Abstract
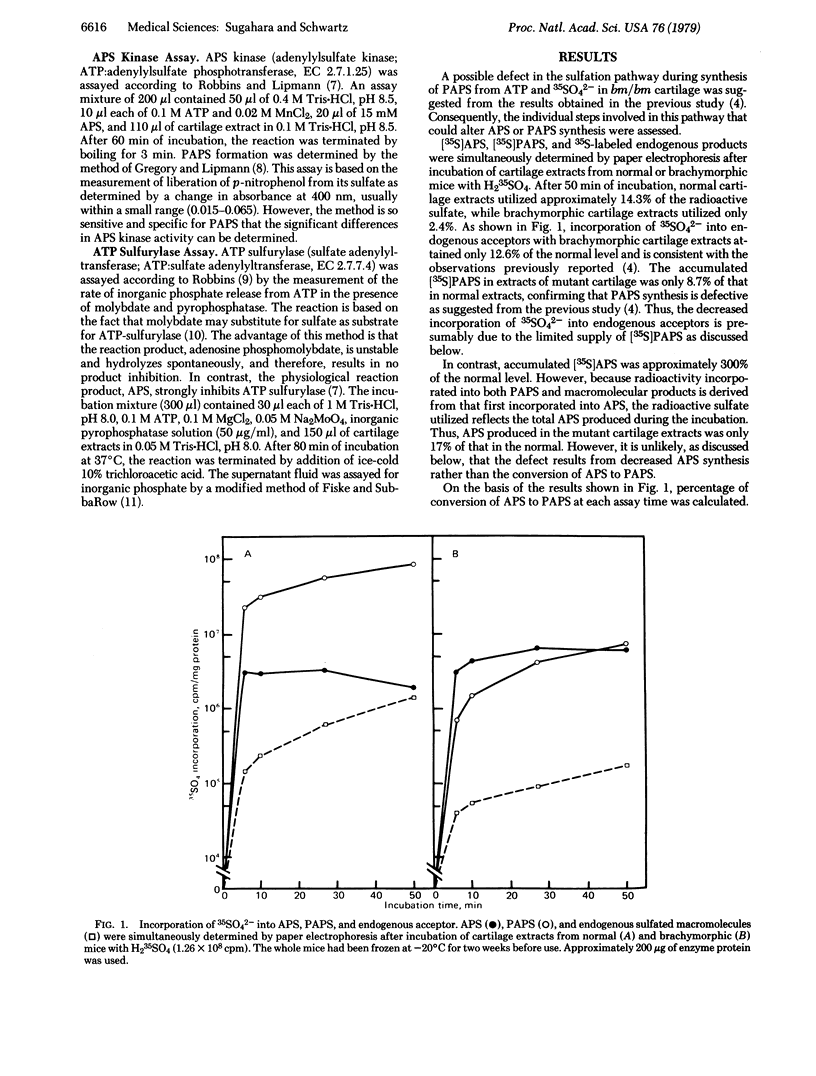
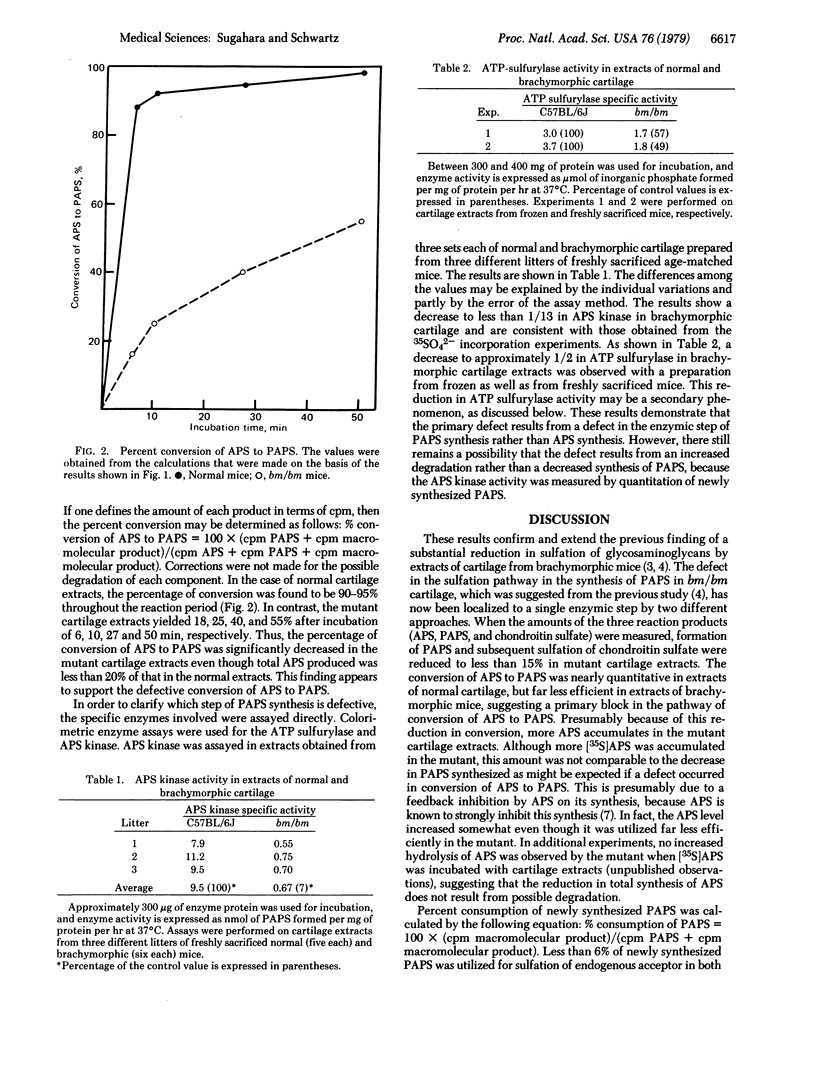
Incorporation of 35SO42- into adenosine 5'-phosphosulfate (APS), 3'-phosphoadenosine 5'-phosphosulfate (PAPS), and chondroitin sulfate was simultaneously assessed with extracts prepared from epiphyseal cartilage of neonatal normal or homozygous brachymorphic mice. Radioactivity measured in APS, PAPS, and chondroitin sulfate of extracts from brachymorphic cartilage was approximately 300%, 9%, and 13% of the normal levels, respectively. Even though more APS accumulated in the mutant cartilage extracts, APS actually synthesized (total 35SO42- incorporated into APS, PAPS, and macromolecular products) was only 17% of that in the normal. However, of the amount synthesized, 90% and 55% of newly synthesized APS were converted to PAPS by cartilage extracts of normal and brachymorphic mice, respectively. Specific assays for ATP sulfurylase (sulfate adenylyltransferase; ATP:sulfate adenylyltransferase, EC 2.7.7.4) and APS kinase (adenylylsulfate kinase; ATP:adenylylsulfate 3'-phosphotransferase, EC 2.7.1.25) showed that the sulfurylase enzyme activity is reduced to approximately 1/2 and the kinase to approxomately 1/14 in brachymorphic mice. These results suggest that the production of an undersulfated proteoglycan in brachymorphic cartilage results from a defective conversion of APS to PAPS.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunham J. S., Hynes R. O. Differences in the sulfated macromolecules synthesized by normal and transformed hamster fibroblasts. Biochim Biophys Acta. 1978 Jan 19;506(2):242–255. doi: 10.1016/0005-2736(78)90395-4. [DOI] [PubMed] [Google Scholar]
- Farooqui A. A., Rebel G., Mandel P. Sulphatide metabolism in brain. Life Sci. 1977 Feb 15;20(4):569–583. doi: 10.1016/0024-3205(77)90459-3. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Max S. R., Tallman J. F., Brady R. O., Maclaren N. K., Cornblath M. Deficient Ganglioside Biosynthesis: a novel human sphingolipidosis. Science. 1975 Jan 10;187(4171):68–70. doi: 10.1126/science.803227. [DOI] [PubMed] [Google Scholar]
- GREGORY J. D., LIPMANN F. The transfer of sulfate among phenolic compounds with 3',5'-diphosphoadenosine as coenzyme. J Biol Chem. 1957 Dec;229(2):1081–1090. [PubMed] [Google Scholar]
- Greene R. M., Brown K. S., Pratt R. M. Autoradiographic analysis of altered glycosaminoglycan synthesis in the epiphyseal cartilage of neonatal brachymorphic mice. Anat Rec. 1978 May;191(1):19–29. doi: 10.1002/ar.1091910103. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane P. W., Dickie M. M. Three recessive mutations producing disproportionate dwarfing in mice: achondroplasia, brachymorphic, and stubby. J Hered. 1968 Sep-Oct;59(5):300–308. doi: 10.1093/oxfordjournals.jhered.a107725. [DOI] [PubMed] [Google Scholar]
- Orkin R. W., Pratt R. M., Martin G. R. Undersulfated chondroitin sulfate in the cartilage matrix of brachymorphic mice. Dev Biol. 1976 May;50(1):82–94. doi: 10.1016/0012-1606(76)90069-5. [DOI] [PubMed] [Google Scholar]
- Orkin R. W., Williams B. R., Cranley R. E., Poppke D. C., Brown K. S. Defects in the cartilaginous growth plates of brachymorphic mice. J Cell Biol. 1977 May;73(2):287–299. doi: 10.1083/jcb.73.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Separation of the two enzymatic phases in active sulfate synthesis. J Biol Chem. 1958 Sep;233(3):681–685. [PubMed] [Google Scholar]
- Schwartz N. B., Ostrowski V., Brown K. S., Pratt R. M. Defective PAPS-synthesis in epiphyseal cartilage from brachymorphic mice. Biochem Biophys Res Commun. 1978 May 15;82(1):173–178. doi: 10.1016/0006-291x(78)90592-2. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]



