Abstract
Background
Minimally invasive pancreatic surgery has evolved rapidly, but total laparoscopic pancreaticoduodenectomy has not been widely adopted owing to its technical complexity. Hybrid laparoscopy-assisted pancreaticoduodenectomy (HLAPD) combines the relative ease of open surgery with the benefits of a minimally invasive approach. This study evaluates the safety and effectiveness of the hybrid approach compared with open surgery.
Methods
We retrospectively analyzed data of consecutive patients undergoing either hybrid or open pancreaticoduodenectomy (OPD) at our institution between September 2009 and December 2013. Demographic, operative and oncologic data were collected to compare outcomes between HLAPD and OPD.
Results
Our analysis included 33 patients (HLAPD: n = 13; OPD: n = 20). There were no differences in patient demographics, comorbidities or surgical indications. The HLAPD group had significantly lower intraoperative blood loss (450 mL v. 1000 mL, p = 0.023) and shorter length of hospital stay (8 v. 12 d, p = 0.025) than the OPD group. Duration of surgery did not differ significantly between the groups. There were no differences in postoperative analgesic requirements, Clavien grade I/II or grade III/IV complications or 90-day mortality. Oncologic outcomes showed no significant differences in tumour size, R1 resection rate or number of lymph nodes harvested.
Conclusion
In select patients, HLAPD is a safe and effective procedure with comparable outcomes to conventional open surgery. Wider adoption of the hybrid approach will allow a greater number of patients to benefit from a less invasive procedure while facilitating the transition toward purely minimally invasive pancreaticoduodenectomy.
Abstract
Contexte
La chirurgie pancréatique minimalement effractive a rapidement évolué, mais la pancréatoduodénectomie laparoscopique totale n’a pas été largement adoptée en raison de sa complexité technique. La pancréatoduodénectomie hybride sous laparoscopie (PDHL) allie la relative facilité de la chirurgie ouverte aux avantages d’une approche minimalement effractive. Cette étude compare l’innocuité et l’efficacité de l’approche hybride à celles de la chirurgie ouverte.
Méthodes
Nous avons analysé de manière rétrospective les données concernant des patients consécutifs soumis à une pancréatoduodénectomie hybride ou ouverte (PDO) dans notre établissement entre septembre 2009 et décembre 2013. Les données démographiques, opératoires et oncologiques ont été recueillies pour comparer les résultats entre la PDHL et la PDO.
Résultats
Notre analyse a inclus 33 patients (PDHL : n = 13; PDO : n = 20). Il n’y avait aucune différence quant aux caractéristiques démographiques, comorbidités ou indications chirurgicales chez les patients. Le groupe soumis à la PDHL a connu des pertes sanguines peropératoires significativement moindres (450 mL c. 1000 mL, p = 0,023) et un séjour hospitalier significativement plus bref (8 j c. 12 j, p = 0,025) comparativement au groupe soumis à la PDO. La durée de la chirurgie n’a pas significativement différé entre les groupes. On n’a noté aucune différence sur le plan des besoins en analgésiques postopératoires, des complications de grade I/II ou III/IV sur l’échelle de Clavien ou de la mortalité à 90 jours. Quant aux paramètres oncologiques, aucune différence significative n’a été notée pour ce qui est de la taille de la tumeur, du taux de résection R1 ou du nombre de ganglions recueillis.
Conclusion
Pour certains patients, la PDHL est une intervention sécuritaire et efficace qui donne des résultats comparables à la chirurgie ouverte classique. L’adoption à plus grande échelle de l’approche hybride permettra à plus de patients de bénéficier d’une intervention moins effractive et facilitera la transition complète vers la pancréatoduodénectomie minimalement effractive.
Recent advances in laparoscopic techniques have led to an increased interest in minimally invasive pancreatic surgery. Compared with conventional open surgery, minimally invasive procedures allow for decreased postoperative pain, shorter hospital stay and improved cosmesis.1–3 Despite these benefits, the adoption of total laparoscopic pancreaticoduodenectomy (TLPD) has been hindered by concerns regarding the technical complexity of laparoscopic reconstruction. Since the first report by Gagner and Pomp in 1994,4 only a few centres worldwide have published large TLPD patient series.4–8 A direct transition from open surgery to TLPD may constitute a hazardous and imprudent leap for surgeons without extensive prior laparoscopic experience. In light of the steep learning curve, the transition toward TLPD may be more safely and effectively achieved as a multistep progression using a spectrum of minimally invasive techniques. In this report, we describe hybrid laparoscopy-assisted pancreaticoduodenectomy (HLAPD): a hybrid laparoscopic–open approach in which pancreaticoduodenal resection is performed laparoscopically, while reconstruction is completed via a small upper midline minilaparotomy.9 The hybrid method combines the relative ease of conventional open surgery with the benefits of a minimally invasive approach. Potentially, HLAPD may serve as a valuable stepping stone to facilitate the transition from open to purely minimally invasive pancreaticoduodenectomy without incurring additional risk to the patient. Although the feasibility of HLAPD has been described, the current literature mainly comprises small patient series lacking comparison groups.7,10–12 To our knowledge, only 3 reports have compared the outcomes of patients undergoing HLAPD versus open pancreaticoduodenectomy (OPD).13–15 In the studies by Cho and colleagues13 and Lee and colleagues,15 patients with preoperatively diagnosed periampullary carcinoma automatically underwent OPD; patients who underwent HLAPD displayed only benign or low-grade lesions. To our knowledge, we report the first Canadian study evaluating the safety, feasibility and operative outcomes of HLAPD compared with OPD.
Methods
With institutional review board approval, we performed a retrospective chart review on all patients undergoing HLAPD or OPD at a single institution between September 2009 and December 2013. Demographic, operative and outcome data were collected from a prospectively maintained database. All surgeries were performed by a single experienced pancreatic surgeon (T.V.), with another attending surgeon (S.B.) as first assistant.
Patient selection
Preoperatively, all patients underwent appropriate imaging studies to assess tumour resectability. The selection criteria for HLAPD were tumours of any size without preoperative evidence of major vascular involvement. Patients whose lesions were at high risk of a positive margin or of abutment of major vessels were excluded from the HLAPD group and underwent OPD. Patients were not excluded on the basis of demographic factors, such as age, body mass index (BMI), Charlson Index and American Society of Anesthesiologists (ASA) grade. Prior to surgery, all patients were informed of the potential advantages and complications of both techniques, and they provided written informed consent.
In our initial institutional experience, we imposed a low threshold to convert to an open procedure. To accurately interpret the benefits and shortcomings of the hybrid approach, we conducted a non-intent-to-treat analysis, defining procedures as HLAPD only when all 3 resections (antrectomy, choledochectomy and pancreatectomy) had been performed laparoscopically. Patients whose cases began laparoscopically but were converted to open surgery before completion of the resections were included in the OPD group.
Operative technique
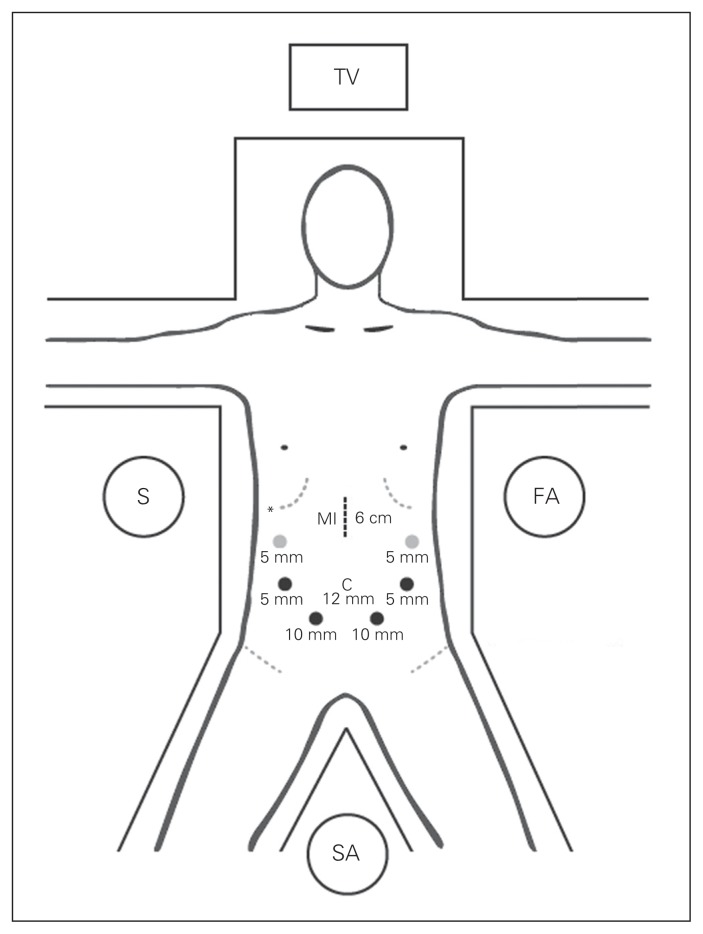
The patient is placed in the supine position on a split-leg table, and CO2 pneumoperitoneum is established via a 12 mm infraumbilical trocar inserted using an open Hasson technique. A 30º camera is used to assess for any evidence of metastatic disease. If no contraindications to resection are found, 6 additional trocars are inserted along a semicircle centred on the head of the pancreas (Fig. 1). The operation is begun by dividing the gastrocolic ligament with a LigaSure (Valleylab). With the stomach and left lateral segment of the liver retracted against the anterior abdominal wall using a miniretractor (Mediflex), the gastroepiploic omentum is separated off the transverse mesocolon. The right gastroepiploic vein is followed to its junction with the infrapancreatic superior mesenteric vein (SMV) and divided. The right colon is mobilized, and a laparoscopic Kocher manoeuvre is performed to the level of the ligament of Treitz. The gastric antrum is transected using serial purple loads of the EndoGIA stapler (US Surgical Corp.). The common hepatic node is identified and resected. The gastrohepatic ligament is opened to expose the common hepatic artery, from which the gastroduodenal artery can be traced down. Flow within the common hepatic artery is verified using a laparoscopic ultrasound probe before transecting the gastroduodenal artery using the white load of the EndoGIA stapler. A retropancreatic tunnel is created by dissecting between the posterior surface of the pancreas and the anterior plane of the SMV in a cephalad direction. The tunnelled pancreas is then encircled using a Penrose tape. A complete hilar lymphadenectomy is undertaken to harvest periportal and peri-pancreatic lymph nodes. A retrograde cholecystectomy is performed, leaving the gallbladder attached to the common bile duct for traction. The hepatoduodenal ligament is dissected to isolate the underlying portal vein. The common bile duct is encircled with an umbilical tape and transected above the junction with the cystic duct using the white load of the EndoGIA stapler. The proximal jejunum is brought back to the right side of the abdomen and transected 10 cm distal to the ligament of Treitz using the white load of the EndoGIA stapler. The pancreas parenchyma is then divided across the neck using the LigaSure starting inferiorly and moving toward the superior border anterior to the portal vein and mesenteric vessels, making sure to immediately identify the pancreatic duct after transection. The uncinate process dissection is performed by dividing the SMV and jejunal branches along the adventitial layers of the superior mesenteric vessels to ensure adequate clearance of the uncinate margin.
Fig. 1.
Hybrid laparoscopic pancreaticoduodenectomy trocar placement and operative setup. Black dots indicate standard trocars, and grey dots represent optional trocars. The asterisk represents the liver retractor port. C = camera port; FA = first assistant; MI = minilaparotomy incision; S = surgeon; SA = second assistant; TV = television monitor.
The reconstruction is begun by creating a 5–6 cm vertical upper midline minilaparotomy incision through which the en bloc resected specimen is retrieved in an endobag. The transected end of the proximal jejunum is brought up to the right upper quadrant through a defect in the transverse mesocolon. A 2-layer duct-to-mucosa pancreaticojejunostomy is constructed in Blumgart fashion using 5–0 polydioxanone sutures (PDS) and through-and-through 3–0 silk stitches.16 Next, an end-to-side hepaticojejunostomy is performed using interrupted 5–0 PDS, and a side-to-side retrogastric antecolic loop gastrojejunostomy is completed using the blue load of the EndoGIA stapler. Two Jackson–Pratt drains (Allegiance Healthcare Corporation) are placed near the biliary and pancreatic anastomoses at the end of the procedure.
Outcomes
The preoperative variables we examined included age, sex, BMI, Charlson Index and ASA grade. Operative data included duration of surgery, intraoperative blood loss and blood transfusions. We also examined oncologic outcomes, such as tumour size and histopathology, margin status and number of lymph nodes harvested. The R1 resection rate reflects the number of patients who had a positive margin out of the total number of patients with a malignant pathology. Seven-day analgesic use consisted of the total amount of narcotics administered over the first 7 postoperative days. Analgesic requirement data were collected from the medical administration record, which documents daily scheduled medications and those administered when necessary (PRN) for each patient. Each medication that is actually taken by the patient is subsequently signed off by the nursing staff. We calculated daily epidural and patient- controlled analgesia rates from specific documentation sheets. All routes of opioid administration (i.e., epidural, oral, intravenous, intramuscular, transdermal) were tabulated and subsequently converted into intravenous (IV) morphine equivalents. Nonopioid analgesics, such as acetaminophen and ibuprofen, were not included in the analysis.
Postoperatively, we analyzed length of hospital stay, and morbidity and mortality were recorded up to 90 days after surgery. We classified complications according to the Clavien system, which grades severity according to the invasiveness of the required treatment.17 For patients with multiple complications, only the most severe one was registered. Pancreatic fistula was defined, according to International Study Group of Pancreatic Fistula (ISGPF) criteria, as any measurable drain output on or after postoperative day 3, with an amylase content greater than 3 times the normal serum level.18 Cases were divided into 4 categories: no fistula; biochemical fistula without clinical sequelae (grade A), fistula requiring any therapeutic intervention (grade B) and fistula with severe clinical sequelae (grade C).
Statistical analysis
Continuous variables were expressed as medians with ranges and compared using the Mann–Whitney U test. Categorical variables were compared using the χ2 test or Fisher exact test. We considered results to be significant at p < 0.05, 2-tailed. We performed all statistical analyses using SPSS version 17.0.
Results
Between September 2009 and December 2013, we performed HLAPD and OPD on 13 and 20 patients, respectively. Of the 22 cases begun laparoscopically, 9 were converted to open surgery before completion of the resections (5 patients had extensive abdominal adhesions, 4 had tumours showing vascular abutment or involvement); they were included in the OPD group. There were no significant differences in age, sex, BMI, ASA grade or Charlson Index between the groups (Table 1).
Table 1.
Demographic and outcome data
| Characteristic | Group; median (range)* | p value | |
|---|---|---|---|
| HLAPD | OPD | ||
| No. of patients | 13 | 20 | |
| Age, yr | 69 (49–88) | 67 (33–78) | 0.45 |
| Sex, male:female, % | 85%:15% | 65%:35% | 0.26 |
| BMI | 24.2 (20.6–32.0) | 25.0 (16.4–33.3) | 0.87 |
| Charlson Comorbidity Index | 1 (0–4) | 2 (0–6) | 0.32 |
| ASA score | 2 (2–3) | 3 (2–3) | 0.36 |
| Operative time, min | 594 (407–779) | 553 (303–892) | 0.06 |
| Estimated blood loss, mL | 450 (100–4000) | 1000 (300–6500) | 0.023 |
| Intraoperative blood transfusion, no. (%) | 5 (38%) | 10 (50%) | 0.72 |
| Total 7-day analgesic use, mg IV, mean ± SD | 174 ± 117 | 288 ± 226 | 0.08 |
| Length of stay, d | 8 (6–14) | 12 (6–26) | 0.025 |
| 90-day mortality, no. (%) | 1 (8%) | 4 (20%) | 0.63 |
ASA = American Society of Anesthesiologists; BMI = body mass index; HLAPD = hybrid laparoscopy-assisted pancreaticoduodenectomy; IV = Intravenous; OPD = open pancreaticoduodenectomy; SD = standard deviation.
Unless otherwise indicated.
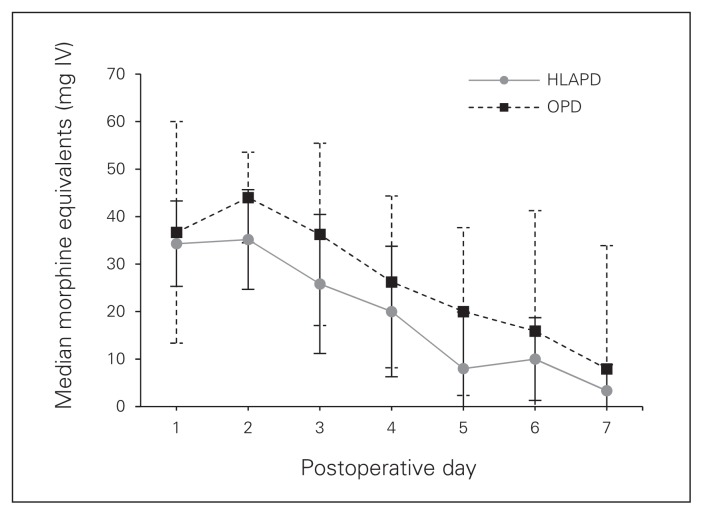
The HLAPD group had a significantly lower estimated intraoperative blood loss (450 mL v. 1000 mL, p = 0.023) and a shorter length of hospital stay (8 v. 12 d, p = 0.025) than the OPD group. There were no significant differences in duration of surgery or intraoperative blood transfusion rates between the groups. There were no intraoperative deaths. Twelve (92%) patients in the HLAPD group used an epidural for postoperative pain control compared with 19 (95%) patients in the OPD group. Mean 7-day analgesic requirements were lower in patients who underwent HLAPD than those who underwent OPD (174 mg v. 288 mg), but this trend did not achieve significance (p = 0.08; Fig. 2). Ninety-day mortality was similar between the HLAPD and OPD groups (8% v. 20%).
Fig. 2.
Seven-day analgesic use. HLAPD = hybrid laparoscopy-assisted pancreaticoduodenectomy; OPD = open pancreaticoduodenectomy.
Pathology findings are summarized in Table 2. Malignant lesions were found in 10 (77%) patients in the HLAPD group compared with 15 (75%) patients in the OPD group. Median tumour size, R1 resection rate and lymph node harvest did not differ significantly between the groups.
Table 2.
Pathology and oncologic outcomes
| Factor | Group; no. (%)* | p value | |
|---|---|---|---|
| HLAPD, n = 13 | OPD, n = 20 | ||
| Tumour size, cm | 3.5 (1.8–4.2) | 3.5 (1.5–6.5) | 0.71 |
| R1 resection margin | 3/10 (30) | 7/15 (47) | 0.68 |
| Lymph node harvest | 22 (14–56) | 20 (7–45) | 0.09 |
| Positive lymph nodes | 9 (69) | 9 (53) | 0.47 |
| Pathology | |||
| Malignant | 10 (77) | 15 (75) | — |
| Pancreatic adenocarcinoma | 9 | 11 | — |
| Ampullary adenocarcinoma | 1 | 3 | — |
| Neuroendocrine tumour | 0 | 1 | — |
| Benign | 3 (23) | 5 (25) | — |
| Intraductal papillary mucinous neoplasm | 1 | 3 | — |
| Duodenal polyp | 1 | 1 | — |
| Autoimmune pancreatitis | 1 | 0 | — |
| Perforated gastric ulcer | 0 | 1 | — |
HLAPD = hybrid laparoscopy-assisted pancreaticoduodenectomy; OPD = open pancreaticoduodenectomy
Unless otherwise indicated.
Within 90 days postoperative, major complications (Clavien grade III/IV) occurred in 2 (15%) patients in the HLAPD group compared with 8 (40%) patients in the OPD group. Six (46%) patients in the HLAPD group experienced minor complications (Clavien grade I/II) compared with 9 (45%) patients in the OPD group (Table 3). One patient in the HLAPD group died due to refractory sepsis following a leak at a gastric staple site, which required surgical repair and drainage. In the OPD group, 4 deaths occurred within 90 days. One patient had acute hepatic and renal failure after 2 subsequent surgeries for portal vein thrombosis; 1 had an acute myocardial infarction; 1 had hemorrhagic shock due to bleeding from the portal vein, which required surgical intervention; and 1 succumbed to abdominal sepsis following operative repair of hepaticojejunostomy and gastrojejunostomy leaks.
Table 3.
Ninety-day complications
| Complication | Group; no. | p value | |
|---|---|---|---|
| HLAPD, n = 13 | OPD, n = 20 | ||
| Clavien I/II* | 6 | 9 | > 0.99 |
| Wound infection | 1 | 2 | |
| Hypotension | 1 | 2 | |
| Intra-abdominal abscess | 1 | 0 | |
| Delayed gastric emptying | 1 | 0 | |
| Thrombocytopenia | 1 | 0 | |
| Urinary retention | 1 | 0 | |
| Anemia | 0 | 3 | |
| Pneumonia | 0 | 2 | |
| Clavien III/IV† | 2 | 8 | 0.25 |
| Intra-abdominal abscess | 1 | 3 | |
| Anastomotic breakdown | 1 | 2 | |
| Portal vein thrombosis | 0 | 1 | |
| Postoperative hemorrhage | 0 | 1 | |
| Acute myocardial infarction | 0 | 1 | |
| Pancreatic fistula | 4 | 6 | > 0.99 |
| Grade A | 3 | 3 | |
| Grade B | 1 | 3 | |
HLAPD = hybrid laparoscopy-assisted pancreaticoduodenectomy; OPD = open pancreaticoduodenectomy.
Not necessitating radiological, endoscopic or operative intervention and not causing organ failure.
Necessitating radiological, endoscopic or operative intervention and/or causing organ failure.
Discussion
The advent of minimally invasive surgery has resulted in increased use of laparoscopic techniques to pancreatic surgery. The benefits of a minimally invasive approach include reduced incisional pain, decreased postoperative complications, shortened hospital stay and improved cosmesis. Although some surgeons have advocated a direct transition from an open to a purely laparoscopic approach, such a shift requires extensive prior laparoscopic experience and has been successfully accomplished in only a few centres. Concerns regarding the complexity of laparoscopic reconstruction and the adequacy of oncologic resection have hindered the adoption of TLPD.19 This report describes the value of HLAPD as a pragmatic stepping stone in the transition from open to purely minimally invasive pancreaticoduodenectomy at our institution. The hybrid method combines the safety and familiarity of conventional open surgery with the benefits of a minimally invasive approach. Given its favourable learning curve, it may be more realistically and widely adopted by hepatobiliary surgeons, even those without extensive laparoscopic experience. The adoption of a multistep approach using a spectrum of minimal access procedures may allow more institutions to successfully implement minimally invasive pancreatic surgery programs.
The main concerns regarding HLAPD are whether smaller incisions are achieved at the expense of the quality of oncologic resection and whether any tangible patient benefit is achieved.20 For pancreaticoduodenectomy, positive margin rates of 20%–40% have been reported in the literature.21,22 Recently, a systematic review of 707 patients undergoing laparoscopic pancreaticoduodenectomy reported an R1 resection rate of 42.5%.23 Our oncological outcomes with HLAPD compare favourably to these standards (R1 resection rate 30%) and confirm the oncologic soundness of the hybrid method. In our study, lymph node retrieval and R1 resection rates did not differ between the OPD and HLAPD groups, further corroborating the adequacy of laparoscopic resection. Certain groups who perform minimally invasive pancreaticoduodenectomy only for benign or low malignant potential disease have reported much lower R1 resection rates. However, these rates are not comparable to those found in our study, in which 77% of HLAPD procedures were performed for malignant indications.13,15 Although certain patients with complex tumours were inherently selected to the open group (including conversions), we nonetheless achieved acceptable oncologic outcomes in HLAPD patients with malignant disease, as compared with values reported in the literature. Our results demonstrate that oncologic principles are not compromised by the use of the hybrid approach, provided careful patient selection.
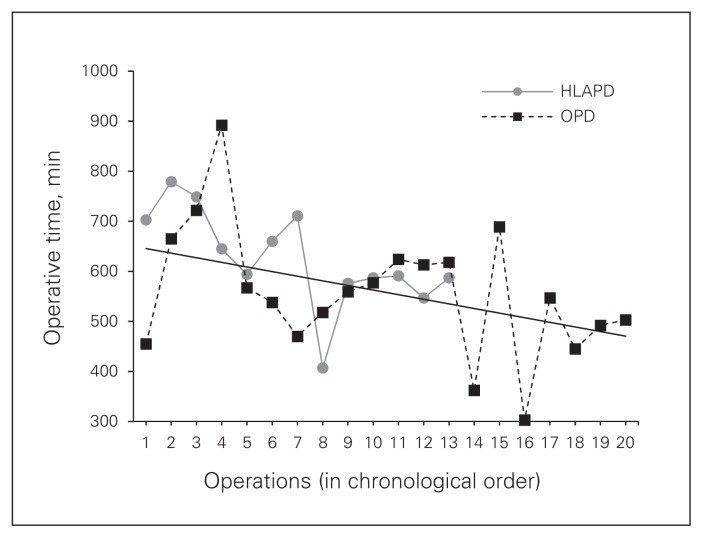
Long learning curves and increased duration of surgery are often invoked as drawbacks of minimally invasive pancreaticoduodenectomy. An advantage of our study is that all operations were performed by the same surgeons, allowing for a more accurate assessment of progression along the learning curve. In our cumulative experience, duration of surgery did not differ significantly between the HLAPD and OPD groups (594 v. 553 min., p = 0.6. Figure 3 depicts the duration of surgery of HLAPD and OPD in chronological order. Initially, we observed significantly longer surgery with HLAPD, as expected during the initial learning phase.24,25 An analysis conducted after 2 years of institutional experience, including 7 HLAPD and 12 OPD procedures, revealed significantly longer surgeries in the HLAPD group than in the OPD group (703 v. 572 min.; p = 0.035). However, the duration of HLAPD decreased from a median 703 minutes in the first 7 patients to 582 minutes in the last 6 patients (p = 0.003), whereas the duration of OPD remained relatively stable. Our results project the continued convergence of the 2 trendlines with increasing operative experience. Importantly, the learning curve appears to affect the duration of the procedure, but is not associated with increased morbidity or compromise of oncologic outcomes. Tseng and colleagues26 reported that surgeons typically achieved significantly decreased estimated blood loss, duration of surgery, length of stay and R1 resection rates after performing approximately 60 OPD procedures. In light of the important learning curve, preference should be given to a hybrid approach before transitioning to total laparoscopic pancreaticoduodenectomy to acquire sufficient experience and ensure patient safety.
Fig. 3.
Operative times in chronological order. HLAPD = hybrid laparoscopy-assisted pancreaticoduodenectomy; OPD = open pancreaticoduodenectomy.
Despite technical advancements and increased surgeon experience, pancreaticoduodenectomy remains associated with high morbidity. We stratified adverse events by severity of the clinical treatment required. Our 90-day Clavien III/IV complication rates for the HLAPD and OPD groups were 15% and 40%, respectively, which compare acceptably to the 40% morbidity reported in previously published studies.8,27 Pancreatic fistula remains the most important morbidity after pancreaticoduodenectomy. In our study, pancreatic fistula rates in the HLAPD and OPD groups were similar: 31% and 30%, respectively. Although the pancreatic fistula rate was not reduced with the hybrid approach, our data suggest that, even within the initial learning curve, complication rates with HLAPD are acceptable and consistent with those reported in large open and TLPD series.6,28 Ninety-day mortality was comparable between the HLAPD and OPD cohorts. Importantly, a very large proportion of patients in both study groups had malignant pathology, which may explain the higher mortality in our study than other studies focusing on benign disease. In addition, our sample size was small, and any calculated rates should be taken in the context of these limited patient numbers.
Patients who underwent HLAPD had a significantly shorter length of hospital stay than those who underwent OPD. Larger series with longer patient follow-up will be required to assess for any tangible benefits, such as quicker return to baseline function. Total analgesic use during the first 7 postoperative days was consistently lower and tapered off faster in the HLAPD group than in the OPD group, but this trend did not achieve statistical significance (Fig. 2). The decreased analgesic requirements following a minilaparotomy versus a standard subcostal incision likely reflect the correlation between postoperative pain and incision length. Furthermore, while TLPD constitutes the least invasive procedure, it classically requires a 5 cm Pfannenstiel incision for specimen extraction. The difference in morbidity from a Pfannenstiel versus a minilaparotomy incision may be of limited clinical importance, thus attenuating some benefits of directly transitioning to a purely minimally invasive approach.
Our study’s small sample size does not allow for definitive conclusions to be drawn regarding the comparative effectiveness of either technique. However, our objective was not to define the better procedure, but rather to assess whether the hybrid procedure is feasible and effective without incurring additional risk to the patient. Our study is limited methodologically by its nonrandomized and retrospective design. Selection bias is inherent, given that patients with major vascular involvement, which poses additional technical challenges, were excluded from the HLAPD group. We had an important conversion rate in our study, as 9 of 22 (41%) cases begun laparoscopically were converted to laparotomy. It is important to highlight that these conversions largely occurred early during the procedure: 4 cases were converted before any resection, 4 after gastrectomy only and 1 after choledochectomy only. As such, the surgery performed in these converted cases is more comparable to an open than to a hybrid procedure, and the associated outcomes are more representative when included in the OPD group. A subanalysis of strictly open versus converted patients was undertaken to compare patient outcomes (Table 4). Oncologic outcomes, such as R1 resection rate and lymph node harvest, were similar between the open and converted groups. The duration of surgery in the converted group was also longer. Although this difference did not achieve statistical significance in our study, the duration of surgery is undoubtedly affected by the process of converting from laparoscopic to open surgery. Patients in the converted group had significantly higher estimated blood loss (1650 mL v. 700 mL; p = 0.020) than those who began with open surgery. Importantly, however, since no case was converted due to excessive bleeding, this difference in blood loss likely reflects inherently difficult pathology and surgical complexity rather than complications of laparoscopic resection or the act of conversion. As such, these outcomes may have remained largely unchanged even if they had initially been begun by laparotomy. Because similar conversion rates have been reported in the literature, further studies evaluating the validity of more rigorous selection criteria are warranted to reduce conversion rates going forward.19
Table 4.
Comparison of cases begun open versus converted cases
| Factor | Open | Converted | p value |
|---|---|---|---|
| No. of patients | 11 | 9 | |
| Age, yr | 62 (33–76) | 72 (47–78) | 0.06 |
| Sex, male/female | 55%/45% | 78%/22% | 0.37 |
| BMI | 26.2 (18.5–33.3) | 23.5 (16.4–31.6) | 0.53 |
| ASA score | 2 (2–3) | 3 (2–3) | 0.36 |
| Operative time, min. | 518 (303–665) | 613 (470–892) | 0.07 |
| Estimated blood loss, mL | 700 (300–6500) | 1650 (800–5050) | 0.020 |
| Tumour size, cm | 3.1 (1.8–4.5) | 3.7 (2.8–6.5) | 0.27 |
| R0 resection margin, no. (%) | 3/8 (38%) | 4/7 (57%) | 0.62 |
| Lymph node harvest | 20 (7–30) | 19 (15–45) | 0.56 |
| Length of stay, d | 11 (6–23) | 15.5 (7–26) | 0.31 |
| Clavien I/II, no. (%) | 5 (45%) | 4 (44%) | > 0.99 |
| Clavien III/IV, no. (%) | 4 (36%) | 4 (44%) | > 0.99 |
| 90-day mortality, no. (%) | 1 (10%) | 3 (33%) | 0.30 |
ASA = American Society of Anesthesiologists; BMI = body mass index.
Robotic-assisted pancreaticoduodenectomy (RAPD) has gained increasing acceptance because it offers 3-dimensional visualization, superior ergonomics and enhanced suturing capabilities.29 Since 2011, our centre has progressed from an open to a robotic reconstruction with favourable results, and the laparoscopic experience initially acquired with HLAPD has been valuable in this transition. All patients eligible for a minimally invasive procedure now undergo RAPD, provided robot availability. We reserve HLAPD for those patients for whom the robotic platform is unavailable for logistic reasons.
Conclusion
Hybrid laparoscopy-assisted pancreaticoduodenectomy is a safe, feasible and effective procedure with comparable outcomes to OPD in select patients. The favourable learning curve makes HLAPD a pragmatic procedure that may allow a greater number of patients to benefit from a minimally invasive approach. The transition from open to purely minimally invasive pancreaticoduodenectomy may be more effectively achieved as a multistep process using the hybrid approach as a valuable stepping stone.
Footnotes
Abstract presented at the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) meeting, Baltimore, MD, April 2013 and the Canadian Association of General Surgeons (CAGS) meeting, Ottawa, ON, September 2013.
Funding: This work was supported by a medical student research bursary from the McGill University Faculty of Medicine.
Competing interests: None declared.
Contributors: Y. Wang, S. Bergman and T. Vanounou designed the study. Y. Wang acquired the data, which all authors analyzed. All authors wrote and reviewed the article and approved it for publication.
References
- 1.Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–7. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–9. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 3.Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–84. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 4.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreaticoduodenectomy. Surg Endosc. 1994;8:408–10. doi: 10.1007/BF00642443. [DOI] [PubMed] [Google Scholar]
- 5.Palanivelu C, Jani K, Senthilnathan P, et al. Laparoscopic pancrea-ticoduodenectomy: technique and outcomes. J Am Coll Surg. 2007;205:222–30. doi: 10.1016/j.jamcollsurg.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Kendrick ML, Cusati D. Total laparoscopic lancreaticoduodenectomy; feasibility and outcome in an early experience. Arch Surg. 2010;145:19–23. doi: 10.1001/archsurg.2009.243. [DOI] [PubMed] [Google Scholar]
- 7.Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc. 2006;20:1045–50. doi: 10.1007/s00464-005-0474-1. [DOI] [PubMed] [Google Scholar]
- 8.Gumbs AA, Rodriguez Rivera AM, Milone L, et al. Laparoscopic pancreatoduodenectomy: a review of 285 published cases. Ann Surg Oncol. 2011;18:1335–41. doi: 10.1245/s10434-010-1503-4. [DOI] [PubMed] [Google Scholar]
- 9.Uyama I, Ogiwara H, Iida S, et al. Laparoscopic minilaparotomy pancreaticoduodenectomy with lymphadenectomy using an abdominal wall-lift method. Surg Laparosc Endosc. 1996;6:405–10. [PubMed] [Google Scholar]
- 10.Suzuki O, Kondo S, Hirano S, et al. Laparoscopic pancreaticoduodenectomy combined with minilaparotomy. Surg Today. 2012;42:509–13. doi: 10.1007/s00595-011-0064-x. [DOI] [PubMed] [Google Scholar]
- 11.Staudacher C, Orsenigo E, Baccari P, et al. Laparoscopic assisted duodenopancreatectomy. Surg Endosc. 2005;19:352–6. doi: 10.1007/s00464-004-9055-y. [DOI] [PubMed] [Google Scholar]
- 12.Pugliese R, Scandroglio I, Sansonna F, et al. Laparoscopic pancreaticoduodenectomy: a retrospective review of 19 cases. Surg Laparosc Endosc Percutan Tech. 2008;18:13–8. doi: 10.1097/SLE.0b013e3181581609. [DOI] [PubMed] [Google Scholar]
- 13.Cho A, Yamamoto H, Nagata M, et al. Comparison of laparoscopy-assisted and open pylorus-preserving pancreaticoduodenectomy for periampullary disease. Am J Surg. 2009;198:445–9. doi: 10.1016/j.amjsurg.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Kuroki T, Adachi T, Okamoto T, et al. A non-randomized comparative study of laparoscopy-assisted pancreaticoduodenectomy and open pancreaticoduodenectomy. Hepatogastroenterology. 2012;59:570–3. doi: 10.5754/hge11351. [DOI] [PubMed] [Google Scholar]
- 15.Lee JS, Han JH, Na GH, et al. Laparoscopic pancreaticoduodenectomy assisted by mini-laparotomy. Surg Laparosc Endosc Percutan Tech. 2013;23:e98–102. doi: 10.1097/SLE.0b013e3182777824. [DOI] [PubMed] [Google Scholar]
- 16.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Grobmyer SR, Kooby D, Blumgart LH, et al. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg. 2010;210:54–9. doi: 10.1016/j.jamcollsurg.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6,336 patients and result of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagner M, Pomp A. Laparoscopic pancreatic resection: Is it worthwhile? J Gastrointest Surg. 1997;1:20–6. doi: 10.1007/s11605-006-0005-y. [DOI] [PubMed] [Google Scholar]
- 20.Park A, Schwartz R, Tandan V, et al. Laparoscopic pancreatic surgery. Am J Surg. 1999;177:158–63. doi: 10.1016/s0002-9610(98)00325-0. [DOI] [PubMed] [Google Scholar]
- 21.Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–8. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Verbeke CS. Resection margins and R1 rates in pancreatic cancer — are we there yet? Histopathology. 2008;52:787–96. doi: 10.1111/j.1365-2559.2007.02935.x. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M, Nakashima H. Laparoscopic distal pancreatectomy and pancreatoduodenectomy: Is it worthwhile? A meta-analysis of laparoscopic pancreatectomy. J Hepatobiliary Pancreatic Sci. 2013;20:421–8. doi: 10.1007/s00534-012-0578-7. [DOI] [PubMed] [Google Scholar]
- 24.Fisher WE, Hodges SE, Wu MF, et al. Assessment of the learning curve for pancreaticoduodenectomy. Am J Surg. 2012;203:684–90. doi: 10.1016/j.amjsurg.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Hardacre JM. Is there a learning curve for pancreaticoduodenectomy after fellowship training? HPB Surg. 2010;2010:230287. doi: 10.1155/2010/230287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng JF, Pisters PW, Lee JE, et al. The learning curve in pancreatic surgery. Surgery. 2007;141:694–701. doi: 10.1016/j.surg.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Zureikat AH, Breaux JA, Steel JL, et al. Can laparoscopic pancreaticoduodenectomy be safely implemented? J Gastrointest Surg. 2011;15:1151–7. doi: 10.1007/s11605-011-1530-x. [DOI] [PubMed] [Google Scholar]
- 28.Cullen JJ, Sarr MG, Ilstrup DM. Pancreatic anastomotic leak after pancreaticoduodenectomy: incidence, significance, and management. Am J Surg. 1994;168:295–8. doi: 10.1016/s0002-9610(05)80151-5. [DOI] [PubMed] [Google Scholar]
- 29.Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–84. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]