Abstract
Attention induces synchronicity in neuronal firing for the encoding of a given stimulus at the exclusion of others. Recently, we reported decreased variability in scalp-recorded cortical evoked potentials to attended compared with ignored speech in adults. Here we aimed to determine the developmental time course for this neural index of auditory attention. We compared cortical auditory-evoked variability with attention across three age groups: preschoolers, school-aged children and young adults. Results reveal an increased impact of selective auditory attention on cortical response variability with development. Although all three age groups have equivalent response variability to attended speech, only school-aged children and adults have a distinction between attend and ignore conditions. Preschoolers, on the other hand, demonstrate no impact of attention on cortical responses, which we argue reflects the gradual emergence of attention within this age range. Outcomes are interpreted in the context of the behavioral relevance of cortical response variability and its potential to serve as a developmental index of cognitive skill.
Introduction
Scalp-recorded cortical evoked potentials are conventionally assessed according to average peak characteristics, which change over the course of maturation. In addition to decreasing in both magnitude and timing from childhood to adulthood, children's responses demonstrate only a broad positivity (the P1) followed by a broad negativity (the N2) whereas adolescents and adults produce a distinct P1-N1-P2 complex (Cunningham, Nicol, Zecker & Kraus, 2000; Pang & Taylor, 2000; Ponton, Eggermont, Kwong & Don, 2000). Although these peaks are not always identifiable in responses to individual trials, they are visible in averaged waveforms that comprise responses to many identical stimuli (on the order of dozens to hundreds). While this method permits the analysis of response properties that are consistent over the course of an experiment, it relies on the assumption that trial-by-trial variability can be disregarded as noise. It is clear, however, that responses to an unchanging stimulus will demonstrate variability on a trial-by-trial basis due to variations in background brain activity as well as unpredictability within the response mechanism itself (Arieli, Sterkin, Grinvald & Aertsen, 1996; Faisal, Selen & Wolpert, 2008). Given that variability in cortical evoked potentials can be as robust as responses to the stimuli themselves (Schiller, Finlay & Volman, 1976), trial-by-trial response variability may reflect functionally relevant aspects of signal processing.
Sensory processing requires the direction of perceptual resources toward a signal of interest amidst competing inputs. Selective attention plays a dynamic gatekeeping role in this process, focusing on a single source within multiple sensory streams. Although attention clearly enhances aspects of averaged sensory-evoked activity (e.g. heightened N100 amplitudes to attended compared with ignored inputs) (Desimone & Duncan, 1995; Hillyard, Hink, Schwent & Picton, 1973; Woldorff, Gallen, Hampson, Hillyard, Pantev, Sobel & Bloom, 1993), the functional relevance of scalp-recorded cortical response variability remains only minimally considered.
We originally predicted decreased cortical response variability with attention given its impact on neural synchrony, both within and across brain regions involved in a task's execution (Fries, Reynolds, Rorie & Desimone, 2001; Fries, Womelsdorf, Oostenveld & Desimone, 2008; Gregoriou, Gotts, Zhou & Desimone, 2009; Steinmetz, Roy, Fitzgerald, Hsiao, Johnson & Niebur, 2000). We recently tested this prediction in young adults, who demonstrated decreased cortical auditory-evoked response variability to attended compared with ignored sound streams (Strait & Kraus, 2011). Here, we aimed to determine the developmental time course for this neural index of auditory attention by comparing attended and ignored evoked response variability across a wide age range, from age 3 to adulthood. By employing a paradigm that afforded the assessment of response variability to attended and ignored speech streams, we were not only able to define the developmental trajectory for selective attention but also for its suppression correlate, inhibitory control. We hypothesized that the difference in cortical response variability to attended and ignored speech increases with development. Given that attention is thought to be established within the first decade of life (Booth, Burman, Meyer, Lei, Trommer, Davenport, Li, Parrish, Gitelman & Mesulam, 2003; Tipper, Bourque, Anderson & Brehaut, 1989), we predicted that older children and adults would demonstrate a significant decrease in the variability of cortical evoked activity with attention whereas the youngest children, in whom attention abilities are still emerging (Levy, 1980; Plude, Enns & Brodeur, 1994), would not. We further predicted developmental increases in variability to the ignored speech stream between older children and adults due to the ongoing development of inhibitory control (Booth et al., 2003; Luna, Thulborn, Munoz, Merriam, Garver, Minshew, Keshavan, Genovese, Eddy & Sweeney, 2001; Tipper et al., 1989; Williams, Ponesse, Schachar, Logan & Tannock, 1999).
Methods
Participants
All experimental procedures were approved by the Northwestern University Institutional Review Board. Eighty-one normal hearing children and adults (< 20 dB pure tone thresholds at octave frequencies from 125 to 8000 Hz) between the ages of 3 and 35 years participated in this study and were grouped into three age groups: preschoolers (3–5-year-olds, N = 24), school-aged children (7–13-year-olds, N = 28) and adults (18–35-year-olds, N = 22). Participants and legal guardians, in the case of minors, provided informed consent and assent. Participants were monetarily compensated for their time. No participant reported a history of neurological or learning abnormalities.
Electrophysiology
Stimulus
The evoking stimulus was a six-formant, 170 ms speech syllable (/da/) produced using a Klatt-based synthesizer (Klatt, 1980) with a 5 ms voice onset time and a level 100 Hz fundamental frequency. The first, second and third formants were dynamic over the first 50 ms (F1, 400–720; F2, 1700–1240; F3, 2580–2500 Hz) but maintained frequency for the rest of the sound's duration. The fourth, fifth and sixth formants were constant throughout (F4, 3300; F5, 3750; F6, 4900 Hz). The stimulus was presented using NeuroScan Stim2 (Compumedics, Charlotte, NC, USA).
Recording parameters
Auditory-evoked potentials were recorded to the speech sound/da/using a 31-channel tin-electrode cap (Electrocap International, Eaton, OH, USA) in NeuroScan Acquire 4.3 (Compumedics) while participants were seated in a sound-attenuated booth. Only 14 of the possible 31 channels were applied in preschoolers in order to limit their testing time (see Figure 2 for channels employed). Single electrodes were placed on the earlobes and on the superior and outer canthi of the left eye, thereby acting as reference and eye-blink monitors, respectively. Contact impedance for all electrodes was under 5 kO for school-aged children and adults and under 20 kO for preschoolers with less than 5 kO difference across channels. Neural recordings were off-line filtered from 0.1 to 100 Hz and digitally sampled at a rate of 500 Hz.
Figure 2.
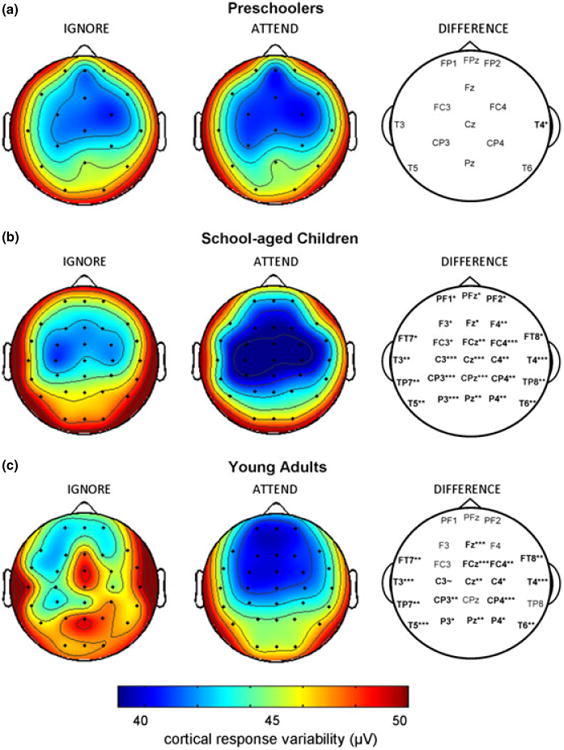
The scalp-topography of cortical auditory-evoked response variability to ignored and attended speech streams. Whereas school-aged children and adults have decreased response variability with attention across the scalp, preschoolers do not. The right column indicates significant differences in response variability between attended and ignored conditions by electrode site (∼p < .10; *p < .05; **p < .01; ***p < .005).
The evoking stimulus was presented in the context of short stories played through two wall-mounted loudspeakers located 1 m to the left and right of the participant. Participants were asked to attend to one of the two simultaneously presented stories, which differed in direction (left/right speaker), voice (male/female) and content, and to direct their gaze at a wall-mounted screen located 1.5 m ahead. This procedure was adapted from Coch et al. (Coch, Sanders & Neville, 2005), who have since established its viability in children as young as age three (Sanders, Stevens, Coch & Neville, 2006). Instructions described both the direction of the attended story and its speaker's sex. The attended voice and its initial direction were randomized across participants to control for potential advantages or disadvantages of attending to one voice over the other. Although adults listened to the stories without visual stimulation, children viewed projected still images that corresponded to the attended story, much like pictures in a book (as in Sanders et al., 2006). New images were presented every 10–50 seconds (M = 27.7, SD = 20.8). Their frequency and inter-onset intervals did not differ between child age groups (F = 2.0, p = .2) or stories presented (F = 1.1, p = .4).
The evoking stimulus was presented randomly to the left or right (i.e. to the attended or ignored) sides of the head with randomized inter-stimulus intervals (ISIs) that were either 600, 900, or 1200 ms. The stories and the evoking stimulus were presented with a 10 dB difference between the stories (65 dB SPL) and the stimulus (75 dB SPL). For older children and adults, the recording took place over four 8-minute (adults) or 4-minute (school-aged children) blocks. For preschoolers, only two 5-minute blocks were presented. After each block, participants were questioned on the content of the attended story; the stories then changed directions and participants were asked to change their attended side (left/right) in order to continue with the same voice and story. The entire recording session yielded 550 (adults), 350 (school-aged children) or 250 (preschoolers) responses recorded concurrently in both attended and ignored conditions (adults heard four 8-minute stories but data collection stopped after 300 sweeps were collected in each block, although/da/stimuli continued to be presented; this enabled adults to hear the entire attended story). Throughout the experiment, preschool children were monitored by an experimenter seated in the testing booth to confirm that subjects remained still and engaged in the task. The experimenter sat quietly, out of direct view of the participant, and was not involved in the task.
All participants were able to perform the selective attention task as indicated by performance on quizzes that addressed story content. Adults correctly answered ≥ four out of five questions on a written multiple-choice quiz after each block (i.e. ≥16 out of 20 questions over the course of the experiment). School-aged children and preschoolers correctly answered ≥ two out of three orally administered free-answer questions on the attended story after each block (i.e. at least 8 out of 12 and 4 out of 6 questions, respectively). Although the images presented to school-aged children and preschoolers may have provided clues to facilitate question-answering, the images only followed the themes of the stories; images alone would not have been sufficient for accurate replies (e.g. although one story's main character was a mouse who was often pictured with flowers, the question, ‘What was the mouse in the story's name’ must have been answered, ‘Chrysanthemum’, which a preschooler could not identify from the flower's cartoon).
Data processing and analysis
Continuous neural data for attended and ignored conditions were epoched from –100 to 500 ms, referenced to the presentation of the stimulus (0 ms); responses were baseline corrected and epochs demonstrating amplitudes beyond 100 uV were rejected as muscular artifact and the first 500 (adults), 300 (school-aged children) or 180 (preschoolers) artifact-free responses from each participant were subjected to analysis.
Averaged event-related potentials
Prior to assessing response variability, we compared the average evoked responses in attended and ignored conditions for each age group. The main objective was to confirm whether the scalp recordings in each age group reflected well-established developmental characteristics (a broad positivity in children versus the emergence of the P1-N1 complex in adults). For the generation of averaged responses, continuous recordings were first bandpass-filtered from 0.1 to 40 Hz (12 dB/octave, zero phase shift), prior to the epoching stage described above. The removal of eye-blink artifacts was conducted using the spatial filtering algorithm in Neuroscan Edit 4.3 (Compumedics). Following the epoching procedure, responses were subjected to a noise reduction algorithm described in Abrams, Nicol, Zecker and Kraus (2008). The algorithm computes the degree of similarity between each epoch and the average of all epochs using Pearson's correlations. Individual responses were ranked according to their Pearson's r-values and the most poorly correlated 30% were discarded. The remaining 70% were averaged, making up the final averaged evoked response for each subject in each condition.
Mean amplitudes were calculated over peak maxima according to average response characteristics at Cz for each group. In adults, mean amplitudes were calculated corresponding to the N1 (100–150 ms) and N2 (295– 320 ms) peaks. In school-aged children, mean amplitudes were calculated corresponding to the first large positivity (80–120 ms) and for the later negativity, including the N2 (300–400 ms). In preschool children, mean amplitudes were again calculated corresponding to the first large positivity (90–130 ms) and for the later negativity (250– 325 ms). Time ranges were determined based on known characteristics of auditory-evoked potentials in these age ranges in addition to visual inspection of individual waveforms. All peaks were observed within the respective time ranges in all participants.
Variability in event-related potentials
Response variability was computed for each subject in each condition following a procedure described in Smith and Goffman (1998). Subaverages of 25 (adults and school-aged children) or 15 (preschoolers) individual responses were generated for each condition.
Response variability over the first 300 ms post-stimulus onset was determined through calculation of amplitude variances across subaverages within each condition. Rather than comparing amplitudes on a point-by-point basis, we averaged amplitudes across 50 adjacent 6-ms increments (comprising three points each) in each sub-average, computed the variances across the subaverages for each of the 50 increments, and summed the 50 variances. This generated a single index of variability for each subject in each condition to facilitate the assessment of variance over the initial cortical evoked response, including early evoked potentials that are not observable in single-trial evoked responses (i.e. the P1/N1). All data processing was executed with scripts generated in Matlab 7.5.0 (The Mathworks, Natick, MA, USA).
Statistical analysis
Initially, differences in response variability between attend and ignore conditions were compared for the 14 electrode sites that were employed across all subjects using a RMANOVA with condition as within-subjects factor and age group as between-subjects factor. Following an interaction between age and condition we assessed each age group separately using RMANOVAs with condition as within-subjects factor including all available electrode sites (14 in preschoolers, 26 in school-aged children and adults). Post-hoc paired t-tests were conducted for all electrode sites except for occipital channels, F7 and F8 in school-aged children and adults, which did not demonstrate clear responses characteristic of cortical auditory-evoked activity.
Age groups were compared using post-hoc independent samples t-tests. To limit the number of comparisons, electrode sites were grouped according to region (see Table 2 for groupings). Relationships to age were examined with Pearson's correlations (SPSS Inc., Chicago, IL, USA). All results reported herein reflect two-tailed values and normality for all data was confirmed using the Kolmogorov-Smirnov test for equality.
Table 2. Correlations between age and cortical response variability to attended and ignored speech streams.
| Relationships to age | ||
|---|---|---|
|
| ||
| Region | Ignore r (p-value) | Attend r (p-value) |
| Prefrontal (FP1, FPz, FP2) | −0.36 (.001) | −0.32 (.004) |
| Frontal (Fz) | 0.39 (<.001) | 0.13 (.28) |
| Central (FC3, FC4, Cz) | 0.31 (.005) | 0.09 (.41) |
| Parietal (CP3, CP4, Pz) | 0.20 (.07) | 0.18 (.10) |
| Left (T3, T5) | 0.19 (.09) | 0.33 (.003) |
| Right (T4, T6) | 0.28 (.01) | 0.28 (.01) |
Results
We observed an increased impact of attention on cortical response variability with development, with adults demonstrating the greatest difference in response variability between attended and ignored speech streams across the scalp. Preschoolers, in whom attention is just emerging, demonstrate no difference in response variability to attended and ignored sounds. Developmental differences in neural function with attention appear to be driven by responses to ignored sounds, which become more variable with age.
Averaged evoked response characteristics
The averaged evoked potentials across age groups demonstrated characteristic maturational changes, with smaller amplitudes and earlier latencies visible with development (Figure 1). Furthermore, whereas children demonstrate a broad positivity (the P1) followed by a broad negativity (the N2), the distinct P1-N1-P2 complex emerges during adolescence (Cunningham et al., 2000; Pang & Taylor, 2000; Ponton et al., 2000). The response morphologies and time ranges within which these peaks occur largely depend on stimulation rate (Paetau, Ahonen, Salonen & Sams, 1995; Sharma, Kraus, McGee & Nicol, 1997).
Figure 1.
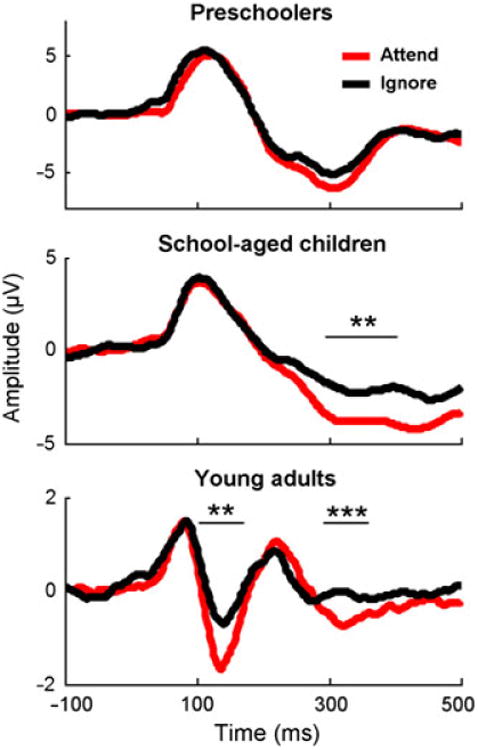
Evoked potentials demonstrate characteristic maturational changes, such as the emergence of the P1/N1/P2 morphology with development. Evoked responses to ignored and attended speech streams (shown at Cz) differ within school-aged children and adults only. Asterisks denote time regions over which amplitudes to attended and ignored stimuli differ (*p < .05; **p < .01; ***p < .005).
A RMANOVA with age group as between-subjects factor and attention and peak as within-subjects factors demonstrated main effects of attention (F(1,74) = 437.1, p < .0001) and peak (F(1,74) = 8.0, p < .01) with interactions between attention × age group (F(2,74) = 128.8, p < .0001) and attention × peak (F(1,74) = 3.8, p < .05). Post-hoc paired t-tests revealed that responses to attended stimuli had greater negative amplitudes at ∼100 ms (N1) and ∼300 ms (N2) post-stimulus onset than to ignored stimuli (N1: t = −2.0, p < .05; N2: t = − 2.9, p < .01, see Tables 1a and 1b). Regarding the attention × age group interaction, post-hoc paired t-tests within each age group revealed that adult responses to attended stimuli had greater negative amplitudes 100 ms post-stimulus onset than to ignored stimuli (i.e. the N1; t = − 2.9, p < .01), consistent with Coch et al. (2005). They also demonstrated greater negative amplitudes to attended stimuli 300 ms post-stimulus onset (t = −3.7, p < .001). Although school-aged children did not show discrepant N1 amplitudes between attend and ignore conditions, they similarly demonstrated more negative amplitudes for the attend condition between 300 and 400 ms post-stimulus onset (t = −3.0, p < .01). Preschoolers' response amplitudes to attended and ignored stimuli were equivalent both 100 and 300 ms post-stimulus onset (all t < 1.0, all p > .3).
Table 1. Paired-samples t-values between attend and ignore peak magnitudes for each age group.
| a. | |||
|---|---|---|---|
|
| |||
| Channel-by-channel effects of attention on the N1 | |||
|
| |||
| Young adults | School-aged | Preschoolers children | |
| FP1 | 1.07 | 0.12 | 0.45 |
| FP2 | 0.33 | 0.96 | 0.48 |
| T3 | −0.61 | 0.02 | −0.23 |
| T4 | −0.98 | 3.67** | 1.39 |
| T5 | −0.11 | −0.60 | 0.11 |
| T6 | −0.22 | −1.50 | 2.13* |
| Cz | −2.90** | −0.73 | −0.75 |
| Fz | −3 78*** | −0.90 | −0.30 |
| Pz | −1.72 | −1.65 | 1.41 |
| CP3 | −1.84∼ | −0.15 | 0.08 |
| CP4 | −2.22* | −1.55 | 1.13 |
| FC3 | −2.74** | 0.04 | −0.78 |
| FC4 | −3.09** | 1.47 | −0.59 |
| Fpz | 0.70 | 2.30* | 0.16 |
| b. | |||
|---|---|---|---|
|
| |||
| Channel-by-channel effects of attention on the N2 | |||
|
| |||
| Young adults | School-aged | Preschoolers children | |
| FP1 | 0.02 | 0.03 | 1.57 |
| FP2 | 0.74 | 2.00 | 1.06 |
| T3 | −1.40 | −0.69 | 0.95 |
| T4 | 0.74 | 0.38 | −0.30 |
| T5 | −0.11 | 0.21 | −0.76 |
| T6 | −0.08 | −0.39 | 0.28 |
| Cz | −3 70*** | −2.87** | −1.06 |
| Fz | −3.12** | −2.70** | −0.38 |
| Pz | −1.99* | −1.44 | −1.01 |
| CP3 | −2.05* | −2.56* | −1.40 |
| CP4 | −2.06* | −1.37 | −1.03 |
| FC3 | −3.28** | −3.12** | −0.93 |
| FC4 | −3.36** | −1.25 | −1.31 |
| Fpz | 0.67 | 0.89 | 0.37 |
p < .05;
p < .01;
p < .005
Impact of attention on cortical response variability
A 3 × 2 × 14 RMANOVA with attention and electrode site as within-subject factors and age group as between-subject factor revealed main effects of age and attention on response variability (age: F(2,74) = 3.6, p = .03; attention: F(1,74) = 28.0, p < .001) as well as age × attention and electrode site × attention interactions (age × attention: F(2,74) = 3.4, p = .03; electrode × attention: F(13,62) = 4.7, p < .001). We also observed a three-way interaction between age × attention × electrode site (F(26,126) = 3.34, p < .0001). We subsequently performed RMANOVAs within each age group with attention and electrode site as within-subject factors (14 electrodes for preschoolers, and 26 for school-aged children and adults). Whereas school-aged children and adults demonstrated main effects of attention (children: F(1,26) = 26.4, p < .001; adults: F(1,20) = 10.6, p < .005), pre-schoolers did not (F(1,22) = 1.0, p = .32). These outcomes held when we constrained our analyses in school-aged children and adults to their first 90 artifact-free sweeps in each of the first two blocks (for a total of 180 individual responses, matching preschoolers' data collection parameters; main effects of attention in children: F(1,26) = 25.0, p < .001 and adults: F(1,20) = 11.6, p < .005). This indicated that procedural differences between preschoolers and school-aged children/adults related to stimulus presentation could not account for the lack of attention effect observed in preschoolers.
Channel-by-channel post-hoc paired t-tests indicated that school-aged children and adults demonstrate more cortical response variability to ignored relative to attended speech streams at most electrode sites except for over prefrontal cortex, where only school-aged children show this effect (Figure 2, right column). There were no attention × channel interactions for either child group (children: F(1,26) = 2.2, p = .17; preschoolers: F(1,22) = 0.4, p = .92), although this interaction was observed in adults (F(1,20) = 17.0, p < .001).
Cortical response variability to ignored sounds increases with age
Pearson's correlations across scalp regions indicated that age related to cortical response variability more often (i.e. over more channels) in the ignore than in the attend condition (Table 2; see table for channel assignments to prefrontal, frontal, central, parietal, left and right groupings). Whereas variability in central, parietal and frontal recordings only correlated with age in the ignore condition (greater ignore variability with increased age), this relationship to age was observed in both ignore and attend conditions over temporal sites. Overall, variability in the ignore condition was higher for older participants with the exception of prefrontal electrode sites, which demonstrated the inverse relationship (see Strait & Kraus, 2011, for further evidence for distinct patterns of variability over prefrontal electrode sites using this same paradigm). Independent samples t-tests indicated that average cortical variability to ignored speech across all but prefrontal channels increased with development (Figure 3; adults > preschoolers: t = 3.3, p < .005.; adults > school-aged children: t = 2.4, p = .01; school-aged children/preschoolers: p = ns). In contrast, all age groups had equivalent cortical variability in response to attended speech streams. While comparisons across school-aged children and adults considered all available electrode sites, comparisons involving preschoolers only considered electrode sites that were available in all age groups.
Figure 3.
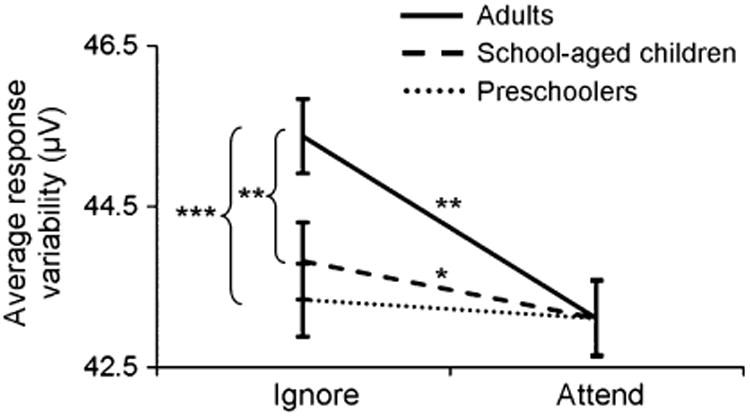
Mean response variability across the scalp excluding prefrontal channels. Adults and school-aged children demonstrate increased cortical response variability to the ignored compared with the attended stimuli (*p < .05; **p < .01; ***p < .005).
Discussion
Our results provide two conceptual advances. First, our previous findings relating selective attention to cortical response variability is extended to children. Like adults (Strait & Kraus, 2011), school-aged children have decreased variability in response to attended compared with ignored speech streams. This relationship unfolds with development, as preschoolers have equivalent response variability to attended and ignored speech streams. Second, we suggest that the maturation of neural mechanisms involved in suppressing ignored input is a key component of attention development. Outcomes are consistent with the protracted maturation of neural mechanisms underlying inhibitory control relative to sustained attention (Booth et al., 2003; Tipper et al., 1989).
Developmental characteristics of cortical response variability with selective auditory attention
Our results demonstrate distinct developmental characteristics for cortical response variability to attended versus ignored speech streams, with responses to ignored speech being more shaped by maturation. This is consistent with the maturation of sustained attention performance by mid-childhood (Booth et al., 2003; Tipper et al., 1989), with a prolonged developmental trajectory for inhibitory control (Becker, Isaac & Hynd, 1987; Bialystok, 2005; Booth et al., 2003; Luna et al., 2001; Tipper et al., 1989; Williams et al., 1999). We originally hypothesized that the impact of attention on cortical response variability increases with development, with more variable responses to ignored compared with attended speech streams. Although this was observed, variability in evoked potentials to ignored speech increased with age while variability to attended speech remained equivalent across all three age groups. We could have reasonably predicted either a general increase or decrease in cortical response variability with development, as both could be supported by known characteristics of brain maturation. Decreased response variability might have been expected in light of the extensive neural pruning that occurs over development, decreasing overall synaptic density and gray matter volume (Huttenlocher, 1979; O'Leary, 1992; Pfefferbaum, Mathalon, Sullivan, Rawles, Zipursky & Lim, 1994). From a network dynamics perspective, however, increased cortical response variability could reflect a more functionally adaptive nervous system (Fingelkurts, 2004; McIntosh, Kovacevic & Itier, 2008). Our results demonstrate that how maturation shapes cortical response variability relates to the specific cognitive demands of the task at hand. Because of this, we argue that neither rationale sufficiently accounts for the impact of development on cortical response variability.
Similar to our findings in the ignore condition, McIntosh et al. observed increased variability in cortical evoked activity from ages 8 to 33 during the performance of a visual memory task (McIntosh et al., 2008). The authors reported greatest cortical response variability for subjects with the best task performance, indicating that response variability provides a metric of behavioral proficiency. Their paradigm did not, however, permit the comparison of cortical evoked potentials recorded during task performance to any other condition. Although follow-up work from the same group has examined the impact of cognitive task performance on variability in the BOLD response using fMRI (Garrett, Kovacevic, McIntosh & Grady, 2012), ours is the first study to examine task-dependent variability in the brain's electrical response to attended and ignored stimuli. Our results reveal that maturation's manifestation in the brain's electrical activity on a trial-by-trial basis reflects dynamic cognitive processes that vary depending on the specific demands of the task at hand, with greater variability observed with development in responses to ignored but not attended stimuli.
Attending and ignoring: not two sides of the same coin
Scientists have long explored how attention shapes perception's neural underpinnings (Coch et al., 2005; Hillyard et al., 1973; Woldorff et al., 1993). Although analyses depend on comparisons of brain activity elicited by attend and ignore (or, in some cases, passive) states, results are often framed according to how attention shapes brain function. This approach allows the challenges imposed by attending to overshadow the cognitive demands of suppressing sensory input; ignored input is automatically processed at the sensory level (Cowan & Wood, 1997), although the extent to which our awareness of it is suppressed is a matter of task difficulty and cognitive capacity (Gisselgard, Petersson & Ingvar, 2004). Here, we show that maturation's impact on cortical response variability recorded in the context of an attention task is evident to ignored but not attended stimuli. This may reflect the gradual increase in one's ability to suppress incoming input with development, such as suppressing noise presented amidst a speech signal of interest (Elliott, 1979; Fallon, Trehub & Schneider, 2000; Hall, Grose, Buss & Dev, 2002).
Given our results, we join others in suggesting that the executive control required to ignore sensory input may play a crucial role in the development of everyday attention performance (Becker et al., 1987; Booth et al., 2003; Luna et al., 2001; Tipper et al., 1989; Williams et al., 1999). The pivotal role that ignoring plays in attention development is supported by literature concerning bilinguals, who have strengthened response inhibition that drives heightened attention-related task performance compared to monolinguals (Bialystok, 2011; Bialystok & Viswanathan, 2009; Carlson & Meltzoff, 2008; Krizman, Marian, Shook, Skoe & Kraus, 2012). In fact, bilinguals' accelerated development of inhibitory control can be observed as early as the preschool years (Bialystok, 1999). That bilinguals have enhanced inhibitory control is not surprising given their perpetual engagement in the act of neural suppression, attending to one language system while ignoring another (Marian & Spivey, 2003; Thierry & Wu, 2007). Although selectively attending and ignoring undoubtedly rely on shared cognitive processes, the control over suppression appears to be most strongly mediated by development and may be most amenable to training (e.g. language learning).
Cortical response variability as a developmental index of cognitive skill
The present results depend on an active recording paradigm in which subjects attend to one speech stream while simultaneously ignoring a competing stream. Although all participants successfully performed the task, cortical response variability and the magnitudes of average evoked potentials only differed between attend and ignore conditions in older children and adults. This is consistent with the maturation of sustained attention abilities by mid-childhood (Booth et al., 2003; Tipper et al., 1989). Although Sanders et al. (2006) reported cortical response magnitude differences in preschoolers to attended and ignored speech using this same paradigm, our data suggest that 3–5-year-olds do not necessarily demonstrate discrepant responses. Preschoolers' equivalent responses between conditions may reflect the gradual emergence of attention and inhibitory control within this age range (Levy, 1980; Plude et al., 1994; Zelazo & Jacques, 1996); as such, it would not be surprising for effects of attention on preschoolers' neural processing to be either inconsistent or slight, depending on the population tested. Response inhibition is considerably difficult for preschoolers (Diamond & Taylor, 1996; Miller, Shelton & Flavell, 1970); the neural variability measure employed here may provide a biological basis for this difficulty. Future work should explore whether within-group differences in the development of inhibitory control and/or within-subject variation in sustaining that control over the course of a task accounts for preschoolers' lack of variability in response to ignored stimuli.
That all subjects could perform the task regardless of age was crucial to our ability to compare neural response characteristics across age groups as it provided assurance that all participants were similarly engaged in the task. Given that this task can be performed by participants spanning a wide age range, we propose that cortical response variability with attention may provide a useful neural metric of the development of attention. This proposition will need to be tested by subsequent studies; while cortical response variability related to attention performance in that response variability was decreased to attended compared with ignored speech, we did not measure attention performance on a continuous scale. Future work will need to assess relationships between cortical response variability and degrees of attention proficiency. Given our results, we predict that children and adults with the strongest attention task performance would have more variable responses to ignored stimuli. In light of our presentation of attended story images to children but not adults, future work might also consider the effects of visual stimulation on task performance and neural response characteristics in addition to assessing these same age groups using equivalent visual parameters.
Spatial characteristics of cortical response variability as a function of attention and development
Our observation of decreased cortical response variability to attended relative to ignored speech streams was consistent across the scalp in school-aged children and young adults except over prefrontal cortex, where only children showed this relationship. In fact, rather than increasing prefrontal response variability, maturation appears to decrease variability over prefrontal cortex for both ignored and attended responses. This may reflect the unique role of prefrontal cortex in directing and sustaining selective attention, for which less variability engenders more consistent task performance over the course of a sustained task. This interpretation is consistent with other work that relates variability in the activation of neural attention networks, including prefrontal cortex, to behavioral disadvantages such as attention lapses (Weissman, Roberts, Visscher & Woldorff, 2006) and symptoms of an attention impairment (Depue, Burgess, Willcutt, Bidwell, Ruzic & Banich, 2010).
That the characteristics of prefrontal response variability were unique compared with the rest of the scalp is not surprising given our previous report in adults, who do not consistently demonstrate decreased prefrontal response variability with attention. Rather, decreased prefrontal response variability with attention only emerges with extensive auditory training such as that engendered by musical practice (Strait & Kraus, 2011). Further studies should define the training-induced malleability of this neural measure over development, with special attention given to prefrontal response characteristics. Furthermore, relationships between cortical response variability and cognitive and psychoeducational performance should be assessed as a function of development and training and/or life experience (e.g. language experience).
Variability in evoked potentials recorded over left and right temporal sites also demonstrated unique characteristics, specifically with regard to how they related to age. Whereas variability in central, parietal and frontal recordings only correlated with age in the ignore condition (greater ignore variability with increased age), this relationship to age was observed in both ignore and attend conditions over temporal sites. Although different methods are required to more accurately identify anatomical contributors to the variability observed over these sites, it is possible that variability in primary auditory cortex increases with development without consideration for the task at hand. This would be in line with McIntosh et al. (2008), who observed increased cortical response variability with development but without comparison between attend and ignore conditions. Still, response variability over these sites related to attentional state in school-aged children and adults, with lower response variability to attended relative to ignored speech streams.
Conclusions and future directions
Here, we reveal that the impact of selective auditory attention on cortical response variability increases with development from age 3 to early adulthood. Although preschoolers, school-aged children and young adults have equivalent response variability to attended speech, only school-aged children and adults have more variable evoked activity in responses to ignored relative to attended speech streams. Preschoolers, on the other hand, demonstrate no impact of attention on cortical responses, which is consistent with the gradual emergence of attention within this age range (Plude et al., 1994).
Although these outcomes reflect the behavioral relevance of cortical response variability, which may provide a biological index of cognitive development, they cannot determine the neural mechanisms responsible for introducing variability into sensory encoding. For example, does the act of ignoring introduce variability into the system or does it move the system toward its default state, during which neural responses are not synchronized to an external stimulus? While the former can be argued, we trend toward the latter interpretation, relating the degree of response variability to variations in resting state dynamics (Arieli et al., 1996; Faisal et al., 2008). Future work aiming to define the cellular mechanisms that underlie relationships between response variability and inhibitory control may yield insights into the neurobiology of attention and its development.
Research highlights.
Attention induces synchronicity in neuronal firing for the encoding of a given stimulus at the exclusion of others. Recently, we reported decreased variability in scalp-recorded cortical evoked potentials to attended compared with ignored speech in adults.
We aimed to determine the developmental time course for this neural index of auditory attention by assessing preschoolers, school-aged children and adults.
Results reveal an increased impact of selective auditory attention on cortical response variability with development.
Cortical response variability may provide a developmental index of cognitive skill.
Acknowledgments
This research was funded by the National Institutes of Health grant F31DC011457-01 to DS, the National Science Foundation grant BCS-0921275 to NK and a grant from the Grammy Foundation. The authors thank Samantha O'Connell and Karen Chan for their assistance with data collection as well as Alexandra Parbery-Clark, Trent Nicol, Jennifer Krizman, Samira Anderson and Karen Chan for their comments on a previous version of this manuscript.
References
- Abrams DA, Nicol T, Zecker S, Kraus N. Right-hemisphere auditory cortex is dominant for coding syllable patterns in speech. Journal of Neuroscience. 2008;28(15):3958–3965. doi: 10.1523/JNEUROSCI.0187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273(5283):1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Becker MG, Isaac W, Hynd GW. Neuropsycho-logical development of nonverbal behaviors attributed to ‘frontal lobe’ functioning. Developmental Neuropsychology. 1987;3:275–298. [Google Scholar]
- Bialystok E. Cognitive complexity and attentional control in the bilingual mind. Child Development. 1999;70(3):636–644. [Google Scholar]
- Bialystok E. Bilingualism across the lifespan: the rise and fall of inhibitory control. International Journal of Bilingualism. 2005;9(1):103–119. [Google Scholar]
- Bialystok E. Reshaping the mind: the benefits of bilingualism. Canadian Journal of Experimental Psychology. 2011;65(4):229–235. doi: 10.1037/a0025406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E, Viswanathan M. Components of executive control with advantages for bilingual children in two cultures. Cognition. 2009;112(3):494–500. doi: 10.1016/j.cognition.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Neural development of selective attention and response inhibition. NeuroImage. 2003;20(2):737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Meltzoff AN. Bilingual experience and executive functioning in young children. Developmental Science. 2008;11(2):282–298. doi: 10.1111/j.1467-7687.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch D, Sanders LD, Neville HJ. An event-related potential study of selective auditory attention in children and adults. Journal of Cognitive Neuroscience. 2005;17(4):605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- Cowan N, Wood NL. Constraints on awareness, attention, processing, and memory: some recent investigations with ignored speech. Consciousness and Cognition. 1997;6(2–3):182–203. doi: 10.1006/ccog.1997.0300. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Nicol T, Zecker S, Kraus N. Speech-evoked neurophysiologic responses in children with learning problems: development and behavioral correlates of perception. Ear and Hearing. 2000;21(6):554–568. doi: 10.1097/00003446-200012000-00003. [DOI] [PubMed] [Google Scholar]
- Depue BE, Burgess GC, Willcutt EG, Bidwell LC, Ruzic L, Banich MT. Symptom-correlated brain regions in young adults with combined-type ADHD: their organization, variability, and relation to behavioral performance. Psychiatry Research. 2010;182(2):96–102. doi: 10.1016/j.pscychresns.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Diamond A, Taylor C. Development of an aspect of executive control: development of the abilities to remember what I said and to ‘do as I say, not as I do’. Developmental Psychobiology. 1996;29(4):315–334. doi: 10.1002/(SICI)1098-2302(199605)29:4<315::AID-DEV2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Elliott LL. Performance of children aged 9 to 17 years on a test of speech intelligibility in noise using sentence material with controlled word predictability. Journal of the Acoustical Society of America. 1979;66(3):651–653. doi: 10.1121/1.383691. [DOI] [PubMed] [Google Scholar]
- Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nature Reviews Neuroscience. 2008;9(4):292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon M, Trehub SE, Schneider BA. Children's perception of speech in multitalker babble. Journal of the Acoustical Society of America. 2000;108(6):3023–3029. doi: 10.1121/1.1323233. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA. Making complexity simpler: multivariability and metastability in the brain. International Journal of Neuroscience. 2004;114(7):843–862. doi: 10.1080/00207450490450046. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291(5508):1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. Journal of Neuroscience. 2008;28(18):4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The modulation of BOLD variability between cognitive states varies by age and processing speed. Cerebral Cortex. 2012;23(3):684–693. doi: 10.1093/cercor/bhs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselgard J, Petersson KM, Ingvar M. The irrelevant speech effect and working memory load. NeuroImage. 2004;22(3):1107–1116. doi: 10.1016/j.neuroimage.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324(5931):1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JW, 3rd, Grose JH, Buss E, Dev MB. Spondee recognition in a two-talker masker and a speech-shaped noise masker in adults and children. Ear and Hearing. 2002;23(2):159–165. doi: 10.1097/00003446-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182(108):177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex: developmental changes and effects of aging. Brain Research. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Klatt D. Software for a cascade/parallel formant synthesizer. Journal of the Acoustical Society of America. 1980;67:13–33. [Google Scholar]
- Krizman J, Marian V, Shook A, Skoe E, Kraus N. Subcortical encoding of sound is enhanced in bilinguals and relates to executive function advantages. Proceedings of the National Academy of Sciences, USA. 2012;109(20):7877–7881. doi: 10.1073/pnas.1201575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F. The development of sustained attention (vigilance) in children: some normative data. Journal of Child Psychology and Psychiatry. 1980;21(1):77–84. doi: 10.1111/j.1469-7610.1980.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Kovacevic N, Itier RJ. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Computational Biology. 2008;4(7):e1000106. doi: 10.1371/journal.pcbi.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian V, Spivey M. Competing activation in bilingual language processing: within-and between-language competition. Bilingualism: Language and Cognition. 2003;6:97–115. [Google Scholar]
- Miller SA, Shelton J, Flavell JH. A test of Luria's hypotheses concerning the development of verbal self-regulation. Child Development. 1970;41(3):651–665. [Google Scholar]
- O'Leary DD. Development of connectional diversity and specificity in the mammalian brain by the pruning of collateral projections. Current Opinion in Neurobiology. 1992;2(1):70–77. doi: 10.1016/0959-4388(92)90165-h. [DOI] [PubMed] [Google Scholar]
- Paetau R, Ahonen A, Salonen O, Sams M. Auditory evoked magnetic fields to tones and pseudowords in healthy children and adults. Journal of Clinical Neurophysiology. 1995;12(2):177–185. doi: 10.1097/00004691-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Pang EW, Taylor MJ. Tracking the development of the N1 from age 3 to adulthood: an examination of speech and non-speech stimuli. Clinical Neurophysiology. 2000;111(3):388–397. doi: 10.1016/s1388-2457(99)00259-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Enns JT, Brodeur D. The development of selective attention: a life-span overview. Acta Psychologica (Amst) 1994;8622(2–3):7–272. doi: 10.1016/0001-6918(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clinical Neurophysiology. 2000;111(2):220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Sanders LD, Stevens C, Coch D, Neville HJ. Selective auditory attention in 3- to 5-year-old children: an event-related potential study. Neuropsychologia. 2006;44(11):2126–2138. doi: 10.1016/j.neuropsychologia.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Finlay BL, Volman SF. Short-term response variability of monkey striate neurons. Brain Research. 1976;105(2):347–349. doi: 10.1016/0006-8993(76)90432-7. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kraus N, McGee TJ, Nicol TG. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephalography and Clinical Neurophysiology. 1997;104(6):540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Smith A, Goffman L. Stability and patterning of speech movement sequences in children and adults. Journal of Speech, Language, and Hearing Research. 1998;41(1):18–30. doi: 10.1044/jslhr.4101.18. [DOI] [PubMed] [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404(6774):187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- Strait DL, Kraus N. Can you hear me now? Musical training shapes functional brain networks for selective auditory attention and hearing speech in noise. Frontiers in Psychology. 2011;2:113. doi: 10.3389/fpsyg.2011.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry G, Wu YJ. Brain potentials reveal unconscious translation during foreign-language comprehension. Proceedings of the National Academy of Sciences, USA. 2007;104(30):12530–12535. doi: 10.1073/pnas.0609927104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper SP, Bourque TA, Anderson SH, Brehaut JC. Mechanisms of attention: a developmental study. Journal of Experimental Child Psychology. 1989;48(3):353–378. doi: 10.1016/0022-0965(89)90047-7. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35(1):205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D, Bloom FE. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proceedings of the National Academy of Sciences, USA. 1993;90:8722–8726. doi: 10.1073/pnas.90.18.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Jacques S. Children's rule use: representation, reflection, and cognitive control. In: Vasta R, editor. Annals of child development: A research annual. London: Jessica Kingsley Publishers; 1996. pp. 119–176. [Google Scholar]


