Abstract
Overweight and obesity before conception as well as excessive weight gain during pregnancy are associated with endocrinological changes of mother and fetus. Insulin resistance physiologically increases during pregnancy, additional obesity further increases insulin resistance. In combination with reduced insulin secretion this leads to gestational diabetes which may develop into type-2-diabetes. The adipose tissue produces TNF-alpha, interleukins and leptin and upregulates these adipokines. Insulin resistance and obesity induce inflammatory processes and vascular dysfunction, which explains the increased rate of pregnancy-related hypertension and pre-eclampsia in obese pregnant women. Between 14 and 28 gestational weeks, the fetal adipose tissue is generated and the number of fat lobules is determined. Thereafter, an increase in adipose tissue is arranged by an enlargement of the lobules (hypertrophy), or even an increase in the number of fat cells (hyperplasia). Human and animal studies have shown that maternal obesity “programmes” the offspring for further obesity and chronic disease. Pregnant women, midwives, physicians and health care politicians should be better informed about prevention, pathophysiological mechanisms, and the burden for society caused by obesity before, during and after pregnancy.
Key words: obesity, pregnancy, adipose tissue, perinatal programming, prevention
Abstract
Zusammenfassung
Übergewicht und Adipositas vor der Konzeption sowie überhöhte Gewichtszunahme während der Schwangerschaft sind mit einer Reihe von Veränderungen des endokrinen Systems von Mutter und Kind verbunden. In der Schwangerschaft steigt die Insulinresistenz ohnehin, bei zusätzlicher Adipositas wird eine schon vorhandene Insulinresistenz verstärkt. Zusammen mit einer verminderten Insulinsekretion führt dies zu einem Gestationsdiabetes, der in einen manifesten Typ-2-Diabetes mellitus konvertieren kann. Das Fettgewebe produziert TNF-alpha, Interleukine und Leptin und sorgt für eine Hochregulierung dieser Adipokine. Insulinresistenz und Adipositas sind mit inflammatorischen Prozessen und vaskulärer Dysfunktion assoziiert, die auch die erhöhte Rate von schwangerschaftsassoziiertem Hypertonus und Präeklampsie adipöser Schwangerer erklärt. Beim Feten entsteht Fettgewebe zwischen den Schwangerschaftswochen 14 und 28. In dieser Zeitspanne wird die Zahl der Fettlobulae festgelegt. Eine spätere Zunahme des Fettgewebes erfolgt durch Vergrößerung der Läppchen (Hypertrophie). Bei Entwicklung einer Adipositas kann es jedoch auch zu einer Zunahme der Zahl der Fettzellen (Hyperplasie) kommen. Studien an Mensch und Tier haben gezeigt, dass mütterliche Adipositas die Nachkommenschaft bereits in der Schwangerschaft für Adipositas und chronische Erkrankungen „programmiert“. Hierüber sollten nicht nur die betroffenen Mütter, sondern auch Hebammen, Ärzte und Gesundheitspolitiker informiert sein, um Risiken zu verhindern und die damit verbundenen Kosten zu limitieren.
Schlüsselwörter: Adipositas, Schwangerschaft, Fettgewebe, perinatale Programmierung, Prävention
Introduction
The prevalence of overweight and obesity has risen dramatically in the past 20 years also including women of child-bearing age. According to the German National Consumption Study, in 2005 and 2006 29 % of 20 to 29-year-old German women were overweight and 8.7 % were obese. In women of 30 to 39 years even 35.3 % were overweight and in addition, 14.3 % were obese 1. There is still an increasing trend representing a challenge for future generations.
Overweight and obesity have an impact on a variety of physiological changes and molecular biological processes during pregnancy. In Part 1 of our two publications we concentrate on the pathophysiological and molecular mechanisms of a high BMI and its effects on maternal metabolism and epigenetic effects on the fetus. We have to be aware of the associated short and long-term risks.
In Part 2, we evaluate evidence-based studies and international guidelines to document steps in diagnosis, prevention and risk reduction. The WHO-classification of overweight and obesity is explained in Part 2, Tab. 2. Experimental and epidemiological data may complement each other and reflect the reality.
Overweight and Obesity during Pregnancy
Pregnancy, overweight and obesity all cause increased insulin resistance, an initial hyperinsulinism and a reduced insulin secretion by pancreatic beta cells, leading to type-2 diabetes (T2D) 2, 3. In pregnancy, mild changes may be physiologic 4. In overweight and obese pregnant women there is a high risk that physiological changes turn into a pathological condition of gestational diabetes (GDM) 5. In observational studies, a close correlation was found between overweight (BMI 25–29.9 kg/m2) or obesity (BMI ≥ 30 kg/m2) and the risk of GDM 6, 7. GDM is present in only 2.3 % of pregnant women within a BMI between 18.5 and 24.9 kg/m2, but in 9.5 % of obese patients 8. A meta-analysis found a 3.76-fold increased risk for GDM in obese compared to non-obese pregnant women, with an 0.82 % increase in prevalence per BMI-gain of 1 kg/m2 9.
Insulin resistance in obesity has been related to (pro-)inflammatory processes and subclinical inflammation 10, 11. These are associated with vascular dysfunction, explaining the increased risk of pre-eclampsia in obese pregnant women 12, 13, 14.
Molecular Biological Mechanisms
The molecular basis of endocrine changes is explained by the fact that adipose tissue stores triglycerides and represents a metabolically highly-active tissue 15, 16, 17, 18, 19, 20. An increase in adipose tissue is associated with an inflammatory reaction inducing insulin resistance and cardiovascular disorders 18 (Fig. 1).
Fig. 1.

Adipokines, obesity and risk factors. Fatty acids are stored as triglycerides (TG) by adipocytes. Hypertrophy of adipocytes results in obesity. This increases secretion of monocyte chemotactic protein 1 (MCP-1) and an accumulation of macrophages leading to a pro-inflammatory status with an increase in adipokines secreted by macrophages (TNF-α, IL-6 and -1). Chronic inflammation with high ectopic levels of muscular lipids, disruptions in mitochondrial β-oxidation and insulin-stimulated glucose transportation are the consequence. Adipokines increase the production of endothelial adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1) and of C-reactive protein (hs CRP) contributing to endothelial dysfunction and atherosclerosis (modified in accordance with 18).
Adipose tissue consists of adipocytes (fat cells) and connective tissue stroma cells, which include endothelial cells, fibroblasts and haematopoetic cells. Immature adipose tissue develops in the fetus between 14 and 16 gestational weeks. Pre-adipocytes gradually differentiate from mesenchymal cell clusters, developing into fat lobules with characteristic lipid vacuoles in the cytoplasm. These fat lobules are surrounded by dense septae of perilobular mesenchymal tissue 21, 22.
Fetal adipose tissue starts to be visible in the head and throat, and later as part of the fetal body and the upper and lower extremities 23. At around 28 gestational weeks, the adipose tissue formation is completed including the number of fat lobules. Thereafter, adipose lobules increase in size (hypertrophy). However, if obesity during childhood develops, even the number of fat cells (hyperplasia) may increase 24. Only in extreme obesity during adulthood, the number of fat cells may still increase 25.
Two types of adipose tissue can be differentiated: white and brown adipose tissue, which have specific functions in fat storage, metabolic activity and thermogenesis respectively 26. In humans, visceral (VAT) and subcutaneous adipose tissue (SAT) are formed. The VAT plays a key role in the development of a metabolic syndrome 16, 17, 21, 27.
In 1993, Hotamisligil et al. demonstrated that fat cells in the adipose tissue of mice produce tumour necrosis factor (TNF)-alpha and that obese mice have a greater TNF-alpha expression and higher TNF-alpha levels 28. They also discovered the association between the TNF-alpha production in adipose tissue and insulin resistance. Only one year later, leptin, the product of the ob gene, was identified as the main secreted product of adipose tissue 15. Excessive TNF-alpha production in human obesity leads to a subclinical inflammation; leptin regulates metabolic processes 29, 30. Additionally, signalling cascades of the cellular metabolism are functionally disturbed. Anti-inflammatory cytokines such as adiponectin which, paradoxically, is reduced in obesity, and interleukin (IL)-10 are produced in the VAT 16, 31, 32. In obesity, adipokines and their receptors are upregulated rather in the VAT than in the SAT 33, 34 which explains that excessive VAT is more closely associated with a metabolic syndrome than excessive SAT 35. Adipokines from the VAT are directly absorbed by the liver via drainage through the portal veins. Thereby, the VAT has a direct impact on hepatic glucose homeostasis and insulin sensitivity 36. Pro-inflammatory cytokines in adipose tissue are positively correlated with liver fat content and systemic arterial dysfunction and negatively correlated with insulin sensitivity 37, 38.
TNF alpha, IL-6, IL-10, leptin and adiponectin are part of more than 50 different “adipokines”, peptides which are produced from the white adipose tissue. They circulate in the maternal blood and play an important role in obesity-specific morbidity 16, 17, 28, 31, 39 (Table 1).
| TNF-alpha | Inflammation, apoptosis, impact on insulin resistance, stimulation of endothelial dysfunction and atherogenesis |
| IL-6 | Inflammation, immune regulation (modulation of the insulin receptor), insulin resistance, atherogenesis |
| Adiponectin | Stimulation of insulin secretion, increase in insulin sensitivity, stimulation of glucose uptake in the muscle, inflammation reduction, plasma lipid reduction, atheroprotective effect |
| Leptin | Saturation, increase in energy utilization, weight control, modulation of insulin sensitivity, reduction of insulin secretion, angiogenesis |
The placenta produces adipokines similar to the white adipose tissue, only lacking adiponectin, a marker for increased insulin sensitivity 40, 41. Challier et al. 42 demonstrated that the number of CD14+ and CD68+ macrophages in the placenta of obese women was three-fold increased compared to normal weight women. These macrophages produce pro-inflammatory cytokines as TNF-alpha and IL-6. Local inflammatory changes in obese pregnant women are also reflected in increased plasma concentrations of C-reactive peptide (CrP) and IL-6. Interestingly, the CD14+ macrophages were of a maternal but not fetal origin 43.
Up to now few studies have investigated the effect of overweight and obesity on inflammation during pregnancy. Ramsay et al. 13 found higher serum concentrations of leptin, CrP and IL-6 in obese compared to normal weight women. These circulating pro-inflammatory cytokines were also correlated to higher levels of TNF-alpha- and IL-6-mRNA produced by maternal peripheral mononuclear cells 42. However, the invasion of macrophages into the VAT have up to now only been demonstrated in non-pregnant obese adults 44, 45. In obese baboons, Farley et al. demonstrated a marked macrophage infiltration into the adipose tissue 46. With increasing degree of obesity macrophages increasingly produce transcription factors, adipokines and inflammatory molecules 47 which also results in insulin resistance 44.
Visceral obesity associated with glucose intolerance and insulin resistance 13, 48, may lead to GDM in obese women 20. Unfortunately, there is a paucity of research related to biochemical pathways of GDM in pregnant women. According to Kirwan et al. 49 TNF-alpha levels in normal weight pregnant women can predict insulin resistance during later stages of pregnancy. Disturbances in the insulin signalling cascade in obese pregnant women with normal glucose tolerance could be demonstrated in both the adipose tissue as well as in the skeletal muscle 50. The negative effects of overweight and obesity become more obvious in glucose intolerance and insulin resistance 21, 51, 52. The inflammatory processes are closely related to the prevalence of pregnancy-induced hypertension or pre-eclampsia in obese pregnant women 53.
Maternal obesity associated with GDM and hypertension is thus related to an inflammatory reaction in the white adipose tissue, in the plasma and the placenta. This “pro-inflammatory state” is supposed to be the primary mechanism underlying the insulin resistance and hypertension in obese pregnant women 20.
Perinatal Programming of Overweight and Obesity
“Perinatal programming” has meanwhile been established as a field of research for dealing with the impact of the intrauterine and early postnatal environment on fundamental mechanisms of health and disease 54, 55, 56, 57, 58, 59 (Fig. 2). The main focus is the phenomenon of an epigenetic, maternal-fetal transmission of acquired conditions.
Fig. 2.
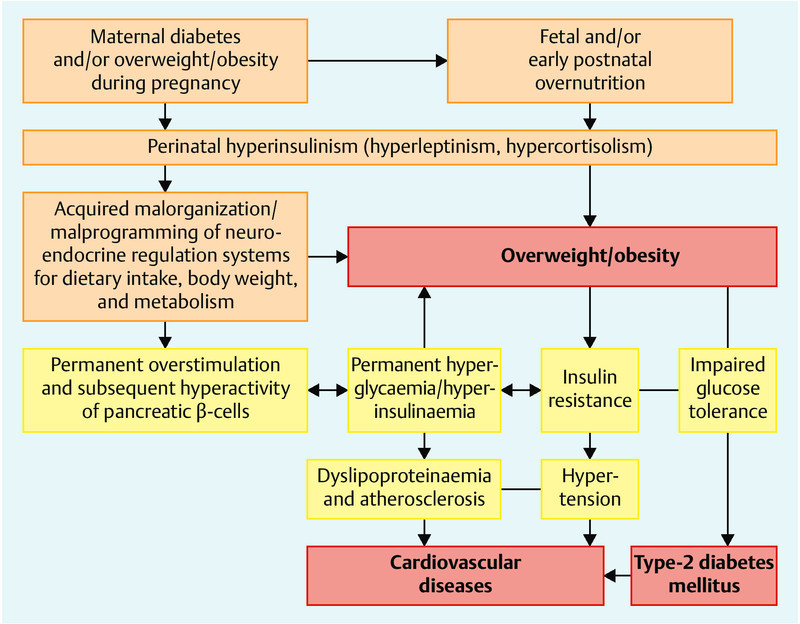
Pathogenetic mechanisms, and consequences of perinatal malprogramming. Aetiology of pre- and neonatal overfeeding and hyperinsulinism for excessive weight gain, overweight/obesity, type-2 diabetes mellitus and subsequent cardiovascular diseases in later life (modified in accordance with 58).
Results from epidemiological, clinical and animal experimental studies indicate the impact of nutrition during the pre-natal and early post-natal development and its impact on the occurrence of overweight, obesity, T2D and cardiovascular diseases in later life 60, 61. The metabolic state during pregnancy and the kind of nutrition in the neonatal period (e.g. breast-feeding and its long-term effect) may both have negative or positive sequelae for the growing child up to adulthood 62, 63, 64, 65.
Günter Dörner at Charité postulated in the 1970s that T2D is “transmitted” more frequently from the maternal line than from the paternal line 66. He developed the concept of “perinatal programming” and established a “functional teratology” 67. In the 1990s the groups of Hales and Barker described the concept of “prenatal origin of adult disease” in growth-retarded fetuses and newborns 68.
Apart from genetic factors, maternal diet and nutritional status during pregnancy have a critical impact on intrauterine growth and birthweight. In developed countries, overall birthweight increased by 126 g during the last 20 to 30 years. Accordingly, the rate of macrosomia rose by 25 % per decade 69, 70, 71, 72 whereby epigenetic causes are supposed to be the underlying mechanism.
The risk of macrosomia (birthweight > 4000 g) is more than doubled or even tripled in children of obese or morbid obese women 73, and mainly combined with excessive weight gain 69, 76. In Part 2, we explain the clinical consequences and complications 74. Regardless of the pre-conceptional weight, maternal weight gain during pregnancy positively correlates with the birthweight 69, 75.
Epidemiological studies showed a positive correlation between birthweight and the body weight in adulthood signifying that maternal obesity-related fetal macrosomia is associated with obesity in later life 77, 78. A meta-analysis of a total of 643 902 individuals between 1 and 75 years of age from 26 countries revealed a positive linear connection between birth weight and the later overweight in 59/66 (89.4 %) of studies. In four of the studies (6.1 %), no correlation was observed. In three studies (4.5 %) a U-shaped relation was described, i.e. a similar risk increase in low and high birth weight. However, no linear inverse relation was described. Based on adjusted estimation, it was shown that the risk of overweight in later life in children with high birth weight was almost doubled (OR 1.96, 95 % CI 1.43–2.67) compared to children of normal birthweight (2500–4000 g) 78.
A meta-analysis on the correlation between birth weight and the subsequent risk of T2D accordingly revealed a U-shaped relation in all published studies explaining that children with low birth and high birth weight exhibit an increased risk for T2D in later life 79.
It seems to be a vicious circle that obesity during pregnancy causes maternal GDM and an increased birth weight and obesity in the offspring who then develop associated diabetic metabolic disorders, e.g., GDM in females 80, 81, 82, 83, 84.
Similarly, animal studies showed that not only maternal overweight and obesity but also the nutrition during pregnancy have an impact on the offspring: Pregnant female Japanese macaques that received either a high-fat (35 % fat) or a normal diet (13 % fat) during pregnancy showed differences in the juvenile microbiome of the gastro-intestinal tract during the first year of life 85. Campylobacter was not detectable in the juvenile microbiome after the high-fat-diet. These changes were very stable and could not even be corrected postnatally by a normal diet.
Maternal obesity and a high-fat diet during pregnancy seem to lead to a malprogramming of the hepatic fatty acid metabolism resulting in upregulated lipogenesis and obesity in the offspring which is indicated by an increased desaturation index (DI), e.g. a ratio of unsaturated (C16 and C18) versus saturated fatty acids. Rats that either received a high-fat or a normal diet did not differ in birth weight, however showed a reduced DI in the offspring of obese rats 86. At the age of six months, the male offspring were significantly heavier (800 vs. 659 g), more obese (26 vs. 20 %) and showed an increased DI in the plasma (0.1 vs. 0.06) and in the liver (0.09 vs. 0.06). A high-fat diet during pregnancy initially suppresses the DI of the newborns but subsequently upregulates the DI despite a normal postnatal diet. Bytautiene et al. demonstrated significantly shorter telomeres and a reduced expression of the Klotho gene in the female and male offspring of obese mice in comparison to normal weight mice, which is a surrogate parameter of an accelerated ageing process 87. A dysfunctional VAT in the offspring of obese mice was described by the same group 88. Obesity before conception was generated by a high-fat diet. The offspring received a normal diet until the age of six months. A reduced expression of the hypoxia-inducible factor 1 alpha (HIF-1 α) and of the angiotensin 2 receptor as well as an increased expression of angiotensin (ANG) were found in the VAT of the offspring of obese mice. These alterations may disturb developmental cascades leading to fetal programmed hypertension and a metabolic syndrome in later life even in following generations.
Perinatal mal-programming may also involve the central nervous regulatory centres of metabolism and body weight control. Thus, maternal overweight and/or maternal diabetes (hyperglycaemia) during pregnancy and early postnatal overnutrition lead to increased insulin, glucose, protein and/or leptin levels during critical development stages (e.g. fetal hyperinsulinism). Malprogramming via epigenetic mechanisms results in a life-long disposition for overweight, obesity and diabetic metabolic disorders across generations 54, 58, 63, 89, 90.
Epigenetic mechanisms are DNA-methylation, the modification of histones and the regulation of microRNAs. Methylation of cytidine bases in cytosine-guanosine nucleotide dimers (CpG) (DNA methylation) has been investigated 91. Highly-methylated DNA (especially the so-called promoter regions) reduces the gene expression 91. Only a few studies have investigated the contribution of epigenetic mechanisms to fetal metabolic programming on a molecular level 92, 93, 94, 95, 96. In placentas of women with gestational diabetes, modifications in the methylation of the leptin and adiponectin genes (LEP and ADIPOQ) have been found. LEP and ADIPOQ are classified as candidate genes for obesity and GDM 93, 96. Furthermore, it was shown that total methylation in the placentas of obese women is significantly higher than in normal-weight women 97. These changes have lasting effects on the regulation of the metabolism of the offspring if they reflect DNA methylation in other tissues, thus triggering the development of chronic metabolic disorders.
In maternal obesity without glucose intolerance the microRNA expression remains unchanged. In a study on 16 obese and 20 normal-weight pregnant women, with a normal glucose tolerance, the microRNA expression in the umbilical cord blood did not differ significantly 98. The authors concluded that in fetal programming other mechanisms are more relevant.
Future prevention should start before conception to avoid maternal obesity, GDM, and the epigenetic modifications. In pregnant rats it was demonstrated that an exercise programme during pregnancy could have positive effects on the metabolic phenotype of the offspring 99. The percentage of fat-free body mass in the male offspring was increased and the fat mass reduced. Interventions and longitudinal studies might help to detect whether we can also modify epigenetics and perinatal programming of GDM, overweight or obesity.
Conclusions
Overweight and obesity lead to an increase in maternal and perinatal morbidity and mortality. The molecular biological mechanisms as gestational diabetes or metabolic syndrome, have not yet been sufficiently investigated. Local and systemic inflammatory processes, triggered by the adipose tissue, seem to play a key role. Despite the increasing prevalence of overweight and obesity in pregnant women in Western countries including Germany, clinical research on mechanisms and interventions are scarce although the diagnosis is easy. Further investigations of the biology of adipose tissue and its association with insulin resistance, the formation of adipokines and endothelial dysfunction are necessary.
Primary prevention programmes have to be implemented. Avoiding overweight and obesity in childhood and adolescence and appropriate weight gain during pregnancy 100 are essential. Educational programs prior to conception are important. The promotion of breast-feeding for newborns should also be considered.
Footnotes
Conflict of Interest None.
Supporting Information
German supporting informations for this article
Literatur
- 1.Max Rubner-Institut Nationale Verzehrsstudie II. Ergebnisbericht, Teil 1. 2008Online:http://www.mri.bund.delast access: 20.01.2014
- 2.Abbasi F, Brown B W jr., Lamendola C. et al. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–943. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin T, Allison G, Abbasi F. et al. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism. 2004;53:495–499. doi: 10.1016/j.metabol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Catalano P M, Tyzbir E D, Roman N M. et al. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165(6 Pt 1):1667–1672. doi: 10.1016/0002-9378(91)90012-g. [DOI] [PubMed] [Google Scholar]
- 5.Catalano P M, Huston L, Amini S B. et al. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 6.Athukorala C, Rumbold A R, Willson K J. et al. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth. 2010;10:56. doi: 10.1186/1471-2393-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ovesen P, Rassmussen S, Kesmodel U. Effect of prepregnancy overweight and obesity on pregnancy outcome. Obstet Gynecol. 2011;118(2 Pt 1):305–312. doi: 10.1097/AOG.0b013e3182245d49. [DOI] [PubMed] [Google Scholar]
- 8.Weiss J L, Malone F D, Emig D. et al. FASTER Research Consortium . Obesity, obstetric complications and cesarean delivery rate – a population-based screening study. Am J Obstet Gynecol. 2004;190:1091–1097. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 9.Torloni M R, Betran A P, Horta B L. et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10:194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 10.Challis J R, Lockwood C J, Myatt L. et al. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;2010:535918. doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangge H, Almer G, Truschnig-Wilders M. et al. Inflammation, adiponectin, obesity and cardiovascular risk. Curr Med Chem. 2010;17:4511–4520. doi: 10.2174/092986710794183006. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay J E, Ferrell W R, Crawford L. et al. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87:4231–4237. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 14.Roberts J M, Bodnar L M, Patrick T E. et al. The role of obesity in preeclampsia. Pregnancy Hypertens. 2011;1:6–16. doi: 10.1016/j.preghy.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Proenca R, Maffei M. et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 16.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006;64:355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 17.Fischer-Posovszky P, Wabitsch M, Hochberg Z. Endocrinology of adipose tissue - an update. Horm Metab Res. 2007;39:314–321. doi: 10.1055/s-2007-976539. [DOI] [PubMed] [Google Scholar]
- 18.Gelsinger C, Tschoner A, Kaser S. et al. [Adipokine update – new molecules, new functions] Wien Med Wochenschr. 2010;160:377–390. doi: 10.1007/s10354-010-0781-6. [DOI] [PubMed] [Google Scholar]
- 19.Denison F C, Roberts K A, Barr S M. et al. Obesity, pregnancy, inflammation, and vascular function. Reproduction. 2010;140:373–385. doi: 10.1530/REP-10-0074. [DOI] [PubMed] [Google Scholar]
- 20.Catalano P M. Trying to understand gestational diabetes. Diabet Med. 2014;31:273–281. doi: 10.1111/dme.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poissonnet C M, Burdi A R, Bookstein F L. Growth and development of human adipose tissue during early gestation. Early Hum Dev. 1983;8:1–11. doi: 10.1016/0378-3782(83)90028-2. [DOI] [PubMed] [Google Scholar]
- 22.Martin R J, Hausman G J, Hausman D B. Regulation of adipose cell development in utero. Proc Soc Exp Biol Med. 1998;219:200–210. doi: 10.3181/00379727-219-44333. [DOI] [PubMed] [Google Scholar]
- 23.Poissonnet C M, Burdi A R, Garn S M. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev. 1984;10:1–11. doi: 10.1016/0378-3782(84)90106-3. [DOI] [PubMed] [Google Scholar]
- 24.Knittle J L, Timmers K, Ginsberg-Fellner F. et al. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest. 1979;63:239–246. doi: 10.1172/JCI109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spalding K L, Arner E, Westermark P O. et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 26.Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab. 2009;297:E977–E986. doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- 27.Després J P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil G S, Shargill N S, Spiegelman B M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 29.Lago F, Dieguez C, Gómez-Reino J. et al. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007;18:313–325. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Maury E, Brichard S M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Hu E, Liang P, Spiegelman B M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 32.Milan G, Granzotto M, Scarda A. et al. Resistin and adiponectin expression in visceral fat of obese rats: effect of weight loss. Obes Res. 2002;10:1095–1103. doi: 10.1038/oby.2002.149. [DOI] [PubMed] [Google Scholar]
- 33.Huber J, Kiefer F W, Zeyda M. et al. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008;93:3215–3221. doi: 10.1210/jc.2007-2630. [DOI] [PubMed] [Google Scholar]
- 34.Fain J N, Madan A K, Hiler M L. et al. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 35.Demerath E W, Reed D, Rogers N. et al. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. Am J Clin Nutr. 2008;88:1263–1271. doi: 10.3945/ajcn.2008.26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tordjman J, Poitou C, Hugol D. et al. Association between omental adipose tissue macrophages and liver histopathology in morbid obesity: influence of glycemic status. J Hepatol. 2009;51:354–362. doi: 10.1016/j.jhep.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 37.Apovian C M, Bigornia S, Mott M. et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poulain-Godefroy O, Lecoeur C, Pattou F. et al. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1–R7. doi: 10.1152/ajpregu.00926.2007. [DOI] [PubMed] [Google Scholar]
- 39.Staiger H, Häring H U. Adipocytokines: fat-derived humoral mediators of metabolic homeostasis. Exp Clin Endocrinol Diabetes. 2005;113:67–79. doi: 10.1055/s-2004-830555. [DOI] [PubMed] [Google Scholar]
- 40.Radaelli T, Varastehpour A, Catalano P. et al. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52:2951–2958. doi: 10.2337/diabetes.52.12.2951. [DOI] [PubMed] [Google Scholar]
- 41.Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27:794–798. doi: 10.1016/j.placenta.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Challier J C, Basu S, Bintein T. et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29:274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basu S, Leahy P, Challier J C. et al. Molecular phenotype of monocytes at the maternal-fetal interface. Am J Obstet Gynecol. 2011;205:2650–2.65E10. doi: 10.1016/j.ajog.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu H, Barnes G T, Yang Q. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hotamisligil G S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 46.Farley D, Tejero M E, Comuzzie A G. et al. Feto-placental adaptations to maternal obesity in the baboon. Placenta. 2009;30:752–760. doi: 10.1016/j.placenta.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lumeng C N, Bodzin J L, Saltiel A R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin A M, Berger H, Nisenbaum R. et al. Abdominal visceral adiposity in the first trimester predicts glucose intolerance in later pregnancy. Diabetes Care. 2009;32:1308–1310. doi: 10.2337/dc09-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirwan J P, Hauguel-De Mouzon S, Lepercq J. et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 50.Colomiere M, Permezel M, Riley C. et al. Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur J Endocrinol. 2009;160:567–578. doi: 10.1530/EJE-09-0031. [DOI] [PubMed] [Google Scholar]
- 51.Metzger B E, Lowe L P, Dyer A R. et al. HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 52.Boomsma C M, Eijkemans M J, Hughes E G. et al. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–683. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 53.Bodnar L M, Ness R B, Harger G F. et al. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol. 2005;162:1198–1206. doi: 10.1093/aje/kwi334. [DOI] [PubMed] [Google Scholar]
- 54.Plagemann A. ‘Fetal programming and ʼfunctional teratogenesisʼ: on epigenetic mechanisms and prevention of perinatally acquired lasting health risks. J Perinat Med. 2004;32:297–305. doi: 10.1515/JPM.2004.055. [DOI] [PubMed] [Google Scholar]
- 55.Catalano P M, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204:479–487. doi: 10.1016/j.ajog.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plagemann A, Harder T, Schellong K. et al. Early postnatal life as a critical time window for determination of long-term metabolic health. Best Pract Res Clin Endocrinol Metab. 2012;26:641–653. doi: 10.1016/j.beem.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Pinney S E, Simmons R A. Metabolic programming, epigenetics, and gestational diabetes mellitus. Curr Diab Rep. 2012;12:67–74. doi: 10.1007/s11892-011-0248-1. [DOI] [PubMed] [Google Scholar]
- 58.Plagemann A. Berlin, Boston: de Gruyter; 2012. Toward a unifying Concept on perinatal Programming: vegetative Imprinting by Environment-dependent Biocybernetogenesis; pp. 243–282. [Google Scholar]
- 59.Ruchat S M, Hivert M F, Bouchard L. Epigenetic programming of obesity and diabetes by in utero exposure to gestational diabetes mellitus. Nutr Rev. 2013;71 01:S88–S94. doi: 10.1111/nure.12057. [DOI] [PubMed] [Google Scholar]
- 60.Plagemann A. Perinatal programming and functional teratogenesis: impact on body weight regulation and obesity. Physiol Behav. 2005;86:661–668. doi: 10.1016/j.physbeh.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 61.Plagemann A, Harder T, Dudenhausen J W. The diabetic pregnancy, macrosomia, and perinatal nutritional programming. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:91–102. doi: 10.1159/000113179. [DOI] [PubMed] [Google Scholar]
- 62.Harder T, Rodekamp E, Schellong K, München: Springer; 2010. Adipositas und perinatale Programmierung; pp. 72–81. [Google Scholar]
- 63.Plagemann A. Maternal diabetes and perinatal programming. Early Hum Dev. 2011;87:743–747. doi: 10.1016/j.earlhumdev.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 64.Plagemann A, Harder T. Fuel-mediated teratogenesis and breastfeeding. Diabetes Care. 2011;34:779–781. doi: 10.2337/dc10-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stupin J H, Harder T, Plagemann A. [Fetal programming during diabetic pregnancy.] Adipositas. 2011;5:134–140. [Google Scholar]
- 66.Dörner G, Mohnike A. Further evidence for a predominantly maternal transmission of maturity-onset type diabetes. Endokrinologie. 1976;68:121–124. [PubMed] [Google Scholar]
- 67.Dörner G. Basel: Karger; 1975. Perinatal Hormone Levels and Brain Organization; pp. 245–252. [Google Scholar]
- 68.Hales C N, Barker D J. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 69.Bergmann R L, Richter R, Bergmann K E. et al. Secular trends in neonatal macrosomia in Berlin: influences of potential determinants. Paediatr Perinat Epidemiol. 2003;17:244–249. doi: 10.1046/j.1365-3016.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- 70.Harder T, Plagemann A. The intrauterine environmental adipogenesis. J Pediatr. 2004;144:551–552. doi: 10.1016/j.jpeds.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 71.Hesse V, Voigt M, Sälzler A. et al. Alterations in height, weight, and body mass index of newborns, children, and young adults in eastern Germany after German reunification. J Pediatr. 2003;142:259–262. doi: 10.1067/mpd.2003.85. [DOI] [PubMed] [Google Scholar]
- 72.Sewell M F, Huston-Presley L, Super D M. et al. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–1103. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 73.Cedergren M I. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 74.Mission J F, Marshall N E, Caughey A B. Obesity in pregnancy: a big problem and getting bigger. Obstet Gynecol Surv. 2013;68:389–399. doi: 10.1097/OGX.0b013e31828738ce. [DOI] [PubMed] [Google Scholar]
- 75.Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr. 2000;71(5 Suppl.):1242S–1248S. doi: 10.1093/ajcn/71.5.1242s. [DOI] [PubMed] [Google Scholar]
- 76.Helms E, Coulson C C, Galvin S L. Trends in weight gain during pregnancy: a population study across 16 years in North Carolina. J Obstet Gynecol. 2006;194:e32–e34. doi: 10.1016/j.ajog.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 77.Harder T, Schellong K, Stupin J. et al. Where is the evidence that low birthweight leads to obesity? Lancet. 2007;369:1859. doi: 10.1016/S0140-6736(07)60847-2. [DOI] [PubMed] [Google Scholar]
- 78.Schellong K, Schulz S, Harder T. et al. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS One. 2012;7:e47776. doi: 10.1371/journal.pone.0047776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harder T, Rodekamp E, Schellong K. et al. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165:849–857. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- 80.Dabelea D, Hanson R L, Lindsay R S. et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 81.Pettitt D J, Baird H R, Aleck K A. et al. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983;308:242–245. doi: 10.1056/NEJM198302033080502. [DOI] [PubMed] [Google Scholar]
- 82.Plagemann A, Harder T, Kohlhoff R. et al. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes Relat Metab Disord. 1997;21:451–456. doi: 10.1038/sj.ijo.0800429. [DOI] [PubMed] [Google Scholar]
- 83.Plagemann A, Harder T, Kohlhoff R. et al. Glucose tolerance and insulin secretion in children of mothers with pregestational IDDM or gestational diabetes. Diabetologia. 1997;40:1094–1100. doi: 10.1007/s001250050792. [DOI] [PubMed] [Google Scholar]
- 84.Silverman B L, Rizzo T, Green O C. et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40 02:121–125. doi: 10.2337/diab.40.2.s121. [DOI] [PubMed] [Google Scholar]
- 85.Prince A, Ma J, Bader D. et al. Maternal diet persistently alters the developing juvenile microbiome in a primate model of obesity (abstract) Am J Obstet Gynecol. 2014;210 01:S30. [Google Scholar]
- 86.Seet E, Yee J, Ross M. et al. Programmed adipogenesis and obesity in offspring of obese dams (abstract) Am J Obstet Gynecol. 2014;210 01:S33. [Google Scholar]
- 87.Bytautiene E, Kechichian T, Syes T. et al. Accelerated aging in the offspring of mothers with pre-pregnancy obesity in a mouse model of developmental programming of metabolic syndrome (abstract) Am J Obstet Gynecol. 2014;210 01:S30–S31. [Google Scholar]
- 88.Bytautiene E, Banerjee D, Kechichian T. et al. Adipose tissue dysfunction in a model of developmental programming of metabolic syndrome. Am J Obstet Gynecol. 2014;210 01:S97. [Google Scholar]
- 89.Dörner G, Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus, obesity and enhanced cardiovascular risk in later life. Horm Metab Res. 1994;26:213–221. doi: 10.1055/s-2007-1001668. [DOI] [PubMed] [Google Scholar]
- 90.Plagemann A, Harder T, Brunn M. et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol. 2009;587(Pt 20):4963–4976. doi: 10.1113/jphysiol.2009.176156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 92.Heijmans B T, Tobi E W, Stein A D. et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bouchard L, Thibault S, Guay S P. et al. Leptin gene epigenetic adaptation to impaired glucose metabolism during pregnancy. Diabetes Care. 2010;33:2436–2441. doi: 10.2337/dc10-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tobi E W, Heijmans B T, Kremer D. et al. DNA methylation of IGF2, GNASAS, INSIGF and LEP and being born small for gestational age. Epigenetics. 2011;6:171–176. doi: 10.4161/epi.6.2.13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Filiberto A C, Maccani M A, Koestler D. et al. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics. 2011;6:566–572. doi: 10.4161/epi.6.5.15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bouchard L, Hivert M F, Guay S P. et al. Placental adiponectin gene DNA methylation levels are associated with mothersʼ blood glucose concentration. Diabetes. 2012;61:1272–1280. doi: 10.2337/db11-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nomura Y, Lambertini L, Rialdi A. et al. Global methylation in the placenta and umbilical cord blood from pregnancies with maternal gestational diabetes, preeclampsia, and obesity. Reprod Sci. 2014;21:131–137. doi: 10.1177/1933719113492206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghaffari N, Parry S, Durnwald C. The role of miRNA in fetal programming of obesity. Am J Obstet Gynecol. 2014;210 01:S70. [Google Scholar]
- 99.Nathanielsz P W, Ford S P, Long N M. et al. Interventions to prevent adverse fetal programming due to maternal obesity during pregnancy. Nutr Rev. 2013;71 01:S78–S87. doi: 10.1111/nure.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Institute of Medicine (IOM) . Washington: National Research Council; 2009. Weight Gain during Pregnancy: re-examining the Guidelines. Committee to Reexamine IOM Pregnancy Weight Guidelines. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
German supporting informations for this article


