Abstract
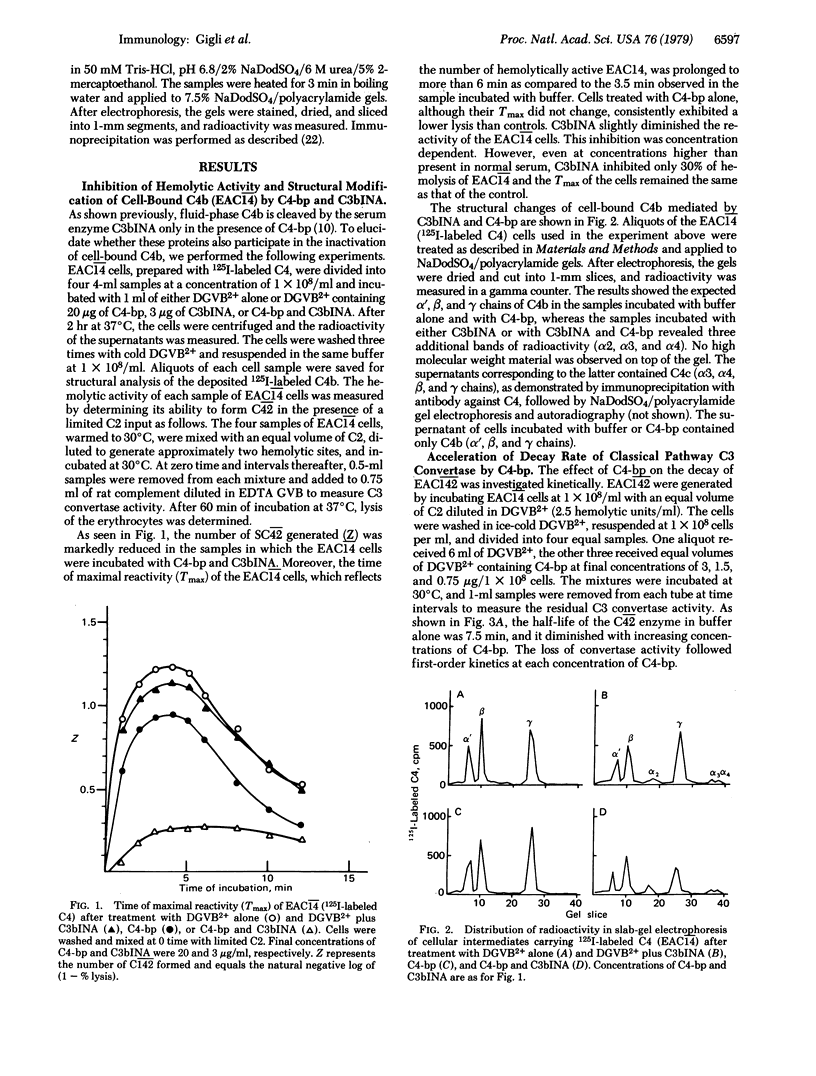
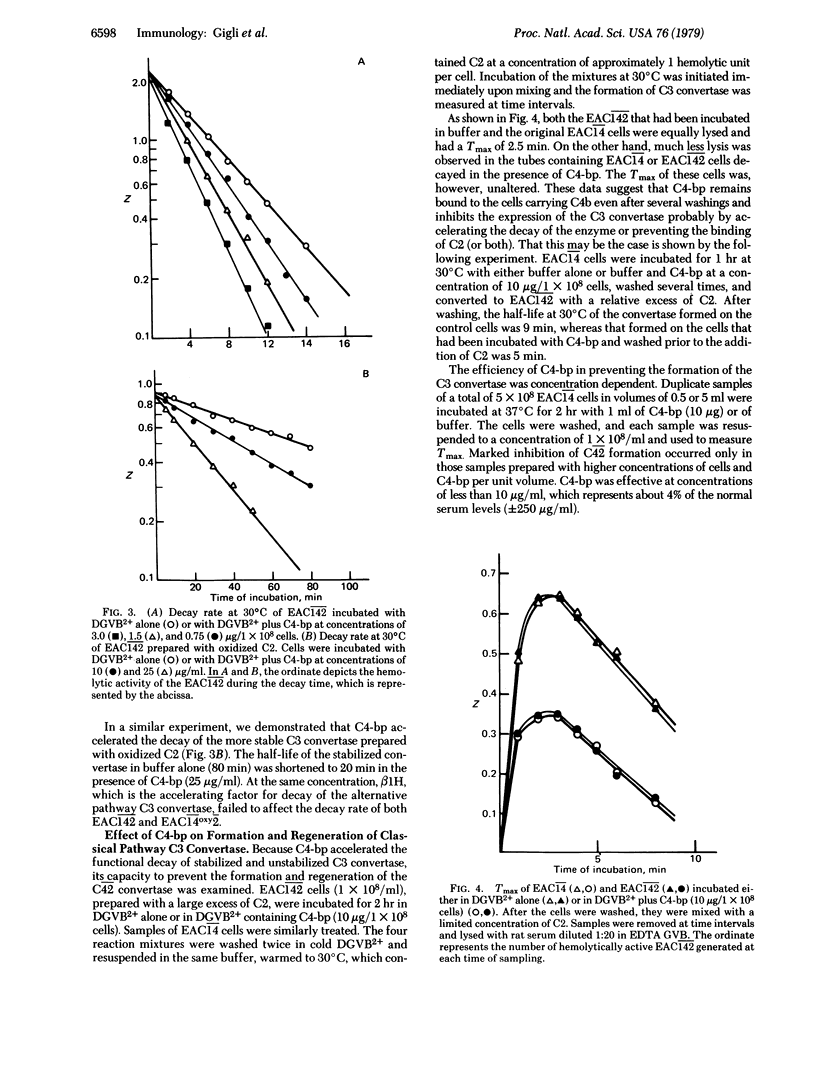
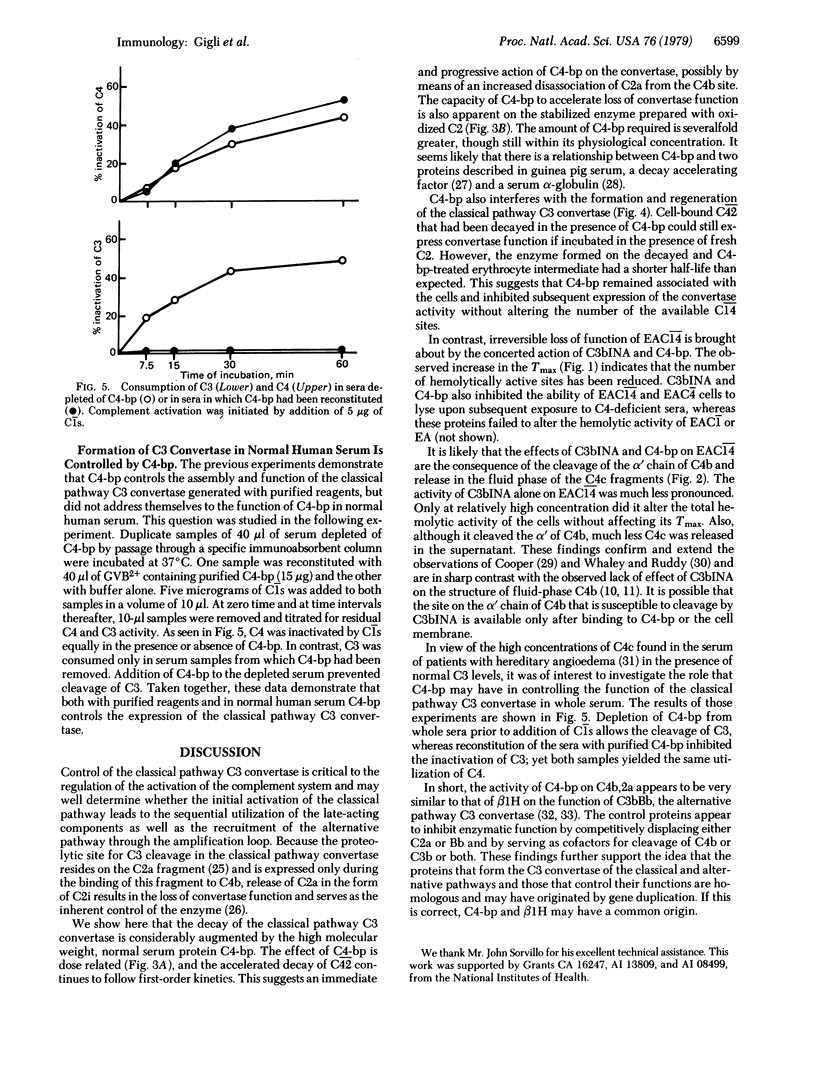
We recently described the isolation from human serum of a serum protein (C4 binding protein) that functions as an essential cofactor for C3b inactivator in the proteolysis of fluid-phase C4b and to a much lesser extent, C3b. We show here the role of C4 binding protein in the formation and function of the classical pathway C3 convertase (C42). C4 binding protein interferes with the assembly of the membrane-bound C3 convertase of the classical pathway and accelerates the decay of C42 in a dose-dependent fashion. Its removal from serum by means of specific immune absorption promotes the vigorous consumption of C3 after addition of C1; this effect is abolished by reconstitution with purified C4 binding protein. Although C4 binding protein inhibits the hemolytic function of cell-bound C4b, we did not detect any change in the structure of C4b even after prolonged incubations of EAC14 with C4 binding protein. For this reason, and on the basis of studies of the time required for maximal reactivity (Tmax) of cellular intermediates generated in the presence of C4 binding protein and limited amounts of C2, we conclude that the effects of C4 binding protein are probably mediated by displacing C2a from specific binding sites on C4b. In addition, C4 binding protein enhances the cleavage by C3b inactivator of the alpha' chain of cell-bound C4b. When EAC14 cells were incubated with both control proteins, the Tmax of the cells was prolonged and the lysis was markedly diminished. We conclude that C4 binding protein and C3b inactivator control the C3 convertase of the classical pathway in a fashion similar to that described for beta 1H and C3b inactivator in the alternative pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augener W., Grey H. M., Cooper N. R., Müller-Eberhard H. J. The reaction of monomeric and aggregated immunoglobulins with C1. Immunochemistry. 1971 Nov;8(11):1011–1020. doi: 10.1016/0019-2791(71)90489-7. [DOI] [PubMed] [Google Scholar]
- BECKER E. L. Concerning the mechanism of complement action. I. Inhibition of complement activity by diisopropyl fluophosphate. J Immunol. 1956 Dec;77(6):462–468. [PubMed] [Google Scholar]
- Cooper N. R. Isolation and analysis of the mechanism of action of an inactivator of C4b in normal human serum. J Exp Med. 1975 Apr 1;141(4):890–903. [PMC free article] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1683–1687. doi: 10.1073/pnas.74.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A., Nussenzweig V., Gigli I. Structural and functional differences between the H-2 controlled Ss and Slp proteins. J Exp Med. 1978 Nov 1;148(5):1186–1197. doi: 10.1084/jem.148.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Gigli I., Nussenzweig V. Human C4-binding protein. II. Role in proteolysis of C4b by C3b-inactivator. J Exp Med. 1978 Oct 1;148(4):1044–1051. doi: 10.1084/jem.148.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Nussenzweig V. The role of C4-binding protein and beta 1H in proteolysis of C4b and C3b. J Exp Med. 1979 Aug 1;150(2):267–276. doi: 10.1084/jem.150.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Porter R. R., Sim R. B. The unactivated form of the first component of human complement, C1. Biochem J. 1976 Sep 1;157(3):541–548. doi: 10.1042/bj1570541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., von Zabern I., Porter R. R. The isolation and structure of C4, the fourth component of human complement. Biochem J. 1977 Sep 1;165(3):439–446. doi: 10.1042/bj1650439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Porter R. R. The purification and properties of the second component of human complement. Biochem J. 1978 Apr 1;171(1):99–107. doi: 10.1042/bj1710099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPOW I. H., NAFF G. B., TODD E. W., PENSKY J., HINZ C. F. Chromatographic resolution of the first component of human complement into three activities. J Exp Med. 1963 Jun 1;117:983–1008. doi: 10.1084/jem.117.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Polley M. J., Calcott M. A. Formation and functional significance of a molecular complex derived from the second and the fourth component of human complement. J Exp Med. 1967 Feb 1;125(2):359–380. doi: 10.1084/jem.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa S., Stroud R. M. Mechanism of action of the C3b inactivator: requirement for a high molecular weight cofactor (C3b-C4bINA cofactor) and production of a new C3b derivative (C3b'). Immunochemistry. 1977 Nov-Dec;14(11-12):749–756. doi: 10.1016/0019-2791(77)90345-7. [DOI] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. Enharncement of the hemolytic activity of the second component of human complement by oxidation. J Exp Med. 1967 Dec 1;126(6):1013–1025. doi: 10.1084/jem.126.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. The second component of human complement: its isolation, fragmentation by C'1 esterase, and incorporation into C'3 convertase. J Exp Med. 1968 Sep 1;128(3):533–551. doi: 10.1084/jem.128.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfstein J., Ferreira A., Gigli I., Nussenzweig V. Human C4-binding protein. I. Isolation and characterization. J Exp Med. 1978 Jul 1;148(1):207–222. doi: 10.1084/jem.148.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi S., Stroud R. M. Cleavage products of C4b produced by enzymes in human serum. Immunochemistry. 1975 Dec;12(12):935–939. doi: 10.1016/0019-2791(75)90256-6. [DOI] [PubMed] [Google Scholar]
- Shiraishi S., Stroud R. M. Cleavage products of C4b produced by enzymes in human serum. Immunochemistry. 1975 Dec;12(12):935–939. doi: 10.1016/0019-2791(75)90256-6. [DOI] [PubMed] [Google Scholar]
- Sitomer G., Stroud R. M., Mayer M. M. Reversible adsorption of C'2 by EAC'4: role of Mg2+, enumeration of competent SAC'4, two-step nature of C'2a fixation and estimation of its efficiency. Immunochemistry. 1966 Jan;3(1):57–69. doi: 10.1016/0019-2791(66)90282-5. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., Mayer M. M., Miller J. A., McKenzie A. T. C'2ad, an inactive derivative of C'2 released during decay of EAC'4,2a. Immunochemistry. 1966 May;3(3):163–176. doi: 10.1016/0019-2791(66)90182-0. [DOI] [PubMed] [Google Scholar]
- Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of C3b hemolytic activity by a plasma protein distinct from C3b inactivator. Science. 1976 Sep 10;193(4257):1011–1013. doi: 10.1126/science.948757. [DOI] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]


