Abstract
End-to-end ductal anastomosis is a physiologic biliary reconstruction that is commonly used in liver transplantation and less frequently in the surgical treatment of iatrogenic bile duct injuries. Currently, end-to-end ductal anastomosis is the biliary reconstruction of choice for liver transplantation in most adult patients. In recent years, it has also been performed for liver transplantation in children and in select patients with primary sclerosing cholangitis. The procedure is also performed in some patients with iatrogenic bile duct injuries, as it establishes physiologic bile flow. Proper digestion and absorption as well as postoperative endoscopic access are possible in patients who undergo end-to-end ductal anastomosis. It allows endoscopic diagnostic and therapeutic procedures in patients following surgery. This anastomosis is technically simple and associated with fewer early postoperative complications than the Roux-en-Y hepaticojejunostomy; however, end-to-end ductal anastomosis is not possible to perform in all patients. This review discusses the indications for and limitations of this biliary reconstruction, the technique used in liver transplantation and surgical repair of injured bile ducts, suture types and use of a T-tube.
Abstract
L’anastomose termino-terminale du canal biliaire est la technique de reconstruction biliaire physiologique la plus couramment utilisée lors de la greffe du foie; elle est moins souvent utilisée pour le traitement chirurgical des blessures iatrogènes affectant le canal biliaire. À l’heure actuelle, l’anastomose termino-terminale est la reconstruction biliaire privilégiée lors d’une transplantation hépatique chez la plupart des patients adultes. Ces dernières années, on y a également eu recours pour la greffe hépatique chez les enfants et dans certains cas de cholangite sclérosante. L’intervention est également effectuée chez certains patients présentant des traumatismes iatrogènes affectant le canal biliaire, puisqu’elle permet la circulation physiologique de la bile. Une digestion et une absorption adéquates, de même qu’un accès endoscopique postopératoire sont donc possibles chez les patients qui subissent une anastomose termino-terminale. Elle facilite les interventions diagnostiques et thérapeutiques endoscopiques chez les patients après la chirurgie. Cette anastomose est simple au plan technique et associée à moins de complications durant la période postopératoire immédiate comparativement à l’hépaticojéjunostomie Roux en Y. Toutefois, l’anastomose termino-terminale n’est pas réalisable chez tous les patients. La présente analyse aborde les indications et les limites de cette reconstruction biliaire, la technique utilisée lors de la greffe hépatique et lors de la réparation chirurgicale des canaux biliaires lésés, les types de sutures et l’utilisation d’un tube en T.
End-to-end ductal anastomosis is a physiologic biliary reconstruction that is commonly used in liver transplantation and in general surgery, including the treatment of iatrogenic bile duct injuries (IBDI). End-to-end ductal anastomosis and Roux-en-Y hepaticojejunostomy (HJ) are the 2 most common biliary reconstructions, and the former is the most common in patients who have had liver transplantation, including those with primary sclerosing cholangitis (PSC). In recent years, the traditional method of HJ has been challenged by end-to-end biliary reconstruction in these patient groups; however, in patients with IBDI, HJ is performed most frequently.
End-to-end ductal anastomosis has many advantages: it is physiologically simpler and associated with fewer early postoperative complications than HJ. End-to-end ductal anastomosis establishes physiologic bile flow; therefore, proper digestion and absorption are possible following this procedure. Postoperative endoscopic access is also possible, facillitating different diagnostic and therapeutic procedures. Despite its advantages, it is not possible to perform end-to-end ductal anastomosis in all patients.1–5
The aim of this paper was to present the use of end-to-end ductal anastomosis in patients undergoing liver transplantation and gastrointestinal surgery. This review also discusses the limitations in using this biliary reconstruction method and describes a surgical technique of end-to-end ductal anastomosis.
End-to-end ductal anastomosis in liver transplantation
Biliary anastomosis is referred to as the Achilles’ heel of liver transplantation. The noted incidence of biliary complications is 5%–15% after deceased donor liver transplantation (DDLT) and 20%–34% after right-lobe live donor liver transplantation (LDLT).6 Nowadays, different methods of biliary reconstruction are used: Roux-en-Y HJ, end-to-end ductal anastomosis and side-to-side ductal anastomosis. Other biliary reconstructions, such as cholecystoduodenostomy, cholecystojejunostomy or the gallbladder conduit technique, were used in the early experience of liver transplantation, but they were associated with a high risk (up to 70%) of septic complications. Also, many gallbladders had to be removed because of cystic duct obstruction. Subsequently, HJ was introduced as the standard technique in liver transplantation, and it was a common method of biliary reconstruction for a long time. End-to-end ductal anastomosis was performed as the standard biliary reconstruction after DDLT, whereas HJ was the standard technique performed after LDLT. This trend has changed because of the disadvantages of HJ: longer duration of surgery and higher risk of bacterial contamination due to construction of the Roux-Y limb. Moreover, the re-established bilioenteric continuity is not physiologic and does not allow endoscopic access after liver transplantation. Currently, end-to-end ductal anastomosis is the standard biliary reconstruction for both DLDT and LDLT in adults. This method is preferable because of an intact sphincter Oddi that can prevent septic cholangitis due to ascending infections. Moreover, the procedure facillitates subsequent endoscopic diagnostic and therapeutic procedures in patients with biliary complications after liver transplantation. However, end-to-end ductal anastomisis with a small duct (< 4 mm in diameter) is associated with a higher risk of biliary strictures than HJ.4,7,8
Hepaticojejunostomy versus end-to-end ductal anastomosis in liver transplantation
There are a number of studies comparing HJ and end-to-end ductal anastomosis in liver transplantation in the literature. Kasahara and colleages5 compared different biliary reconstructions in 321 recipients of right lobe LDLT. Biliary reconstruction was performed with HJ in 121 patients, end-to-end ductal anastomosis in 192 patients, and combined HJ and end-to-end ductal anastomosis in 8 patients. They found that end-to-end ductal anastomosis showed a significantly lower incidence of leakage and a higher incidence of stricture. However, 74.5% of the stricture was managed with endoscopic treatment. It should be emphasized that in recent years, the traditional method of HJ has been challenged by end-to-end ductal anastomosis biliary reconstruction even in patients undergoing liver transplantation owing to PSC. Damrah and colleagues9 compared HJ and end-to-end ductal anastomosis after liver transplantation in patients who had PSC. They used end-to-end ductal anastomosis when the recipient’s common bile duct was free of gross disease. Morbidity, mortality, disease recurrence and graft and patient survival were comparable between the groups. Based on these results, the authors recommended end-to-end ductal anastomosis for select patients with PSC as the first option for reconstruction. Similar results have been presented in other studies.10–12
End-to-end ductal anastomosis in pediatric liver transplantation
Currently, end-to-end ductal anastomosis is the biliary reconstruction of choice in adults. Its pediatric feasibility has rarely been reported. Tanaka and colleagues13 compared 14 patients who underwent end-to-end ductal anastomosis and 46 patients who underwent HJ; the incidence of biliary leakage was 7.1% and 8.7%, respectively, and that of stricture was 28.6% and 10.9%, respectively, but the differences were not significant. The authors observed that, compared with the HJ group, biliary stricture in the end-to-end ductal anastomosis group tended to require revision surgery with HJ and longer treatment with percutaneous transhepatic biliary drainage. Based on these results, the authors recommended HJ as the preferable reconstruction in children. They recommended that end-to-end ductal anastomosis should be considered when making a new Roux-Y limb is impossible or troublesome owing to abdominal dense adhesion or short bowel syndrome. Liu and colleagues14 analyzed results of end-to-end ductal anastomosis in 7 children undergoing LDLT using a left-lobe graft. The authors concluded that end-to-end ductal anastomosis biliary reconstruction without external stent tube in patients undergoing left-lobe LDLT was feasible in a select group of children with normal extrahepatic bile ducts. In smaller recipients with larger grafts, the use of a transanastomotic biliary tube could prevent anastomotic kinking, although the authors suggested HJ as a better method of biliary reconstruction for this condition. Other studies have also confirmed the usefulness of end-to-end ductal anastomosis for liver transplantation in select pediatric patients.15–18
End-to-end ductal anastomosis in the surgical treatment of iatrogenic bile duct injuries
Iatrogenic bile duct injuries are still an important problem in gastrointestinal surgery. Noninvasive, percutaneous radiological end endoscopic techniques are recommended as initial treatment of IBDI. When these techniques are not effective, surgical management is considered. The goal of surgical treatment is to reconstruct the proper bile flow to the alimentary tract. The long-term results depend on the type of biliary reconstruction performed. Different biliary reconstructions have been reported in the surgical treatment of IBDI: Roux-en-Y HJ, end-to-end ductal biliary anastomosis, choledochoduodenostomy, Lahey HJ, jejunal interposition hepaticoduodenostomy, Blumgart (Hepp) anastomosis, Heinecke–Mikulicz biliary plastic reconstruction and Smith mucosal graft.2,19,20
Hepaticojejunostomy versus end-to-end ductal anastomosis in the surgical treatment of IBDI
Currently, Roux-en-Y HJ is the most common surgical reconstruction of IBDI.1,2 Most authors have reported a preference for HJ owing to the lower number of postoperative anastomosis strictures with HJ than with end-to-end ductal anastomosis. The latter procedure is seldom performed in patients with IBDI because of a higher incidence of postoperative anastomosis strictures (up to 80%) compared with HJ.21 However, after HJ, bile flow into the alimentary tract is not physiologic because the duodenum and upper part of the jejunum are excluded from bile passage. Roux-en-Y HJ is associated with different disturbances in the release of gastrointestinal hormones leading to maldigestion and malabsorption.1,2,22,23 Significantly lower weight gain in patients who had HJ than in those who had end-to-end ductal anastomosis was observed in a previous study.1 Moreover, a higher number of duodenal ulcers has been observed in patients undergoing HJ, and this may be associated with a loss of the neutralizing effect of the bile, including bicarbonates and the secondary gastric hypersecretion. Control endoscopic examination and endoscopic dilatation of strictured biliary anastomosis is not possible after HJ.1,2 End-to-end ductal anastomosis should be considered the treatment of choice in select patients with IBDI because it is a more physiologic procedure than HJ; however, HJ should be considered in patients in whom end-to-end ductal anastomosis is not possible.1
It has been shown that good long-term results can be achieved in a select group of patients following end-to-end ductal anastomosis. Gazzaniga and colleagues24 performed end-to-end ductal anastomosis in the immediate repair procedures only when the injury did not exceed one-third of the duct circumference and was not located more than 2 cm below the ductal confluence (Strasberg E2), or when injury was detected during the primary operation. In this series, injuries were type E2 in 18 patients, type E3 in 29 patients, and type E4 in 15 patients. Direct repair is not recommended when more than one-third of the bile duct circumference is injured. It cannot be carried out when the lesion involves the bifurcation of 1 or both hepatic ducts (Strasberg E3/E4). In such cases a Roux-en-Y HJ is the only procedure available to repair the damage. Reuver and colleagues21 recommendeded end-to-end ductal anastomosis in patients with injuries detected preoperatively when there was not extensive tissue loss. In patients with extensive tissue loss, particularly in those with more proximal injuries within the hepatic bifurcation or intrahepatic lesions, the authors recommend no primary repair. Kohneh and colleagues25 achieved better results with end-to-end ductal anastomosis (100%) than with HJ (71.4%) during early repair procedures (< 30 d after the initial trauma). They performed end-to-end ductal anastomosis in patients with bile duct injuries classified as type II (Bismuth) or E2 (Strasberg). In the Department of Digestive Tract Surgery, Katowice, Poland, end-to-end ductal anastomosis reconstruction was performed when bile duct loss was 0.5–4 cm. Excision of the bile duct stricture, dissection and refreshing of the proximal and distal stumps as far as the tissues are healthy and without inflammation, and the use of nontraumatic, monofilament-interrupted sutures 5–0 yielded good long-term results comparable to the results achieved with HJ. Recurrent stricture was observed in 5.3% of patients after HJ and 9.6% after end-to-end ductal anastomosis.1 Another study revealed that quality of life was also comparable after HJ and end-to-end ductal anastomosis. Moreover, it should be emphasized that physical functioning was significantly better in patients who underwent end-to-end ductal anastomosis than in those who underwent HJ.3 Another essential advantage of end-to-end ductal anastomosis is the possibility of control endoscopic examination and therapeutic procedures in patients after biliary reconstruction. End-to-end ductal anastomosis strictures can be easily dilated endoscopically in contrast to HJ. Fewer early complications have been observed after end-to-end ductal anastomosis than HJ; the complications were associated with opening of the alimentary tract and a higher number of performed anastomoses (biliary-enteric and entero-enteric) in patients who underwent HJ.1
It should be noted that end-to-end ductal anastomosis has some limitations and cannot be performed in patients with all bile duct injuries; it is not possible to perform the procedure in patients with complex vasculobiliary injuries. According to Strasberg and Helton,26 a vasculobiliary injury (VBI) is an injury to both a bile duct and a hepatic artery and/or portal vein; the bile duct injury can be caused by surgical trauma, be ischemic in origin or both, and can or cannot be accompanied by various degrees of hepatic ischemia. Injury of a right hepatic artery (RHA) is the most frequent type of VBI. There are contradictory reports regarding the association between the outcome of bile duct injuries and RHA injuries in the literature. Strasberg and Helton26 reviewed studies on VBI. Koffron and colleagues27 reported an associated injury of the artery in 61% of patients with recurrent strictures after primary bile duct repair. Schmidt and colleagues28 reported that the presence of combined vascular and bile duct injuries and injury at or above the level of the biliary bifurcation were significant independent predictors of poor outcome in patients undergoing Roux-en-Y HJ. Madariaga and colleagues29 described early necrosis of a biliary anastomosis requiring right hepatic lobectomy in the presence of an RHA injury. Sarno and colleagues30 noted that patients with concomittant VBI had worse outcomes after bile duct injury repair. In contrast to the aforementioned studies, Alves and colleagues31 reported comparable incidence of postoperative complications in patients with and without arterial injury. Stewart and colleagues32 did not report any influence of RHA injury on long-term results following biliary reconstruction, but RHA injury was associated with a higher incidence of postoperative abscess, bleeding, hemobilia, hepatic ischemia, and the need for hepatic resection. Results of RHA injury and vasculobiliary injury involving both RHA and bile duct are different. It is associated with the arterial blood supply of the extrahepatic biliary tract.
In an injury to the RHA without biliary injury, occlusion of the RHA results in ischemia of the right liver, but blood flow is restored by preformed collateral arterial shunts. In a combined vasculobiliary injury involving the RHA, E1–3 injuries leave the hilar shunt (hilar plexus) open but obstruct the longitudinal shunt (axial arteries at 3, 9 and 12 o’ clock) and may induce greater hepatic ischemia than RHA occlusion only, and E4 injuries induce greater ischemia than right hepatic injuries alone by obstructing the important hilar shunt and the longitudinal shunt. Therefore, it is not possible to perform end-to-end ductal anastomosis in patients with complex vasculobiliary injuries that require Roux-en-Y HJ and, frequently, hepatic hepatectomy or liver transplantation.26,30
Technique of end-to-end ductal anastomosis
General principles
Two main conditions must be met for proper healing of each biliary anastomosis. The anastomosed edges should be healthy; there should be no inflammation, ischemia or fibrosis; and the anastomosis should be tension-free and properly vascularized.33 Dissection and refreshing of the proximal and distal stumps as far as the tissues are healthy and without inflammation should be performed. However, careful dissection is required to save intact axial arteries within a wall of the common bile and hepatic ducts.34 Biliary reconstruction should be performed when no active inflammation process is present, particularly in patients with IBDI, who frequently have ischemia, fibrosis and inflammation within the bile ducts.1 Ischemia, either associated with graft preservation injury or inflammation due to rejection, has also been observed during liver transplantation.35 Both proximal and distal ductal stumps should be dissected and approximated without tension. End-to-end ductal anastomosis could be recommended for patients when the maximal length loss of the bile duct is 4 cm. The sutured ends have to be healthy and without inflammation and ischemia. The diameter of both anastomosed ends has to be comparable. In the Department of Digestive Tract Surgery, if there was a difference between a diameter of anastomosed ends, the narrower end was incised longitudinally in the anterior surface to extend it. End-to-end ductal anastomosis repair was not carried out in bile ducts that were too narrow (diameter < 4 mm). The approximating of both ends is possible because of a wide Kocher manoeuvre (mobilization of the pancreatic head with the descending, horizontal and ascending part of the duodenum out of the peritoneum). Patients undergoing a first or, exceptionally, second bile duct repair can be a candidate for end-to-end ductal anastomosis. Hepaticojejunostomy should be performed in patients who do not satisfy the aforementioned criteria.1
Suture type
Both continuous (CS) or interrupted (IS) and absorbable (polydioxanone) or nonabsorbable (prolene or polypropylene), 5–0, 6–0 or 7–0 sutures are used for end-to-end ductal anastomosis in patients undergoing liver transplantation.4,14,15,35,36 Initially, IS was the standard for these patients; CS was not adopted for end-to-end ductal anastomosis owing to concern for higher stricture rates than IS. Continuous sutures are quicker to perform than IS. Castaldo and colleagues35 compared CS and IS for end-to-end ductal anastomosis in patients undergoing liver transplantation. The authors reported comparable results with both surgical techniques. There was no difference in biliary complications, graft survival or patient survival between the analyzed groups. The overall biliary complication rate was 15%. There was no difference in the proportion of leaks (CS 7.3% v. IS 8.5%) or strictures (CS 9.8% v. IS 5.1%) between groups. The nontraumatic, monofilament- interrupted 5–0 suture is the technique of choice for end-to-end ductal anastomosis in patients with IBDI.1
T-tube use
The use of a T-tube in end-to-end ductal anastomosis remains controversial. There are contradictory reports in the literature regarding the feasibility of biliary drainage for end-to-end ductal anastomosis in patients undergoing liver transplantation and those undergoing IBDI repair. The advantage of biliary drainage is to limit the inflammation and fibrosis that occur after the surgical procedure. Therefore, some authors believe that the presence of the biliary tube prevents anastomosis stricture.1,28 The disadvantage is the higher risk of postoperative complications.1 Scatton and colleagues37 compared the incidence of biliary complications after liver transplantation in patients undergoing end-to-end ductal anastomosis with or without T-tube in a large multicentre, prospective, randomized trial. The study included 108 patients divided into 2 groups: patients with (n = 90) or without (n = 90) a T-tube who underwent surgery in 6 French liver transplantation centres. The authors reported an increased biliary complication rate in the T-tube group, that was linked to minor complications. The incidence of biliary fistula was 10% in the T-tube group and 2.2% in the group without a T-tube. Therefore, the authors did not recommend the performance of end-to-end ductal anastomosis with a T-tube in patients undergoing liver transplantation. Recently, López-Andújar and colleagues38 compared the incidence and severity of biliary complications due to liver transplantation after end-to-end ductal anastomosis with or without a T-tube in a single-centre, prospective, randomized trial. The study involved 95 patients with a T-tube and 92 patients without a T-tube. Significantly fewer anastomotic strictures were reported in the T-tube group (n = 2 [2.1%]) than in the non-T-tube group (n = 13 [14.1%]). No difference in anastomotic biliary leakage was observed between the groups. The authors concluded that complications in the T-tube group were less severe and required less aggressive treatment than those in the non-T-tube group. The incidence of anastomotic strictures was higher in patients without T-tubes. The authors recommended using a rubber T-tube for end-to-end ductal anastomosis during liver transplantation in risky anastomosis and when the bile duct diameter is less than 7 mm. Contradictory meta-analyses regarding the usefulness of a T-tube in end-to-end ductal anastomosis can also be found in the literature. Sotiropoulos and colleagues39 pooled the outcomes of 1027 patients undergoing end-to-end ductal anastomosis with or without T-tube in 9 of 46 screened trials by means of fixed- or random-effects models. In this meta-analysis, the patients without T-tubes had fewer episodes of cholangitis and peritonitis, and they demonstrated a favourable trend for fewer overall biliary complications. Anastomotic bile leaks or fistulas, end-to-end ductal anastomosis revisions, dilatation and stenting, hepatic artery thromboses, retransplantation and death due to biliary complications were comparable in between the groups. Therefore, the authors did not recommend the use of a T-tube for end-to-end ductal anastomosis in patients undergoing liver transplantation. In contrast, Huang and colleagues40 reviewed 5 randomized control trials (RCTs) and 8 comparative studies. They suggested that the insertion of a T-tube reduced the incidence of biliary stenosis without increasing the incidence of other biliary complications. Based on these results, the use of a T-tube for end-to-end ductal anastomosis in patients undergoing liver transplantation could be recommended.
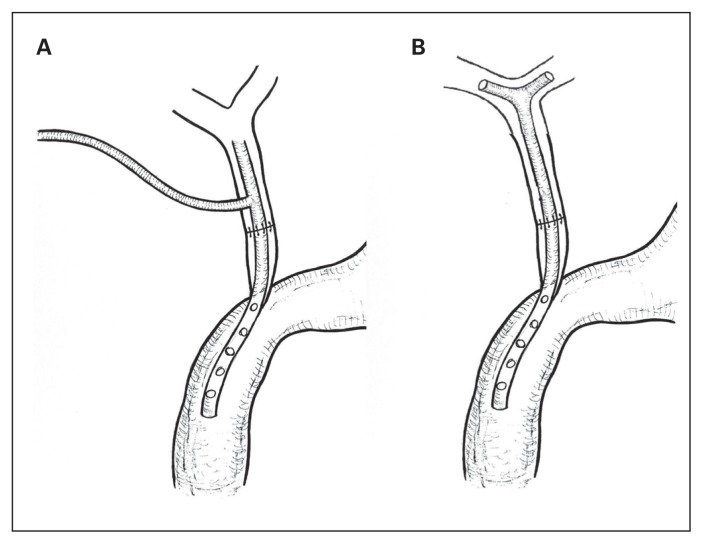
The use and duration of biliary drainage in patients with IBDI is controversial. The advantage of biliary drainage is limitation of the inflammation and fibrosis occurring after the surgical procedure. In some authors’ opinions, the presence of the biliary tube prevents anastomosis stricture.41 The disadvantage of biliary drainage is a higher risk of postoperative complications.2,42 The duration of drainage is also controversial. According to most authors, the optimal duration for biliary drainage is about 3 months. Investigations showed that longer duration of biliary drainage did not provide a greater advantage.2,43 The 2 main types of biliary drainage using T-tube can be distinguished: external T-drainage (Fig. 1A) and internal Y-drainage (Fig. 1B). External T-drainage involves using a typical T-tube with insertion of its short branches into the bile duct and conducting of its long branch through the abdominal wall outside. It can be removed percutaneously after healing of the end-to-end ductal anastomosis. Internal Y-drainage involves insertion of short branches of the T-tube into both the right and left hepatic ducts, splinting of the anastomosis and conducting of its long branch into the duodenum by the papilla of Vater. This drainage can be removed endoscopically after healing of the end-to-end ductal anastomosis. It should be emphasized that the internal Y-drainage is less traumatic (does not involve additional incision of the bile duct wall) than the external T-drainage. Therefore, it should be recommended as the drainage of choice in end-to-end ductal anastomosis.1,2,24
Fig. 1.
Types of biliary drainage using T-tube. (A) External T-drainage. (B) Internal Y-drainage.
Complications of end-to-end ductal anastomosis
An anastomostic fistula and stenosis are the 2 common postoperative complications following end-to-end ductal anastomosis. In patients who have had end-to-end ductal anastomosis, endoscopic control and treatment of these complications are possible. In anastomotic leakages and strictures, endoscopic retrograde cholangiopancreatography (ERCP) with stenting or stricture balloon dilatation is the first-line treatment. Percutaneous transhepatic biliary drainage can also be performed.6 Yoshiya and colleagues44 described the use of rendezvous ductoplasty to treat biliary anastomotic stricture after LDLT. Biliary anastomotic stricture was classified according to ERCP findings after normal pressure contrast injection: type I (n = 32) in which the stricture was visualized; type II (n = 13) in which the common hepatic duct and graft intrahepatic ducts were visualized, but the stricture was not visualized; or type III (n = 8) in which the stricture and graft intrahepatic ducts were not visualized. The number of attempts to pass the guidewire through the stricture was significantly lower in type I than type II or type III. The treatment success rate was 78.1% for type I, 38.5% for type II, and 50.0% for type III. Rendezvous ductoplasty was the first successful treatment in a higher proportion of types II and III patients than type I patients (66.7% vs. 6.3%). Cumulative treatment success rates were not significantly different between the rendezvous ductoplasty and the non–rendezvous ductoplasty groups. Hsieh and colleagues45 described aggressive endoscopy-based treatment with maximal stent placement that allowed 100% resolution of all biliary anastomotic strictures after LDLT without the need for surgical intervention or retransplantation. When less invasive (using endoscopy and interventional radiology) treatment is not successful, surgery is needed.36
Conclusion
End-to-end ductal anastomosis is used for biliary reconstruction in patients undergoing liver transplantation and surgical repair of IBDI. The use of end-to-end ductal anastomosis in patients undergoing liver transplantation is more common than in those undergoing surgical treatment of IBDI. The achievement of good long-term results is possible in patients undergoing both treatments. End-to-end ductal anastomosis should be considered as the biliary reconstruction of choice because it is more physiologic than HJ and it is associated with fewer early postoperative complications.
Footnotes
Competing interests: None declared.
References
- 1.Jabłonska B, Lampe P, Olakowski M, et al. Hepaticojejunostomy vs. end-to-end biliary reconstructions in the treatment of iatrogenic bile duct injuries. J Gastrointest Surg. 2009;13:1084–93. doi: 10.1007/s11605-009-0841-7. [DOI] [PubMed] [Google Scholar]
- 2.Jabłonska B, Lampe P. Iatrogenic bile duct injuries: etiology, diagnosis and management. World J Gastroenterol. 2009;15:4097–104. doi: 10.3748/wjg.15.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabłonska B, Olakowski M, Lampe P, et al. Quality-of-life assessment in the treatment of iatrogenic bile duct injuries: hepaticojejunostomy versus end-to-end biliary reconstructions. ANZ J Surg. 2012;82:923–7. doi: 10.1111/j.1445-2197.2012.06243.x. [DOI] [PubMed] [Google Scholar]
- 4.Ishiko T, Egawa H, Kasahara M, et al. Duct-to-duct biliary reconstruction in living donor liver transplantation utilizing right lobe graft. Ann Surg. 2002;236:235–40. doi: 10.1097/00000658-200208000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasahara M, Egawa H, Takada Y, et al. Biliary reconstruction in right lobe living-donor liver transplantation: comparison of different techniques in 321 recipients. Ann Surg. 2006;243:559–66. doi: 10.1097/01.sla.0000206419.65678.2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadhawan M, Kumar A, Gupta S, et al. Post-transplant biliary complications — an analysis from a predominantly living donor liver transplant centre. J Gastroenterol Hepatol. 2013;28:1056–60. doi: 10.1111/jgh.12169. [DOI] [PubMed] [Google Scholar]
- 7.Neuhaus P, Blumhardt G, Bechstein WO, et al. Technique and results of biliary reconstruction using side-to-side choledochocholedochostomy in 300 orthotopic liver transplants. Ann Surg. 1994;219:426–34. doi: 10.1097/00000658-199404000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SF, Huang ZY, Chen XP. Biliary complications after living donor liver transplantation. Liver Transpl. 2011;17:1127–36. doi: 10.1002/lt.22381. [DOI] [PubMed] [Google Scholar]
- 9.Damrah O, Sharma D, Burroughs A, et al. Duct-to-duct biliary reconstruction in orthotopic liver transplantation for primary sclerosing cholangitis: a viable and safe alternative. Transpl Int. 2012;25:64–8. doi: 10.1111/j.1432-2277.2011.01371.x. [DOI] [PubMed] [Google Scholar]
- 10.Heffron TG, Smallwood GA, Ramcharan T, et al. Duct-to-duct biliary anastomosis for patients with sclerosing cholangitis undergoing liver transplantation. Transplant Proc. 2003;35:3006–7. doi: 10.1016/j.transproceed.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 11.Esfeh JM, Eghtesad B, Hodgkinson P, et al. Duct-to-duct biliary reconstruction in patients with primary sclerosing cholangitis undergoing liver transplantation. HPB (Oxford) 2011;13:651–5. doi: 10.1111/j.1477-2574.2011.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz V, Neumann UP, Puhl G, et al. Surgical complications and long-term outcome of different biliary reconstructions in liver transplantation for primary sclerosing cholangitis-choledochoduodenostomy versus choledochojejunostomy. Am J Transplant. 2006;6:379–85. doi: 10.1111/j.1600-6143.2005.01173.x. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka H, Fukuda A, Shigeta T, et al. Biliary reconstruction in pediatric live donor liver transplantation: duct-to-duct or Roux-en-Y hepaticojejunostomy. J Pediatr Surg. 2010;45:1668–75. doi: 10.1016/j.jpedsurg.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Loong CC, Hsia CY, et al. Duct-to-duct biliary reconstruction in selected cases in pediatric living-donor left-lobe liver transplantation. Pediatr Transplant. 2009;13:693–6. doi: 10.1111/j.1399-3046.2008.01040.x. [DOI] [PubMed] [Google Scholar]
- 15.Haberal M, Sevmis S, Emiroglu R, et al. Duct-to-duct biliary reconstruction in pediatric liver transplantation: one center’s results. Transplant Proc. 2007;39:1161–3. doi: 10.1016/j.transproceed.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 16.Shirouzu Y, Okajima H, Ogata S, et al. Biliary reconstruction for infantile living donor liver transplantation: Roux-en-Y hepaticojejunostomy or duct-to-duct choledochocholedochostomy? Liver Transpl. 2008;14:1761–5. doi: 10.1002/lt.21599. [DOI] [PubMed] [Google Scholar]
- 17.Okajima H, Inomata Y, Asonuma K, et al. Duct-to-duct biliary reconstruction in pediatric living donor liver transplantation. Pediatr Transplant. 2005;9:531–3. doi: 10.1111/j.1399-3046.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 18.Kimura T, Hasegawa T, Ihara Y, et al. Feasibility of duct-to-duct biliary reconstruction in pediatric living related liver transplantation: report of three cases. Pediatr Transplant. 2006;10:248–51. doi: 10.1111/j.1399-3046.2005.00430.x. [DOI] [PubMed] [Google Scholar]
- 19.Jabłonska B, Lampe P, Olakowski M, et al. Surgical treatment of iatrogenic bile duct injuries — early complications. Pol J Surg. 2008;80:299–305. [Google Scholar]
- 20.Jabłonska B, Lampe P, Olakowski M, et al. Long-term results in the surgical treatment of iatrogenic bile duct injuries. Pol J Surg. 2010;82:354–61. [Google Scholar]
- 21.de Reuver PR, Bush ORC, Rauws EA, et al. Long-term results of a primary end-to-end anastomosis in peroperative detected bile duct injury. J Gastrointest Surg. 2007;11:296–302. doi: 10.1007/s11605-007-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen ML, Jensen SL, Malstrom J, et al. Gastryn and gastric acid secretion in hepaticojejunostomy Roux-en-Y. Surg Gynecol Obstet. 1980;150:61–4. [PubMed] [Google Scholar]
- 23.Imamura M, Takahashi M, Sasaki I, et al. Effects of the pathway of bile flow on the digestion of FAT and the release of gastrointestinal hormones. Am J Gastroenterol. 1988;83:386–92. [PubMed] [Google Scholar]
- 24.Gazzaniga GM, Filauro M, Mori L. Surgical treatment of iatrogenic lesions of the proximal common bile duct. World J Surg. 2001;25:1254–9. doi: 10.1007/s00268-001-0105-5. [DOI] [PubMed] [Google Scholar]
- 25.Kohneh Shahri N, Lasnier C, Paineau J. Bile duct injuries at laparoscopic cholecystectomy: early repair results. Ann Chir. 2005;130:218–23. doi: 10.1016/j.anchir.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Strasberg SM, Helton WS. An analytical review of vasculobiliary injury in laparoscopic and open cholecystectomy. HPB (Oxford) 2011;13:1–14. doi: 10.1111/j.1477-2574.2010.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koffron A, Ferrario M, Parsons W, et al. Failed primary management of iatrogenic biliary injury: incidence and significance of concomitant hepatic arterial disruption. Surgery. 2001;130:722–8. doi: 10.1067/msy.2001.116682. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt SC, Langrehr JM, Hintze RE, et al. Long-term results and risk factors influencing outcome of major bile duct injuries following cholecystectomy. Br J Surg. 2005;92:76–82. doi: 10.1002/bjs.4775. [DOI] [PubMed] [Google Scholar]
- 29.Madariaga JR, Dodson SF, Selby R, et al. Corrective treatment and anatomic considerations for laparoscopic cholecystectomy injuries. J Am Coll Surg. 1994;179:321–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Sarno G, Al-Sarira AA, Ghaneh P, et al. Cholecystectomy-related bile duct and vasculobiliary injuries. Br J Surg. 2012;99:1129–36. doi: 10.1002/bjs.8806. [DOI] [PubMed] [Google Scholar]
- 31.Alves A, Farges O, Nicolet J, et al. Incidence and consequence of an hepatic artery injury in patients with postcholecystectomy bile duct strictures. Ann Surg. 2003;238:93–6. doi: 10.1097/01.sla.0000074983.39297.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart L, Robinson TN, Lee CM, et al. Right hepatic artery injury associated with laparoscopic bile duct injury: incidence, mechanism, and consequences. J Gastrointest Surg. 2004;8:523–30. doi: 10.1016/j.gassur.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Jabłonska B, Lampe P. Reconstructive biliary surgery in the treatment of iatrogenic bile duct injuries. In: Brzozowski T, editor. New advances in the basic and clinical gastroenterology. Rijeka (HR): InTech; 2012. pp. 477–494. [Google Scholar]
- 34.Jabłonska B. The arterial blood supply of the extrahepatic biliary tract — surgical aspects. Pol J Surg. 2008;80:336–42. [Google Scholar]
- 35.Castaldo ET, Pinson CW, Feurer ID, et al. Continuous versus interrupted suture for end-to-end biliary anastomosis during liver transplantation gives equal results. Liver Transpl. 2007;13:234–8. doi: 10.1002/lt.20986. [DOI] [PubMed] [Google Scholar]
- 36.Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245–57. doi: 10.1159/000144653. [DOI] [PubMed] [Google Scholar]
- 37.Scatton O, Meunier B, Cherqui D, et al. Randomized trial of choledochocholedochostomy with or without a T tube in orthotopic liver transplantation. Ann Surg. 2001;233:432–7. doi: 10.1097/00000658-200103000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López-Andújar R, Montalvá Orón E, Frangi Carregnato A, et al. T-tube or no T-tube in cadaveric orthotopic liver transplantation: the eternal dilemma: results of a prospective and randomized clinical trial. Ann Surg. 2013;258:21–9. doi: 10.1097/SLA.0b013e318286e0a0. [DOI] [PubMed] [Google Scholar]
- 39.Sotiropoulos GC, Sgourakis G, Radtke A, et al. Orthotopic liver transplantation: T-tube or not T-tube? Systematic review and meta-analysis of results. Transplantation. 2009;87:1672–80. doi: 10.1097/TP.0b013e3181a5cf3f. [DOI] [PubMed] [Google Scholar]
- 40.Huang WD, Jiang JK, Lu YQ. Value of T-tube in biliary tract reconstruction during orthotopic liver transplantation: a meta-analysis. J Zhejiang Univ Sci B. 2011;12:357–64. doi: 10.1631/jzus.B1100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pekolj J, Alvarez FA, Palavecino M, et al. Intraoperative management and repair of bile duct injuries sustained during 10,123 laparoscopic cholecystectomies in a high-volume referral center. J Am Coll Surg. 2013;216:894–901. doi: 10.1016/j.jamcollsurg.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 42.Robinson TN, Stiegmann GV, Durham JD, et al. Management of major bile duct injury associated with laparoscopic cholecystectomy. Surg Endosc. 2001;15:1381–5. doi: 10.1007/s00464-001-8156-0. [DOI] [PubMed] [Google Scholar]
- 43.Lillemoe KD, Melton GB, Cameron JL, et al. Postoperative bile duct strictures: management and outcome in the 1990s. Ann Surg. 2000;232:430–41. doi: 10.1097/00000658-200009000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshiya S, Shirabe K, Matsumoto Y, et al. Rendezvous ductoplasty for biliary anastomotic stricture after living-donor liver transplantation. Transplantation. 2013;95:1278–83. doi: 10.1097/TP.0b013e31828a9450. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh TH, Mekeel KL, Crowell MD, et al. Endoscopic treatment of anastomotic biliary strictures after living donor liver transplantation: outcomes after maximal stent therapy. Gastrointest Endosc. 2013;77:47–54. doi: 10.1016/j.gie.2012.08.034. [DOI] [PubMed] [Google Scholar]