) e4 genotype as well as plasma levels of amyloid-β (Aβ)-40 and Aβ-42. All samples were processed using a standard protocol and shipped to the University of Pennsylvania (UPenn) Biomarker Core Laboratory for processing.
2.2.6 Neuropsychological tests
Predictor variables in the neuropsychological test domain included the Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-cog),31 which assesses 13 aspects of cognitive function (traditional range: 0–70; expanded range: 0–85; higher scores reflect worse cognitive function).32 The Rey Auditory-Verbal Learning Test33 assesses verbal learning and memory based on a 16-word list; scores considered included the number of words recalled on the 6th learning trial and following a 30-minute delay (range: 0–16; higher scores reflect better recall). Digit Span Forward and Backward34 were utilized to assess working memory; the score is the longest number of digits repeated forward or backward. Category Fluency35 was assessed for animals and vegetables; scores were the number correct in one minute. The Clock Drawing Test36 was utilized to assess visuospatial/executive function (range: 0 to 5; lower scores reflect greater impairment). The Trail Making Test (Parts A & B)37 was performed to assess processing speed and executive function; scores reflect the time to complete the test (higher scores reflect worse/slower performance). The Digit Symbol Substitution Test34 was utilized to assess processing speed; scores reflect the number of items completed in 90 seconds (higher scores reflect better performance). The Boston Naming Test38 was used to assess naming ability; scores reflect the number of items named correctly (range: 0 to 30; higher scores reflect better performance).
2.3 Conversion to AD
Our primary outcome was conversion to probable AD. As part of ADNI-1, subjects were reassessed at 6, 12, 18, 24 and 36 months. Additional follow-ups are being performed annually as part of ADNI-2.20 Potential conversions from MCI to probable AD were initially detected and reported by physicians at each site. Conversions were then reviewed by a clinical monitor and confirmed by the conversion committee to establish a consensus diagnosis. Diagnoses were based on National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable AD.39 Since the exact date of conversion to AD was not known, we used the midpoint between the last follow-up without an AD diagnosis and the first follow-up with an AD diagnosis for our analyses. Subjects that did not convert were censored at the time of their last interview.
2.4 Statistical analyses
We first examined univariate distributions of all potential predictors to assess for evidence of outlier values. Bivariate associations between potential predictors and the outcome (conversion to AD) were then examined using t-tests or analysis of variance for continuous variables and Chi-square tests for categorical variables. For continuous variables, we assessed for non-linearity using graphical techniques and examining percent conversion over categories. Clinically meaningful categories were utilized when available (e.g., blood pressure); for most continuous variables, quartiles were utilized. Variables with ≤ 5 subjects in a given cell were not considered further.
To develop the final predictive model, we first performed a series of Cox proportional hazards analyses in which all variables within a given domain were considered together. For example, all MRI variables were considered together, competing with each other in a single multivariate model to determine which MRI variables were most strongly associated with our outcome. Those variables within each domain that were associated with conversion to AD at p<0.20 were then carried forward and allowed to compete against each other. A less stringent p-value was used at this step to ensure consideration of a wide range of potential predictors.
In the final stage of model development, all variables that were identified within the 6 domains were simultaneously evaluated in a single multivariate model. All variables that were significantly associated with conversion to AD at p<0.05 in this final Cox model were then retained as independent predictors of conversion. Each variable was then assigned a point value by dividing its model coefficient value by the coefficient for the Clock Test and rounding to the nearest integer. The Clock Test was used because it had the smallest coefficient for a dichotomous variable in the final model. This method for determining points has been successfully used in the development of other clinical prediction tools.17, 19 We used Cox proportional hazards rather than logistic regression to account for differential length of follow-up and withdrawals. Analyses were performed using all available data. Mortality and drop-outs were handled by censoring subjects who did not develop AD at the time of their last interview.
Model discrimination was assessed using Harrell’s c statistic. The final model was validated by bootstrapping the entire model selection process to correct for potential optimism due to overfitting.40 Model calibration was assessed by plotting the predicted vs. actual conversion rates (based on Kaplan-Meier estimates) at 1 year and 3 years as a function of point scores. To determine whether the model was equally predictive in younger and older study participants, we also performed analyses stratified based on the median age.
3. RESULTS
Baseline characteristics of the study population and their association with AD are shown in Table 1. Subjects had a mean (SD) age of 75 (7) years; 36% were women, 91% were non-Hispanic white, 20% had ≤ 12 years of education and 80% were married. More than one-fourth of subjects had a history of depression and approximately half had a history of hypertension or other cardiovascular disease. More than half of subjects had 1 or more APOE e4 alleles. Consistent with their MCI diagnosis, mean scores on most neuropsychological tests were in the normal-to-low range.
Table 1.
Baseline Characteristics of 382 Participants with Amnestic MCI
| Characteristic | No. (%) or Mean ± SD |
|---|---|
| Demographic | |
| Age, years | 75 ± 7 |
| Gender, female | 137 (36) |
| Race, non-Hispanic white | 346 (91) |
| Education, ≤ 12 years | 77 (20) |
| Marital status, married | 307 (80) |
| Family history of AD, positive | 132 (35) |
| Premorbid IQ (ANART), score | 13.7±9.9 |
| Medical history | |
| Depression | 106 (28) |
| Stroke | 13 (3) |
| Hypertension | 188 (49) |
| Other cardiovascular disease | 207 (54) |
| Diabetes | 32 (8) |
| Respiratory condition | 88 (23) |
| Cancer/blood condition | 89 (23) |
| Kidney disease | 24 (6) |
| Smoking | 13 (3) |
| Head injury | 12 (3) |
| Thyroid condition | 56 (15) |
| Symptoms and vital signs | |
| Low energy | 78 (20) |
| Insomnia | 46 (12) |
| Abnormal gait | 35 (9) |
| Systolic blood pressure, mmHg | 132.3 (19.4) |
| Diabolic blood pressure, mmHg | 73.5 (10.8) |
| Pulse, beats/minute | 66±10.7 |
| Body mass index, kg/m2 | 26.1 ± 4.0 |
| Functional dependence (FAQ), score | 3.8±4.5 |
| Neuropsychiatric symptoms (NPI), score | 1.9 (2.7) |
| Depressive symptoms (GDS), score | 1.6 (1.4) |
| MRI variables | |
| Hippocampal subcortical volume, mm3 | 3168±537 |
| Entorhinal cortical volume, mm3 | 1641±381 |
| Entorhinal cortical thickness, mm | 3.0±0.5 |
| Middle temporal cortical thickness, mm | 2.6±0.2 |
| Middle temporal cortical volume, mm3 | 9266.8±1455 |
| Inferior temporal cortical thickness, mm | 2.6±0.2 |
| Inferior parietal cortical thickness, mm | 2.1±0.2 |
| Blood-based biospecimens | |
| Apolipoprotein E, ≥1 e4 allele | 207 (54) |
| Aβ-40, pg/mL | 151±54 |
| Aβ-42, pg/mL | 36±12 |
| Neuropsychological measures | |
| ADAS-cog, total score | 18.7±6.4 |
| RAVLT Trial 6, words recalled | 3.8±3.1 |
| RAVLT delayed, words recalled | 2.8±3.3 |
| Digit Span Forward, no. digits | 8.2±2 |
| Digit Span Backward, no. digits | 6.2±2 |
| Category fluency-animals, no. correct | 15.9±5 |
| Category fluency-vegetables, no. correct | 10.8±3.5 |
| Clock Drawing Test, score<4 | 79 (21) |
| Trail Making Test-A, seconds | 45±22 |
| Trail Making Test-B, seconds | 131±73 |
| Digit Symbol Substitution Test, no. correct | 36.9 (11.1) |
| Boston Naming Test, no. correct | 25.5±4 |
AD, Alzheimer’s disease; ADAS-cog, Alzheimer’s Disease Assessment Scale – cognitive subscale; ANART, American National Adult Reading Test; FAQ, Functional Assessment Questionnaire; GDS, Geriatric Depression Scale; MCI, mild cognitive impairment; NPI, Neuropsychiatric Inventory; RAVLT, Rey Auditory Verbal Learning Test. Data missing as follows: ANART (2), Blood pressure (4), pulse (1), FAQ (3), all MRI (3), Aβ-40 (34), Aβ-42 (34), Trail Making Test-B (4), Boston Naming Test (2), Digit Symbol Substitution Test (1), Digit Span Backward (2), ADAS-cog (3).
A total of 179 (46.9%) study participants converted to probable AD over a mean (SD, range) follow-up period of 2.9 (1.1, 0.5–4.0) years. Of the 203 who did not convert, 71 had <3 years of follow-up data and were censored while 132 were followed for at least 3 years. Subjects had a mean (SD) of 4.7 (1.5) visits, and the mean time to AD was 2.2 years. Twenty-three subjects had only one follow-up visit.
The factors that emerged as being predictive of AD (p<0.20) within each domain are shown in Table 2. Demographic predictors included being female or married. None of the medical history variables considered were associated with conversion to AD. In the symptoms/vital signs domain, greater functional dependence (based on FAQ score), more neuropsychiatric symptomatology (NPI≥4), low BMI (<22) and lack of insomnia all were predictors of conversion to AD. The strongest MRI predictors were hippocampal subcortical volume, entorhinal cortical volume and middle temporal cortical thickness. APOE e4 emerged as the only genetic/blood-based predictor. Finally, many neuropsychological assessment measures were associated with conversion to AD, including ADAS-cog score, RAVLT Trial 6 or delayed word recalled, impaired Clock Test, and Trails B score.
Table 2.
Factors Associated with Conversion to AD (p<0.20) Within Each Domain
| Characteristic | 3-Year Conversion to AD | Domain-Specific HR (95% CI) |
|---|---|---|
| Demographic | ||
| Gender | ||
| Male | 45.1% | 1 |
| Female | 53.5% | 1.33 (0.96,1.84) |
| Marital status | ||
| Not married | 47.4% | 1 |
| Married | 48.3% | 1.34 (0.88, 2.04) |
| Symptoms/vital signs | ||
| Functional dependence (FAQ) score | ||
| Lowest quartile (0) | 21.4% | 1 |
| Second quartile (1–2) | 43.0% | 2.47 (1.46,4.16) |
| Third quartile (3–6) | 57.6% | 3.99 (2.44,6.53) |
| Highest quartile (≥ 7) | 79.9% | 6.81 (4.16,11.17) |
| Neuropsychiatric symptoms (NPI) | ||
| <4 | 44.4% | 1 |
| ≥4 | 66.2% | 1.46 (1.01,2.11) |
| Body mass index (kg/m2) | ||
| ≥22 | 45.3% | 1 |
| <22 | 62.0% | 1.47 (1.01, 2.15) |
| Insomnia | ||
| Yes | 38.9% | 1 |
| No | 49.4% | 1.60 (0.97,2.64) |
| MRI variables | ||
| Hippocampal subcortical volume, mm3 | ||
| Highest quartile (3546–4716.5) | 25.6% | 1 |
| Third quartile (3132.5–3546) | 37.9% | 1.35 (0.80, 2.27) |
| Second quartile (2792.5–3132.5) | 53.7% | 1.60 (0.93, 2.75) |
| Lowest quartile (1640.5–2792.5) | 76.3% | 2.08 (1.18, 3.67) |
| Entorhinal cortical volume, mm3 | ||
| Highest quartile (1908.5–2830) | 23.5% | 1 |
| Third quartile (1640.25–1908.5) | 39.0% | 1.59 (0.92, 2.73) |
| Second quartile (1354.4–1640.25) | 60.6% | 2.01 (1.17, 3.44) |
| Lowest quartile (779–1354.4) | 70.7% | 2.31 (1.30, 4.10) |
| Middle temporal cortical thickness, mm | ||
| Highest quartile (2.761–3.052) | 30.7% | 1 |
| Third quartile (2.624–2.761) | 32.3% | 1.17 (0.71,1.92) |
| Second quartile (2.48–2.624) | 51.3% | 1.69 (1.05,2.72) |
| Lowest quartile (1.781–2.48) | 80.7% | 2.85 (1.80,4.54) |
| Genetic/blood-based biospecimens | ||
| APOE e4 | ||
| No e4 alleles | 35.1% | 1 |
| ≥1 e4 alleles | 59.1% | 1.86 (1.36,2.56) |
| Neuropsychological measures | ||
| ADAS-cog, total score | ||
| Lowest quartile (3–14.33) | 13.7% | 1 |
| Second quartile (14.34–18.67) | 48.5% | 2.90 (1.59,5.27) |
| Third quartile (18.68–23.00) | 65.8% | 3.76 (2.04,6.93) |
| Highest quartile (>23.00) | 68.1% | 3.82 (2.04,7.15) |
| RAVLT Trial 6, words recalled | ||
| Highest quartile (≥ 5) | 13.2% | 1 |
| Third quartile (4–5) | 46.8% | 2.00 (0.95,4.20) |
| Second quartile (2–3) | 65.1% | 2.54 (1.17,5.52) |
| Lowest quartile (0–1) | 65.8% | 2.41 (1.09,5.31) |
| RAVLT delayed, words recalled | ||
| Highest quartile (≥ 5) | 17.6% | 1 |
| Third quartile (3–4) | 45.1% | 1.14 (0.58, 2.25) |
| Second quartile (1–2) | 53.4% | 1.08 (0.55, 2.13) |
| Lowest quartile (0) | 69.6% | 1.63 (0.83, 3.20) |
| Clock Test score | ||
| 4–5 | 42.2% | 1 |
| 0–3 | 71.0% | 1.57 (1.11,2.23) |
| Trail Making Test-B, seconds | ||
| Lowest quartile (0–76) | 35.7% | 1 |
| Second quartile (77–105.5) | 42.7% | 0.98 (0.61, 1.56) |
| Third quartile (105.5–165) | 50.8% | 1.44 (0.92, 2.26) |
| Highest quartile (>165) | 65.8% | 1.61 (1.03, 2.51) |
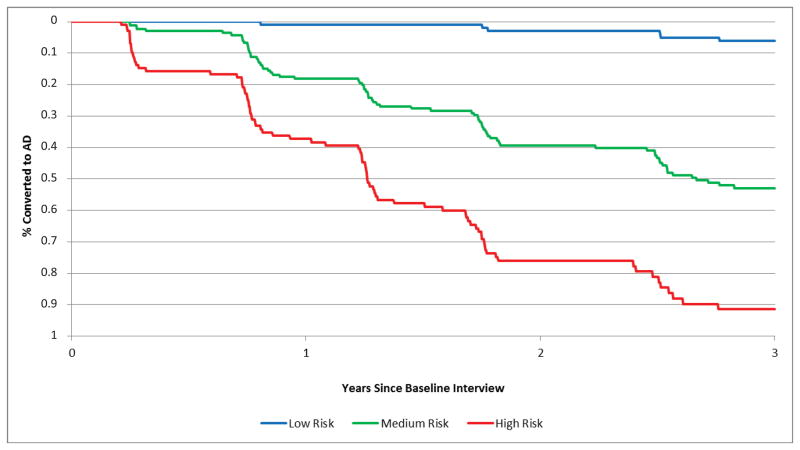
The factors identified in the final model as being most predictive of conversion from MCI to AD are shown in Table 3 along with the coefficient values and number of points for each predictor. Total point score values could range from 0 to 9, with a mean (SD) of 4.8 (2.3). Key predictors included greater functional dependence based on the FAQ (2–3 points); MRI middle temporal cortical thinning (1 point); MRI hippocampal subcortical volume (1 point); worse neuropsychological test performance on the ADAS-cog (2–3 points); and impaired Clock Test (1 point). The total point score was highly predictive of conversion from MCI to AD (Harrell’s c, 0.78; 95% CI: 0.75, 0.81). Furthermore, when subjects were grouped based on their risk scores, 6.2% of subjects with low risk scores (0 to 3 points, n=111) converted to AD over 3 years, compared to 52.9% of those with moderate risk scores (4 to 6 points, n=169) and 91.4% of those with high risk scores (7 to 9 points, n=102) (Figure 1).
TABLE 3.
Final Prognostic Model for Conversion from Amnestic MCI to AD
| Characteristic | Coefficient | Points |
|---|---|---|
| Functional dependence (FAQ) score | ||
| Lowest quartile (0) | 0 | 0 |
| Second quartile (1–2) | 0.95 | 2 |
| Third quartile (3–6) | 1.02 | 2 |
| Highest quartile (≥ 7) | 1.52 | 3 |
| Middle temporal cortical thickness, mm | ||
| Highest quartile (2.761–3.052) | 0 | 0 |
| Third quartile (2.624–2.761) | 0.03 | 0 |
| Second quartile (2.48–2.624) | 0.47 | 1 |
| Lowest quartile (1.781–2.48) | 0.75 | 1 |
| Hippocampal subcortical volume, mm3 | ||
| Highest quartile (3546.0–4716.5) | 0 | 0 |
| Third quartile (3132.5–3546.0) | 0.27 | 0 |
| Second quartile (2792.50–3132.5) | 0.61 | 1 |
| Lowest quartile (1640.5–2792.5) | 0.81 | 1 |
| ADAS-cog, total score | ||
| Lowest quartile (3–14.33) | 0 | 0 |
| Second quartile (14.34–18.67) | 1.29 | 2 |
| Third quartile (18.68–23.00) | 1.64 | 2 |
| Highest quartile (>23.00) | 1.59 | 3 |
| Clock Test score, <4 | 0.56 | 1 |
| Total range | 0–9 | |
| Harrell’s c (95% CI) | 0.78 (0.75,0.81) | |
| Corrected for optimism | 0.74 |
Figure 1. Conversion to AD in Participants with Low, Moderate and High Risk Scores.
Figure 1 shows actual conversion to AD as a function of risk score group, with low risk (0 to 3 points) shown in blue, moderate risk (4 to 6 points) shown in green and high risk (7 to 9 points) shown in red.
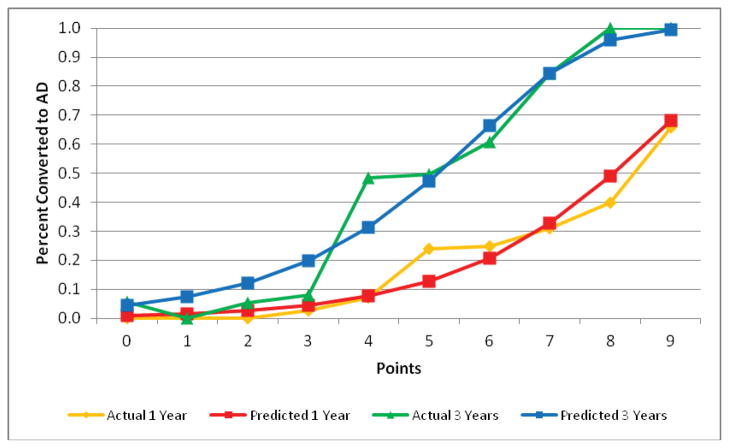
Validation of the final model using boot-strapping techniques estimated optimism as 0.04 (Harrell’s c corrected for optimism, 0.74). Figure 2 shows the actual and predicted rates of conversion to AD at 1 year and 3 years as a function of the point score, suggesting excellent calibration of the final model. The median age of study participants was 75 years, and the model was equally predictive in those <75 (Harrell’s c, 0.80) and those ≥75 years old (0.78).
Figure 2. Actual and Predicted Conversion Rates Over 1 and 3 Years.
Figure 2 shows actual and predicted conversion rates over 1 year and 3 years of follow-up as a function of point score. The grey bars show the number of subjects with each point score value. The overlapping of the actual and predicted curves suggests good calibration of the model.
4. DISCUSSION
We found that a point-score-based combination of functional dependence, neuropsychological test performance and cerebral MRI measures at baseline stratified subjects with amnestic MCI into those with a low, moderate or high risk of converting to probable AD within 1 to 3 years with good accuracy. If validated in other study populations, this point-score may be useful in research settings, where it could potentially be used to identify MCI subjects with a high risk of conversion who could be targeted for secondary prevention trials.
Most prior studies of predictors of conversion from MCI to probable AD have focused on various imaging techniques and biomarkers. However, to date, these approaches have been limited by relatively low overall prognostic accuracy. For example, the Spatial Pattern of Abnormalities for Recognition of Early AD (SPARE-AD), which applies advanced pattern analysis methods to structural MRIs, was found to have c statistics for prediction of conversion from MCI to AD that ranged from 0.66 to 0.73, depending on the sample.41 Furthermore, accuracy of SPARE-AD was not appreciably improved by inclusion of CSF biomarkers, with c statistics of 0.66–0.68.41 Vermuri et al. found only modest prognostic accuracy using structural imaging based on the Structural Abnormality (STAND) Index (c statistic, 0.69) or CSF Aβ1-42 (0.62).42
The key predictors of conversion from MCI to probable AD in our study were greater functional impairment, lower neuropsychological test scores on measures of global cognitive function and executive function, and evidence of atrophy on cerebral MRIs. This is generally consistent with the conceptual model proposed by Jack et al.,16 which hypothesized that impairments in brain structure, memory and function would occur most proximally to the development of clinical AD symptoms, although additional studies are needed to examine the more distal predictors of conversion in the model.
Surprisingly, several recent studies that have compared the effects of different predictors of conversion from MCI to AD have not considered functional measures.43–45 This may be because individuals with MCI, by definition, must be generally functionally independent;1, 2 however, consistent with other studies,46 our findings suggest that even very mild functional limitations are strong predictors of conversion to probable AD in individuals with amnestic MCI.
Our findings are complimentary to several recent publications that have examined multi-domain models for predicting conversion from MCI to AD. Cui at al.30 found that a combination of functional, neuropsychological and MRI measures had a c statistic of 0.78, and addition of CSF biomarkers improved this only slightly to 0.80.30 Gomar et al.47 also found that cognitive and MRI measures were the strongest predictors of conversion, with a c statistic of 0.80. Devanand et al.48 achieved even higher accuracy in models that included functional, cognitive, and MRI measures, with c statistics of 0.86 and 0.94 in two cohorts. Ye et al.49 created the Biosignature-15 that included MRI, cognitive, genetic, functional and lab values and had a c statistic of 0.86. Our findings build on this prior work by creating a point score to stratify individuals with amnestic MCI into those with low, moderate or high risk of conversion to probable AD. Taken together, these studies suggest that multi-domain prognostic models are more accurate than single-domain models; however, in some settings, it may be preferable to accept lower accuracy in exchange for parsimony.
In research settings, interventions and strategies for secondary prevention of AD could potentially restrict enrollment to individuals with MCI who receive a high-risk score. A limitation of many prior RCTs has been that AD conversion rates are lower than expected and, as a result, larger sample sizes are needed to test treatment effects.50 This inefficiency increases the costs of RCTs and also exposes a large number of low-risk individuals to unnecessary side effects. Therefore, restricting RCTs to MCI subjects with a high risk of conversion has the potential to both lower costs and minimize potential harms. Furthermore, a key finding of this study and other recent studies is that multi-domain models have the highest prognostic accuracy, which suggests that multi-domain intervention strategies may also be needed.
Our study has several limitations that should be considered when interpreting the results. Most study participants are white and have high educational attainment and only 36% were women. In addition, individuals with cerebrovascular disease, depression or contraindications to MRI were excluded. Therefore, it is critically important to determine the accuracy of the risk score in other settings and study populations. We also were unable to consider PET imaging or CSF biomarkers as these were measured at baseline in a small subset of ADNI 1 participants. However, other studies that have considered these biomarkers have found that they do not appear to appreciably improve accuracy for predicting conversion from MCI to AD.30, 41 ADNI also does not routinely collect data on other potentially important predictors such as physical performance (e.g., walking speed) or lifestyle factors (e.g., physical activity) that may be predictive of conversion rates. Some variables initially identified as being predictive of conversion within domains were counterintuitive (e.g., being married, lack of insomnia); however, these likely reflect false positives due to our relaxed p-value of 0.20 at that step, as they were not retained in the final model. Finally, our risk score was internally validated using bootstrapping techniques to estimate optimism and should be used in limited research settings until it is externally validated. It is possible that different approaches may be necessary depending on the target population, intervention of interest, and length of follow-up. Also, this risk score is not intended as a diagnostic tool, and patients and family members should be counseled that risk scores are never 100% predictive.
In conclusion, we have created a point score that uses a combination of cognitive, functional and structural MRI measures to stratify individuals with amnestic MCI into those with a low, moderate or high risk of conversion to AD with good accuracy. This tool could potentially be used in research settings to identify individuals for secondary prevention trials.
Research in Context.
Systematic Review
We searched PUBMED using “Alzheimer’s” (major subject heading) AND “mild cognitive impairment OR MCI” (any field) AND (“risk index” OR “risk score” OR “prognostic index” OR risk prediction OR predict conversion) with results restricted to Engligh-language publications in humans aged 65 years or older. A total of 101 publications were retrieved. The references of relevant publications also were reviewed.
Interpretation
Many recent studies have attempted to identify predictors of conversion from MCI to AD. Most studies have focused on various neuroimaging techniques and biomarkers, especially markers of amyloid beta (Aβ) deposition such as Pittsburgh compound B (PiB) positron emission tomography (PET) and cerebrospinal fluid (CSF) Aβ levels and markers of neuronal injury such as CSF total and phosphorylated tau, fluorodeoxyglucose (FDG) PET, and structural magnetic resonance imaging (MRI). However, to date, no single biomarker has emerged that predicts conversion with high accuracy. Several recent studies also have examined multi-domain models. Consistent with our findings, most of these studies have found that cognitive, functional and structural MRI measures are most strongly predictive of conversion from MCI to AD. The current study builds on this prior work by creating a risk prediction score to help classify individuals with MCI into those with a low, moderate or high risk of conversion.
Future Directions
Our model was internally validated using boot-strapping techniques to estimate optimism. If it is validated in other settings and study populations, our risk score may be useful in research settings, where it could potentially be used to identify MCI subjects with a high risk of conversion who could be targeted for secondary prevention trials.
Acknowledgments
We thank Dr. John Boscardin, Professor of Medicine and Epidemiology & Biostatistics at the University of California, San Francisco, for guidance on statistical methods as well as the study participants.
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514.
Study funding: This study was supported by the S.D. Bechtel, Jr. Foundation. Dr. Yaffe was supported in part by K24-AG031155.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: Dr. Barnes reports research support from the National Institutes of Health, Alzheimer’s Association, Department of Veterans Affairs, Department of Defense, NARSAD, University of California School of Medicine, S.D. Bechtel Jr. Foundation, UCSF Osher Center for Integrative Medicine and UCB Pharma, Inc. and serves as a study design consultant for the UCSF Clinical and Translational Sciences Institute. Ms. Cenzer reports no disclosures. Dr. Yaffe reports serving on data safety monitoring boards for Takeda, Inc and a study sponsored by the NIH and has served as a consultant for Novartis, Inc. Dr. Ritchie reports research funding from the National Institutes of Health, The Commonwealth Fund, The Retirement Research Foundation, programmatic funding support from the S.D. Bechtel Foundation and royalties from UptoDate. Dr. Lee reports funding from the National Institute on Aging and the American Federation of Aging Research through the Beeson Career Development Award (K23AG040779) and the S.D. Bechtel Foundation.
Contributor Information
Deborah E. Barnes, Email: deborah.barnes@ucsf.edu.
Irena S. Cenzer, Email: irena.stijacic@ucsf.edu.
Kristine Yaffe, Email: kristine.yaffe@ucsf.edu.
Christine S. Ritchie, Email: christine.ritchie@ucsf.edu.
Sei J. Lee, Email: sei.lee@ucsf.edu.
References
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Archives of neurology. 2009;66:1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dementia and geriatric cognitive disorders. 2006;22:312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 4.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 7.Brodaty H, Heffernan M, Kochan NA, et al. Mild cognitive impairment in a community sample: The Sydney Memory and Ageing Study. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2012 doi: 10.1016/j.jalz.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Archives of neurology. 2011;68:761–767. doi: 10.1001/archneurol.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiology of aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Villemagne VL, Pike KE, Chetelat G, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Annals of neurology. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Annals of neurology. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 13.Drzezga A, Grimmer T, Riemenschneider M, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2005;46:1625–1632. [PubMed] [Google Scholar]
- 14.Risacher SL, Saykin AJ, West JD, et al. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Current Alzheimer research. 2009;6:347–361. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitwell JL, Przybelski SA, Weigand SD, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain: a journal of neurology. 2007;130:1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet neurology. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes DE, Covinsky KE, Whitmer RA, Kuller LH, Lopez OL, Yaffe K. Predicting risk of dementia in older adults: The late-life dementia risk index. Neurology. 2009;73:173–179. doi: 10.1212/WNL.0b013e3181a81636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. The American journal of cardiology. 1976;38:46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA: the journal of the American Medical Association. 2006;295:801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 20.Weiner MW, Veitch DP, Aisen PS, et al. The Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2012;8:S1–68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Annals of neurology. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 22.Sheikh JI, Yesavage JA. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version; pp. 165–173. [Google Scholar]
- 23.Wechsler D. Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 26.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 28.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 29.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui Y, Liu B, Luo S, et al. Identification of conversion from mild cognitive impairment to Alzheimer’s disease using multivariate predictors. PloS one. 2011;6:e21896. doi: 10.1371/journal.pone.0021896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohs RC. Administration and Scoring Manual for the Alzheimer’s Disease Assessment Scale, 1994 Revised Edition. New York: The Mount Sinai School of Medicine; 1994. [Google Scholar]
- 32.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 33.Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 34.Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 35.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 36.Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 37.Reitan RM. Validity of the Trail-Making Test as an indicator of organic brain damage. Perceptual Motor Skills. 1958;8:271–276. [Google Scholar]
- 38.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 39.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 40.Miao Y, Stijacic Cenzer I, Kirby K, Boscardin WJ. Estimating Harrell’s optimism on predictive indices using bootstrap samples. Proceedings of the SAS Global Forum; 2013. [Google Scholar]
- 41.Davatzikos C, Bhatt P, Shaw LM, Batmanghelich KN, Trojanowski JQ. Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiology of aging. 2011;32:2322, e2319–2327. doi: 10.1016/j.neurobiolaging.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ewers M, Walsh C, Trojanowski JQ, et al. Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiology of aging. 2012;33:1203–1214. doi: 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trzepacz PT, Yu P, Sun J, et al. Comparison of neuroimaging modalities for the prediction of conversion from mild cognitive impairment to Alzheimer’s dementia. Neurobiology of aging. 2013 doi: 10.1016/j.neurobiolaging.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 46.Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 47.Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE Alzheimer’s Disease Neuroimaging I. Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s disease neuroimaging initiative. Archives of general psychiatry. 2011;68:961–969. doi: 10.1001/archgenpsychiatry.2011.96. [DOI] [PubMed] [Google Scholar]
- 48.Devanand DP, Liu X, Brown PJ, Huey ED, Stern Y, Pelton GH. A two-study comparison of clinical and MRI markers of transition from mild cognitive impairment to Alzheimer’s disease. International journal of Alzheimer’s disease. 2012;2012:483469. doi: 10.1155/2012/483469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye J, Farnum M, Yang E, et al. Sparse learning and stability selection for predicting MCI to AD conversion using baseline ADNI data. BMC neurology. 2012;12:46. doi: 10.1186/1471-2377-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jelic V, Kivipelto M, Winblad B. Clinical trials in mild cognitive impairment: lessons for the future. Journal of neurology, neurosurgery, and psychiatry. 2006;77:429–438. doi: 10.1136/jnnp.2005.072926. [DOI] [PMC free article] [PubMed] [Google Scholar]