Abstract
MicroRNAs (miRNAs) are short, endogenous RNAs that have essential roles in regulating gene expression through the disruption of target genes. The miRNA-induced suppression can occur through Argonaute-mediated cleavage of target mRNAs or by translational inhibition. System-wide studies have underscored the integral role that miRNAs play in regulating the expression of essential genes within bone marrow stromal cells. The miRNA expression has been shown to enhance or inhibit cell differentiation and activity, and elucidating miRNA targets within bone marrow cells has revealed novel regulations during normal bone development. Importantly, multiple studies have shown that miRNA misexpression mediates the progression of bone-related pathologies, including osteopetrosis and osteoporosis, as well as the development and progression of osteosarcoma. Furthermore, recent studies have detailed the capacity for miRNAs to influence bone metastasis from a number of primary carcinomas. Taken together, these findings reveal the significant clinical potential for miRNAs to regulate bone homeostasis, as well as to mediate bone-related pathologies.
MiRNA biogenesis and function
The past decade has seen a torrent of novel research into the post-translational regulation of genes via microRNA-mediated suppression. The microRNAs (miRNAs) are a class of ∼22-nucleotide-long RNAs that repress gene expression through complementary binding to sites in the 3′-untranslated region (UTR) of target mRNAs.1 Mature miRNAs are generated by the sequential cleavage of longer precursor transcripts, or pri-miRNAs, that are typically transcribed from intragenic or intergenic regions by RNA polymerase II.2 The initial cleavage step, mediated by Drosha and the DGCR8 complex, occurs in the nucleus and produces a shortened (70–100 nucleotides) pre-miRNA hairpin. Pre-miRNAs are exported from the nucleus by Exportin 5, followed by a second cleavage by the ribonuclease Dicer that produces a double-stranded, ∼18–25-nucleotide-long mature miRNA. One miRNA strand will then combine with Argonaute (AGO2) proteins to produce an RNA-induced silencing complex, allowing for directed pairing with target mRNAs.1
The miRNA targeting of mRNAs is regulated through binding of the 2–8 nucleotides from the 5′ end of the miRNA, a region known as the ‘seed sequence', to complementary regions within the target 3′-UTR.1,3 There are currently two validated mechanisms for miRNA-mediated inhibition of target genes: mRNA degradation or translational silencing. Cleavage of target mRNAs is most commonly observed in situations where the miRNA and target mRNA exhibit complete complementarity. In these rare situations it is possible to observe the cleavage and subsequent degradation of a bound mRNA target.3 Conversely, miRNA–mRNA pairings that feature imperfect complementarity result in translational inhibition in the absence of target cleavage.3,4 Thus, even in the absence of perfect binding, the association between a miRNA and a target 3′-UTR still results in the suppression of a target protein. Because of the relatively short length of the seed sequence, each miRNA is capable of binding to hundreds of mRNAs. Although this presents a significant hurdle, recent advances in computational and biochemical methods have aided target prediction, opening the way for studies into miRNA-mediated regulation of target genes.3
MiRNA regulation within bone marrow cells
As important regulators of gene expression, miRNAs themselves are subject to complex control. Initial modulation can occur either through regulation of pri-miRNA transcription or through controlling of processing into a mature strand via alterations in the activity of Drosha or Dicer.2 Within bone homeostasis, alterations in miRNA processing have been shown to have a profound effect on the function of bone marrow cells. For example, in vivo deletion of Dicer in osteoprogenitors, osteoblasts and chondrocytes, using a Col1a1 promoter-driven Cre recombinase, resulted in severe skeletal deformities in fetal mice.5 These mice exhibited a misproportioned cartilage skeleton at E14.5 and displayed a reduction in mineralized tissue formation due to defects in osteoblast maturation. When dicer was ablated by Osteocalcin-Cre, eliminating miRNA processing in mature osteoblasts, the mice were viable but exhibited delayed bone development that corresponded with reduced osteoblast numbers.5 Similarly, Dicer knockout in chondrocytes, using Col2a1-Cre mice, modulated proliferation and differentiation.6 These transgenic mice presented with significant skeletal defects that were associated with the differentiation of cells into postmitotic hypertrophic chondrocytes featuring decreased proliferation. Subsequent analysis has revealed the expression of several hundred miRNAs within chondrocytes under physiological conditions.
Osteoclast activity has been similarly tied to proper miRNA expression. Osteoclast-specific Dicer knockout, utilizing CD11b-Cre transgenic mice, exhibits defective osteoclastogenesis, with a reduction in multinuclear osteoclasts and increased bone mass.7 Cultured bone marrow from these mice was incapable of producing osteoclasts ex vivo, further confirming the defect in osteoclast maturation.8,9 Importantly, this defect was not confined to Dicer; knockdown of DGCR8 and Ago2 using siRNA similarly resulted in decreased osteoclast differentiation and bone resorption, and osteoclast-specific DGCR8 knockout mice display impaired bone development.7,10 Taken together, these results reveal the necessity for proper miRNA regulation during the differentiation and maintenance of multiple essential cell types required for bone homeostasis.
A central role for MiRNAs in bone homeostasis
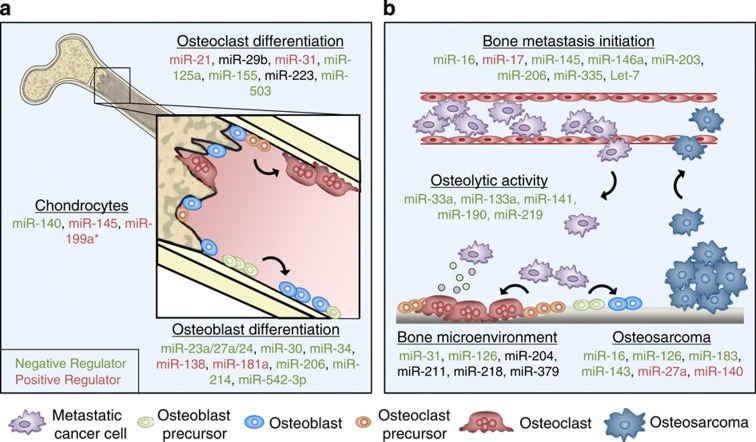
The maintenance of homeostasis within the bone microenvironment depends on the careful orchestration of multiple cell types, each of which maintains precise expression of a multitude of genes that regulate cellular activity.11,12 Importantly, the molecular pathways that regulate differentiation of bone cells are coordinated through cross-talks among neighboring stromal cells.13 Thus, it is not surprising that general disruptions within the miRNA biogenesis machinery described above would have dramatic impacts on physiological bone remodeling. Interestingly, a number of recent studies have revealed the capacity for individual miRNAs to direct the differentiation and activity of cells residing in the bone microenvironment (Figure 1a).
Figure 1.
The miRNA involvement in bone homeostasis and bone metastasis. (a) The miRNA-mediated regulation of osteoclast, osteoblast and chondrocyte differentiation and activity is essential for proper bone development and maintenance. Known positive (red) and negative (green) regulators are indicated. (b) Bone metastasis migration, invasion and homing to the bone microenvironment are controlled by multiple miRNAs. In addition, miRNAs regulate bone metastasis activity, including the tumor–stromal cross-talk that mediates osteolysis. Finally, the development of osteosarcoma depends on proper regulation of miRNAs that can enhance or inhibit tumor progression.
Chondrocytes, essential regulators of longitudinal bone growth, have been shown to be intricately regulated by a series of miRNAs. MiR-140, a positive regulator of chondrogenesis through the inhibition of HDAC4 and Dnpep, contributes to craniofacial development and endochondral bone formation.14,15,16 Consistent with this, mice lacking miR-140 display accelerated bone formation at embryonic and neonatal stages of development.15 Conversely, miR-199a* and miR-145 have been shown to negatively regulate chondrocyte differentiation; miR-199a* downregulates the expression of Smad1,17 whereas miR-145 targets Sox9, a key transcription factor involved in chondrogenesis.18 Thus, miRNAs can serve both negative and positive regulatory roles during chondrocyte differentiation.
Similar miRNA-based regulation has been observed during osteoblast differentiation and activity. MiR-138 inhibits osteogenesis by directly targeting focal adhesion kinase, a critical factor in osteoblast differentiation, leading to attenuated bone formation in vivo.19 Importantly, when human mesenchymal stem cells were treated with anti-miR-138 oligonucleotides, there was a significant increase in osteogenic capacity and bone formation. The miR-23a/-27a/24-2 cluster is similarly tied to osteogenesis through a Runx2 regulatory loop. Expression of the miRNA cluster can be transcriptionally repressed by a Runx2 binding site in the cluster's promoter, whereas miR-23a is capable of directly repressing Runx2 expression.20 Thus, Runx2-mediated inhibition of the miR-23a/-27a/24-2 cluster initiates a positive feedback loop. In addition to increased expression of Runx2, the inhibition of miR-23a/-27a/24-2 also derepresses SATB2, an additional activator of osteogenesis, allowing for progression of the cell through osteoblast differentiation.20 The miR-206 expression was shown to inhibit osteoblast development, whereas miR-206 knockdown promoted differentiation.21 Many additional miRNAs have been shown to play a role in osteogenesis (see Figure 1a and Table 1).
Table 1. The miRNA involvement in bone homeostasis.
| MiRNA biogenesis | Regulatory function | Activity | Reference |
|---|---|---|---|
| Dicer | Essential for development | Knockout inhibits osteoblast, osteoclast, chondrocyte development | 5,6,7,8,9 |
| DGCR8 | Essential for development | Knockout inhibits osteoclast development | 7,10 |
| Ago2 | Essential for development | Knockout inhibits osteoclast development | 7 |
| Osteoclast | |||
| miR-223 | Activates and/or inhibits | Positive and negative regulator of differentiation | 7,22 |
| miR-29b | Activates and/or inhibits | Activates and/or inhibits differentiation, targets Cdc42, Srgap2 | 23,24 |
| miR-31 | Essential for development | Essential for differentiation | 25 |
| miR-21 | Promotes | Promotes differentiation, targets PDCD4 | 9 |
| miR-148a | Promotes | Promotes differentiation, targets MAFB | 88 |
| miR-125a | Inhibits | Inhibits differentiation, targets TRAF6 | 27 |
| miR-155 | Inhibits | Inhibits differentiation, targets SOCS1, MITF | 28,29 |
| miR-503 | Inhibits | Inhibits differentiation, targets RANK | 26 |
| miR-146a | Inhibits | Inhibits differentiation, protects joint destruction during Collagen-induced arthritis | 89 |
| Osteoblast | |||
| miR-138 | Inhibits | Inhibits osteoblast differentiation, targets FAK | 19 |
| miR-23a/27a/24–2 | Inhibits | Inhibits apoptosis, regulates FAK, Runx2, SATB2 | 20,82 |
| miR-20a | Promotes | Promotes differentiation, targets PPARγ, Bambi, Crim1 | 90 |
| miR-181a | Promotes | Promotes osteoblast differentiation, targets Tgfbi | 86 |
| miR-335–5p | Promotes | Promotes differentiation, targets DKK1 | 91 |
| miR-15b | Promotes | Promotes differentiation, targets Smurf1 | 92 |
| miR-17∼92 | Promotes | Promotes proliferation, activity in mouse model | 93 |
| miR-322 | Promotes | Promotes differentiation, targets Tob2 | 94 |
| miR-29a | Promotes | Promotes differentiation, targets Osteonectin, Dkk1, Kremen2, sFRP2 | 36,95,96 |
| miR-34 | Inhibits | Inhibits differentiation, targets SATB2, Runx2, Notch pathway | 30,31,32,84 |
| miR-143 | Inhibits | Inhibits differentiation, targets Osterix | 97 |
| miR-155 | Inhibits | Regulates TNF-α inhibition of osteoblast differentiation, targets SOCS1 | 98 |
| miR-93 | Inhibits | Osteoblast mineralization, targets Sp7 | 99 |
| miR-182 | Inhibits | Inhibits differentiation, targets FoxO1 | 100 |
| miR-764–5p | Inhibits | Inhibits differentiation, targets CHIP/STUB1 | 101 |
| miR-208 | Inhibits | Inhibits differentiation, targets Ets1 | 102 |
| miR-206 | Inhibits | Inhibits differentiation, targets Cx43 | 21 |
| miR-30 | Inhibits | Inhibits differentiation, targets Smad1, Runx2 | 83 |
| miR-214 | Inhibits | Inhibits activity and bone formation, targets ATF4, Osterix | 85,103 |
| miR-542–3p | Inhibits | Inhibits differentiation, targets BMP7 | 87 |
| miR-141, miR-200a | Inhibits | Inhibits differentiation, target Dlx5 | 104 |
| miR-100 | Inhibits | Inhibits differentiation, targets BMPR2 | 105 |
| miR-17–5p, miR-106 | Inhibits | Inhibits differentiation, targets BMP2 | 106 |
| Chondrocytes | |||
| miR-140 | Essential for activity | Essential for proper activity, targets HDAC4, Dnpep | 14,15,16 |
| miR-34a | Inhibits | Inhibits differentiation, regulates apoptosis | 107,108 |
| miR-199a* | Inhibits | Inhibits differentiation, targets Smad1 | 17 |
| miR-145 | Inhibits | Inhibits differentiation, targets Sox9 | 18 |
| miR-17∼92 | Essential for development | Loss induces microcephaly and other skeletal defects in patients | 33 |
Osteoclast differentiation likewise involves the temporally organized coordination of multiple transcription factors and regulatory proteins. Unsurprisingly, the expression of many of these proteins is modulated by miRNA-mediated silencing. MiR-223 has been well studied in osteoclastogenesis, with conflicting studies indicating that miR-223 expression is capable of simultaneously enhancing and suppressing osteoclast differentiation.7,22 Similarly, the miR-29 family has been shown to play a dichotomous role during osteoclastogenesis. In one study, ectopic miR-29b expression inhibited osteoclast activity,23 whereas a more recent report found that miR-29b was able to promote osteoclastogenesis by directly targeting Cdc42 and Srgap2, and knockdown of miR-29 in pre-osteoclasts inhibited differentiation.24 Additional studies have uncovered miRNAs with more easily defined roles during osteoclast differentiation. Mizoguchi et al.25 found that miR-31, which is highly upregulated during osteoclast development, is essential for proper differentiation. Similarly, miR-21 has been shown to positively regulate osteoclastogenesis by targeting the c-Fos inhibitor PDCD4.9 Conversely, RANK targeting by miR-503 inhibited osteoclastogenesis, whereas silencing miR-503 enhanced in vivo bone resorption.26 MiR-125a was also shown to inhibit osteoclast differentiation by suppressing TRAF6 expression,27 whereas miR-155 was shown to regulate cell-fate commitment within macrophages through the downregulation of SOCS1 and MITF, effectively inhibiting differentiation through an osteoclast lineage.28,29
It is important to recognize that the regulation of bone homeostasis depends on carefully orchestrated cross-talk between bone marrow cells. MiR-34c is an essential regulator of Notch signaling in osteoblasts, directly targeting Notch1, Notch2 and Jagged1, as well as Satb2 and Runx2.30 Interestingly, miR-34c has been shown to function with both cell-autonomous and non-cell-autonomous activities. Although upregulation of miR-34c inhibits osteoblast differentiation by decreasing Satb2 and Runx2, the inhibition of Notch signaling might have a role in regulating the RANKL/OPG ratio, leading to increased osteoclast differentiation.30,31 Thus, an individual miRNA is capable of simultaneously regulating multiple bone marrow cells to maintain homeostasis.
MiRNA regulation of other bone-related pathologies
Given the profound effect that miRNA misregulation can have on bone marrow cell differentiation, it is perhaps not surprising that miRNAs have also been shown to regulate a number of bone-related pathologies. In addition to the broad skeletal defects seen from the unbiased ablation of miRNA biogenesis, specific miRNAs have been found to have a role in pathologies related to impaired osteoblast and osteoclast activity. For example, the miR-34 family of miRNAs serves as key regulators of osteoblast maturation. Wei et al.32 found that miR-34 could inhibit osteoblast terminal differentiation through the inhibition of SATB2, and could additionally inhibit proliferation of mature osteoblasts by inhibiting Cyclin D1, CDK4 and CDK6. These effects were confirmed in the pronounced skeletal defects seen in transgenic mice featuring ectopic miR-34c under the control of Col1a1. Similarly, the miR-17∼92 cluster, comprising six well-studied miRNAs, has been tied to skeletal defects in humans. De Pontual et al.33 uncovered hemizygous deletions of the miRNA cluster in patients with microcephaly, featuring shortened stature and digital abnormalities, and miR-17∼92 heterozygous mice phenocopy the human developmental features.
In addition, a number of miRNAs have been shown to function during osteoporosis. High-throughput screening revealed broad changes in miRNA expression during age-related osteoporosis; examination of the overlap between mesenchymal stem cells and bone tissue in a mouse model of osteoporosis revealed the differential expression of 8 upregulated and 30 downregulated miRNAs.34 MiR-2861 is normally transcribed during BMP2-induced osteogenesis, and inhibition results in the derepression of histone deacetylase 5, itself an enhancer of Runx2 degradation, leading to decreased bone formation.35 Importantly, loss of miR-2861 activity was found to be responsible for osteoporosis in two adolescent patients. Interestingly, miR-29a, which had been previously shown to suppress Osteonectin during osteoblastogenesis,36 was found to protect against glucocorticoid-induced bone loss, a frequent pathological repercussion that leads to decreased osteoblast survival and increased osteoclast activity.37 Forced expression of miR-29a attenuated the effects of glucocorticoid treatment, increasing trabecular bone mass and decreasing cortical bone porosity.
MiRNA expression influences growth and progression of osteosarcoma
Osteosarcoma, the most common primary sarcoma of bone, remains the leading cause of cancer-related death in adolescents.38,39 Although questions remain as to the molecular basis of osteosarcoma, patients frequently present with high-grade tumors seemingly of osteoblastic or fibroblastic origin. The miRNA signatures within osteosarcoma have been shown to associate with pathological features, whereas specific miRNAs can regulate tumor progression and metastasis40 (Figure 1b and Table 2). For example, miR-16 was found to function as a tumor suppressor in human osteosarcoma cell lines, decreasing tumor volume and enhancing apoptosis. Similarly, miR-126 is frequently downregulated in osteosarcoma, and ectopic expression inhibited invasion and induced apoptosis in osteosarcoma cells.41 MiR-183 and miR-143, which target Ezrin and MMP-13, respectively, can similarly inhibit osteosarcoma migration and invasion.42,43 Importantly, ectopic expression of miR-143 was able to suppress lung metastasis in a mouse model of human osteosarcoma.43 Conversely, miR-27a enhanced migration and invasion in vitro while simultaneously increasing pulmonary and bone metastasis after intravenous inoculation.40 MiR-21 was similarly shown to function as a driver of osteosarcoma migration and invasion, and suppression of miR-21 reduced motility in osteosarcoma cell lines by direct targeting of RECK.44
Table 2. The miRNA involvement in bone metastasis and osteosarcoma.
| Tumor seeding | Activity | Validated targets | Model | Reference |
|---|---|---|---|---|
| Let-7g | Inhibits migration, bone metastasis | HMGA2 | Human breast cancer cell lines, intracardiac injected bone metastasis in mice | 58 |
| miR-17 | Enhances migration and metastasis | Human breast cancer cell lines, spontaneous bone metastasis in mice | 59 | |
| miR-335 | Inhibits invasion, bone metastasis | Sox4, Tenascin C | Human breast cancer cell lines, intracardiac injected bone metastasis in mice | 60,61 |
| miR-206 | Inhibits invasion, dissemination | Human breast cancer cell lines, intracardiac injected bone metastasis in mice | 60 | |
| miR-203 | Inhibits bone metastasis | Zeb2, Bmi, Survivn, Runx2 | Human prostate cancer cell lines, intracardiac injected bone metastasis in mice | 67 |
| miR-16 | Inhibits bone metastasis | Human prostate cancer cell lines, systemic miR-16 inhibits bone metastasis | 68 | |
| miR-146a | Inhibits proliferation and adhesion to endothelial cells, | ROCK | Human prostate cancer PC3 cells in vitro | 69 |
| miR-145 | Inhibits migration, seeding | HEF1 | Human prostate cancer PC3 cells in vitro | 70,71 |
| Bone marrow microenvironment | ||||
| miR-218 | Osteomimicry in breast cancer cells | SOST, DKK2, SFRP2 | Human breast cancer cells in vitro | 62 |
| miR-126 | Knockdown enhances bone metastasis, inhibits endothelial cell recruitment in lung | IGFBP2, PITPNC1, MERTK | Human breast cancer cell lines, intracardiac injected bone metastasis in mice | 65 |
| miR-31 | Regression of established metastasis | ITGA5, RDX, RhoA | Human breast cancer cell lines, intracardiac injected bone metastasis in mice | 66 |
| miR-33a, miR-133a, miR-141, miR-190, miR-219 | Inhibits osteoclast differentiation in vitro and in vivo | Mmp14, Mitf, Calcr, Traf6 | Human breast cancer cell lines, intracardiac injected bone metastasis in mice | 72 |
| miR-33a | Inhibits lung cancer induced osteoclastogenesis | PTHrP | Human lung cancer cell lines in vitro | 73 |
| Let-7 | Inhibits IL-6 in tumor-associated MSCs | IL-6 | Human prostate cancer PC3 cells in vitro | 74 |
| Osteosarcoma | ||||
| miR-16 | Tumor suppressor | Human osteosarcoma cells in vivo injected in mice | 40 | |
| miR-126 | Inhibits invasion, induces apoptosis | Sox2 | Human osteosarcoma cells in vitro | 41 |
| miR-183 | Inhibits migration/invasion, | Ezrin | Human osteosarcoma cells in vitro | 42 |
| miR-143 | Inhibits migration/invasion, suppresses lung metastasis | MMP13 | Human osteosarcoma cells inoculated in knee, intravenous injection of miR-143 | 43 |
| miR-27a | Enhances migration, bone metastasis | Human osteosarcoma cells in vivo injected in mice | 40 | |
| miR-21 | Enhances migration/invasion | RECK | Human osteosarcoma cells in vitro | 44 |
| miR-140 | Chemoresistance to methotrexate and 5-fluorouracil | HDAC4 | Human osteosarcoma cells in vitro | 46 |
| miR-215 | Chemoresistance to methotrexate and Tomudex | DHFR, TS | Human osteosarcoma cells in vitro | 45 |
Abbreviations: IL-6, interleukin-6; MSC, mesenchymal stem cell.
In addition to a direct role in osteosarcoma pathology, miRNAs might also serve as biomarkers for disease progression and response to chemotherapy. A number of miRNAs, including miR-140 and miR-215, have been shown to regulate osteosarcoma chemoresistance.45,46 Ectopic miR-215 protected osteosarcoma and colon cancer cell lines from methotrexate (MTX) and Tomudex, whereas miR-140 overexpression desensitized osteosarcoma cells to MTX and 5-fluorouracil. More recently, miRNA expression analysis of chemoresistant patient samples and cell lines revealed a miRNA signature consisting of miR-92a, -99b, -132, -193a-5p and -422a that was capable of predicting response to ifosfamide, whereas miR-33a reduced cisplatin-induced apoptosis.47,48
Regulation of multiple myeloma via miRNAs
The progression of multiple myeloma (MM) has also been shown to require the careful coordination of miRNA regulatory pathways (Table 2). MiR-29b has been shown to induce apoptosis in MM cells through inhibition of MCL1,49 and ectopic expression of miR-29b can inhibit DNA methyltransferase activity in human MM cells by directly targeting DNMT3A and DNMT3B.50 Ectopic expression of miR-29b attenuated global DNA methylation, inhibited cell-cycle progression and reduced tumor growth in an in vivo model of MM. A follow-up study uncovered miR-29b-mediated upregulation of SOCS1 due to reduced promoter methylation, leading to increased adhesion of MM cells to bone marrow stromal cells and decreasing migration in vitro.51 Similarly, miR-34a was found to decrease in vivo tumor growth of human MM cells, potentially through the regulation of BCL2, CDK6 and NOTCH1.52 MiR-15a and miR-16, which are frequently suppressed in MM, inhibit proliferation and growth of MM both in vitro and in vivo.53 In addition, both miRNAs were shown to directly regulate VEGF-A expression during MM progression, inhibiting angiogenesis and decreasing tumor growth in vivo.54 Conversely, miR-221 and miR-222 were frequently upregulated in MM patients, and expression of miRNA inhibitors to miR-221/222 decreased the proliferation and tumorigenesis of MM cells in vitro and in vivo.55
Tumor-Intrinsic MiRNA regulation of bone metastasis
Bone metastasis, frequently observed in the advanced stages of breast and prostate cancers, represents the dysregulation of bone developmental pathways and a divergence from normal bone homeostasis. The presentation of metastatic lesions within the bone is responsible for pathological fractures, as well as for severe pain and hypercalcemia, representing a significant clinical concern.56 Importantly, miRNAs can function in multiple aspects of bone metastasis regulation, including cell-autonomous mechanisms within tumor cells themselves, as well as through tumor–stromal interactions within the bone microenvironment (Figure 1b and Table 2).
The miRNA-mediated control of breast cancer bone metastasis
Tumor-intrinsic miRNA regulation has been shown to encompass nearly every aspect of breast cancer tumor progression. During metastasis, miRNAs can enhance or inhibit invasion, colonization to bone and secondary lesion outgrowth.57 Let-7 has been shown to inhibit breast cancer invasion and bone metastasis in a mouse model, likely through the direct inhibition of HMGA2.58 Similarly, miR-17 enhances breast cancer cell line invasion and migration as well as metastasis.59 When miR-17 expression was inhibited, Liu et al.59 observed a decrease in proliferation and in vitro migration. A number of miRNAs have also been shown to negatively influence breast cancer migration and invasion. Ectopic expression of miR-335, which inhibits invasion through direct targeting of Sox4 and Tenascin C, was shown to reduce breast cancer bone metastasis in an orthotopic xenograft model,60 as well as in a model for small-cell lung cancer bone metastasis.61 In addition, miR-206 expression significantly inhibited in vitro migration and invasion as well as tumor dissemination to the bone.60
In addition to regulating cellular motility, miRNAs have also been shown to influence tumor seeding and activity within the bone. MiR-218 stimulates the Wnt pathway in breast cancer cells by directly targeting the Wnt inhibitors SOST, DKK2 and SFRP2 that have the potential to enhance the expression of osteoblastic genes in metastatic cells.62 This osteomimetic gene expression has the potential to influence metastatic cell homing to the bone. An additional study found that miR-204, miR-211 and miR-379 regulate interleukin (IL)-11 expression in breast cancer cells.63 IL-11 has been previously shown to enhance osteolytic bone metastasis in breast cancer cells, and ectopic expression of these miRNAs in tumor cells might represent a novel therapeutic strategy.64 Silencing of miR-126 in the human breast cancer MDA-MB-231 cell line was found to increase metastasis to multiple organs, including to bone.65 MiR-126 expression inhibits endothelial cell recruitment to breast cancer cells, suppressing angiogenesis and distant colonization, through direct targeting of IGFBP2, PITPNC1 and MERTK. Thus, inhibition of metastasis by miR-126 functions through a non-cell-autonomous mechanism resulting from decreased tumor–stromal interactions. Importantly, the signaling defects induced by miR-126 could be rescued by direct co-injection of tumor and endothelial cells.65
These studies underscore the therapeutic potential of miRNAs in inhibiting the molecular events preceding breast cancer bone metastasis. Interestingly, ectopic activation of miR-31 stimulates regression of established bone metastasis lesions, decreasing the number of existing lesions while preventing the outgrowth of residual metastatic nodules.66 Although the exact mechanism behind this inhibition remains unclear, the authors observed a significant decrease in Akt activity after treatment with miR-31 that was tied to indirect inhibition through upstream targeting of ITGA5, RDX and RhoA.
Prostate cancer bone metastasis is mediated by miRNA regulation
Similar to breast cancer, late-stage prostate cancer patients frequently exhibit pathological complications related to bone metastasis. Consistent with findings from breast cancer, prostate cancer cells exhibit precise control of miRNA regulation. For example, miR-203 was found to function in an antimetastatic role in prostate cancer cell lines. Saini et al.67 found direct targeting of a number of important genes for metastasis progression, including Zeb2, Bmi, Survivn and Runx2. Importantly, reintroduction of miR-203, which was repressed in metastatic cell lines relative to normal prostate cells, attenuated bone metastasis in a mouse model. In addition, miR-16 was shown to inhibit bone metastasis in a mouse prostate cancer xenograft model.68 MiR-146a was shown to target ROCK1 in androgen-independent PC3 cells; overexpression of miR-146a led to reduced proliferation and invasion, and impaired adhesion to a monolayer of human bone marrow endothelial cells.69
Finally, multiple studies have shown inhibitory effects from miR-145 on prostate cancer bone metastasis. Guo et al.70 found that miR-145 can directly inhibit HEF1, leading to decreased migration and invasion, and decreased bone seeding of human prostate cancer cell lines. A separate study found that both miR-143 and miR-145 decreased in bone metastatic samples compared with primary tumors, and ectopic expression reduced migration, invasion and bone metastasis.71
MiRNA regulation of tumor–stromal interactions during bone metastasis
In addition to tumor-intrinsic effects, miRNAs have recently been shown to influence the essential tumor–stromal interactions that mediate bone metastasis. During osteolytic metastasis, tumor cells co-opt the regulatory signaling within bone marrow cells to recruit and activate osteoclasts that in turn orchestrate bone degradation. Recently, a number of miRNAs have been shown to directly influence osteoclast activity during bone metastasis. We found that a number of miRNAs are repressed during osteoclast differentiation, including miR-33a, miR-133a, miR-141, miR-190 and miR-219.72 Interestingly, in vitro osteoclastogenesis assays revealed a necessity for this miRNA downregulation, with ectopic expression inhibiting osteoclast differentiation. Systemic inoculation of pre-miRNA oligonucleotides for miR-141 or miR-219 was sufficient to inhibit bone metastasis from human breast cancer cells. At the same time, miR-16 and miR-378, which were upregulated in patient serum samples with bone metastasis, possessed comparable sensitivity relative to the bone turnover marker N-terminal telopeptide (NTX).72 A similar study found that miR-33a could target PTHrP, a known activator of osteolytic bone resorption, decreasing the ability of lung cancer cells to induce osteoclast differentiation and activity.73 Thus, it is possible for miRNAs to inhibit bone metastasis through perturbation of tumor–stromal interactions.
Stromal cell-derived miRNAs are also capable of directly influencing metastatic cells. Let-7c inhibits IL-6 within bone marrow mesenchymal stem cells (MSCs), and ectopic expression of Let-7c was capable of repressing the migration- and invasion-promoting potential of tumor-associated MSCs.74 A separate study found that multiple miRNAs were transferred from bone marrow stromal cells to nearby breast cancer cells upon seeding to the bone.75 MiR-127, miR-197, miR-222 and miR-223, all direct inhibitors of CXCL12, were shown to directly transfer from stromal cells into cancer cells via gap junctions or through secreted exosomes. The transfer of these miRNAs decreased cancer cell proliferation and could represent a mechanism for tumor quiescence within the bone microenvironment.
Exosome-Derived MiRNAs during bone metastasis
In addition to the biological significance of intracellular miRNAs, recent studies have revealed the functional importance of secreted miRNAs. MiRNAs can be released from cancer cells within microvesicles or exosomes that are subsequently taken up by distant cells.76 Although the examination of exosome-delivered miRNAs has been shown in multiple cells, including microvesicles derived from dendritic77 and T cells,78 the process has not been comprehensively studied within bone metastasis. Ectopic expression of miR-192 in bone metastatic lung cancer cells resulted in secretion of the miRNA within microvesicles that could be transferred to endothelial cells in vitro.79 Importantly, the transfer of miR-192 to the endothelial cells reduced angiogenic activity in vitro, and systemic injection decreased osteolytic lesions in a mouse model of bone metastasis.79
Intravenously injected exosomes are detectable in the bone marrow of mice;80 thus, the potential exists for exosome-delivered miRNAs to serve as a therapeutic strategy for bone-related pathologies. Further research is required to monitor the efficiency of targeting to specific cells, both within the bone marrow microenvironment and tumor of origin, and to minimize off-target effects. In addition to therapeutic delivery, the detection of circulating miRNAs within exosomes holds the potential to serve as biomarkers for disease, with specific circulating miRNA signatures potentially serving as an indication of disease progression.81
Conclusions
The miRNA activity represents an essential level of gene regulation during skeletal development and homeostasis. Unbiased ablation of miRNA activity, through the inhibition of miRNA processing pathway components such as Dicer and Drosha, has been shown to inhibit the development of multiple bone marrow stromal cells, resulting in devastating skeletal defects. Misregulation of individual miRNAs is capable of inhibiting the differentiation and activity of multiple cell types and precipitating pathological events. Importantly, miRNA regulation has also been shown to play pivotal roles in regulating the development and progression of osteosarcoma, multiple myeloma and bone metastasis. Thus, proper miRNA expression is essential for bone formation and maintenance.
From a clinical standpoint, miRNAs involved in bone disease, osteosarcoma and bone metastasis might serve as an attractive therapeutic target. However, the development of miRNA-based therapeutics will require a more complete understanding of miRNA–mRNA targeting in diverse tissue types in order to minimize the potential side effects due to the multi-target nature of miRNAs in gene regulation. Nevertheless, preliminary studies have shown promising results in inhibiting osteosarcoma and bone metastasis, suggesting the potential utility of this therapeutic strategy in clinical practice. Taken together, the therapeutic potential of miRNA treatments has the ability to transform clinical paradigms for bone-related diseases.
Footnotes
The authors declare no conflict of interest.
References
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J Loedige I Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010;11:597–610. [DOI] [PubMed] [Google Scholar]
- Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet 2012;13:271–282. [DOI] [PubMed] [Google Scholar]
- Djuranovic S Nahvi A Green R. A parsimonious model for gene regulation by miRNAs. Science 2011;331:550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur T Hussain S Mudhasani R Parulkar I Colby JL Frederick D et al. Dicer inactivation in osteoprogenitor cells compromises fetal survival and bone formation, while excision in differentiated osteoblasts increases bone mass in the adult mouse. Dev Biol 2010;340:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T Lu J Cobb BS Rodda SJ McMahon AP Schipani E et al. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc Natl Acad Sci USA 2008;105:1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugatani T Hruska KA. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J Biol Chem 2009;284:4667–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi F Izu Y Hayata T Hemmi H Nakashima K Nakamura T et al. Osteoclast-specific Dicer gene deficiency suppresses osteoclastic bone resorption. J Cell Biochem 2010;109:866–875. [DOI] [PubMed] [Google Scholar]
- Sugatani T Vacher J Hruska KA. A microRNA expression signature of osteoclastogenesis. Blood 2011;117:3648–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugatani T Hildreth BE 3rd Toribio RE Malluche HH Hruska KA. Expression of DGCR8-dependent microRNAs is indispensable for osteoclastic development and bone-resorbing activity. J Cell Biochem 2014;115:1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ Simonet WS Lacey DL. Osteoclast differentiation and activation. Nature 2003;423:337–342. [DOI] [PubMed] [Google Scholar]
- Harada S Rodan GA. Control of osteoblast function and regulation of bone mass. Nature 2003;423:349–355. [DOI] [PubMed] [Google Scholar]
- Henriksen K Neutzsky-Wulff AV Bonewald LF Karsdal MA. Local communication on and within bone controls bone remodeling. Bone 2009;44:1026–1033. [DOI] [PubMed] [Google Scholar]
- Eberhart JK He X Swartz ME Yan YL Song H Boling TC et al. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat Genet 2008;40:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y Inloes JB Katagiri T Kobayashi T. Chondrocyte-specific microRNA-140 regulates endochondral bone development and targets Dnpep to modulate bone morphogenetic protein signaling. Mol Cell Biol 2011;31:3019–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuddenham L Wheeler G Ntounia-Fousara S Waters J Hajihosseini MK Clark I et al. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett 2006;580:4214–4217. [DOI] [PubMed] [Google Scholar]
- Lin EA Kong L Bai XH Luan Y Liu Cj. miR-199a*, a bone morphogenic protein 2-responsive microRNA, regulates chondrogenesis via direct targeting to Smad1. J Biol Chem 2009;284:11326–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B Guo H Zhang Y Chen L Ying D Dong S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS ONE 2011;6:e21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen T Taipaleenmaki H Stenvang J Abdallah BM Ditzel N Nossent AY et al. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci USA 2011;108:6139–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MQ Gordon JAR Beloti MM Croce CM van Wijnen AJ Stein JL et al. A network connecting Runx2, SATB2, and the miR-23a∼27a∼24-2 cluster regulates the osteoblast differentiaion program. Proc Natl Acad Sci USA 2010;107:19879–19884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inose H Ochi H Kimura A Fujita K Xu R Sato S et al. A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci USA 2009;106:20794–20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugatani T Hruska KA. MicroRNA-223 is a key factor in osteoclast differentiation. J Cell Biochem 2007;101:996–999. [DOI] [PubMed] [Google Scholar]
- Rossi M Pitari MR Amodio N Di Martino MT Conforti F Leone E et al. miR-29b negatively regulates human osteoclastic cell differentiation and function: implications for the treatment of multiple myeloma-related bone disease. J Cell Physiol 2013;228:1506–1515. [DOI] [PubMed] [Google Scholar]
- Franceschetti T Kessler CB Lee SK Delany AM. miR-29 promotes murine osteoclastogenesis by regulating osteoclast commitment and migration. J Biol Chem 2013;288:33347–33360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi F Murakami Y Saito T Miyasaka N Kohsaka H. miR-31 controls osteoclast formation and bone resorption by targeting RhoA. Arthritis Res Ther 2013;15:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C Cheng P Xie H Zhou HD Wu XP Liao EY et al. MiR-503 regulates osteoclastogenesis via targeting RANK. J Bone Miner Res 2014;29:338–347. [DOI] [PubMed] [Google Scholar]
- Guo LJ Liao L Yang L Li Y Jiang TJ. MiR-125a TNF receptor-associated factor 6 to inhibit osteoclastogenesis. Exp Cell Res 2014;321:142–152. [DOI] [PubMed] [Google Scholar]
- Zhang J Zhao H Chen J Xia B Jin Y Wei W et al. Interferon-beta-induced miR-155 inhibits osteoclast differentiation by targeting SOCS1 and MITF. FEBS Lett 2012;586:3255–3262. [DOI] [PubMed] [Google Scholar]
- Mann M Barad O Agami R Geiger B Hornstein E. miRNA-based mechanism for the commitment of multipotent progenitors to a single cellular fate. Proc Natl Acad Sci USA 2010;107:15804–15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y Yang T Zeng HC Campeau PM Chen Y Bertin T et al. miRNA-34c regulates Notch signaling during bone development. Hum Mol Genet 2012;21:2991–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F Yao Z Yang T Zhou G Bertin T Jiang MM et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med 2008;14:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J Shi Y Zheng L Zhou B Inose H Wang J et al. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J Cell Biol 2012;197:509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pontual L Yao E Callier P Faivre L Drouin V Cariou S et al. Germline deletion of the miR-17 approximately 92 cluster causes skeletal and growth defects in humans. Nat Genet 2011;43:1026–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X Zhang W Liao L Fu X Yu Q Jin Y. Identification and characterization of microRNAs by high through-put sequencing in mesenchymal stem cells and bone tissue from mice of age-related osteoporosis. PLoS ONE 2013;8:e71895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H Xie H Liu W Hu R Huang B Tan YF et al. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest 2009;119:3666–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinas K Kessler CB Delany AM. miR-29 suppression of osteonectin in osteoblasts: regulation during differentiation and by canonical Wnt signaling. J Cell Biochem 2009;108:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FS Chuang PC Lin CL Chen MW Ke HJ Chang YH et al. MicroRNA-29a protects against glucocorticoid-induced bone loss and fragility in rats by orchestrating bone acquisition and resorption. Arthritis Rheum 2013;65:1530–1540. [DOI] [PubMed] [Google Scholar]
- Ottaviani G Jaffe N. The epidemiology of osteosarcoma. In: Jaffe N, Bruland OS, Bielack S (eds).Pediatric and Adolescent Osteosarcoma. Cancer Treatment and Research 152:Springer: USA, 2010; p 3–13. [DOI] [PubMed] [Google Scholar]
- Martin JW Squire JA Zielenska M. The genetics of osteosarcoma. Sarcoma 2012;2012:627254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KB Salah Z Del Mare S Galasso M Gaudio E Nuovo GJ et al. miRNA signatures associate with pathogenesis and progression of osteosarcoma. Cancer Res 2012;72:1865–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C Hou C Zhang H Wang D Ma Y Zhang Y et al. miR-126 functions as a tumor suppressor in osteosarcoma by targeting Sox2. Int J Mol Sci 2013;15:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhu J Feng Y Ke Z Yang Z Zhou J Huang X et al. Down-regulation of miR-183 promotes migration and invasion of osteosarcoma by targeting Ezrin. Am J Pathol 2012;180:2440–2451. [DOI] [PubMed] [Google Scholar]
- Osaki M Takeshita F Sugimoto Y Kosaka N Yamamoto Y Yoshioka Y et al. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol Ther 2011;19:1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyan W Shuhua Y Xiufang W Xiaoyun L. MicroRNA-21 is involved in osteosarcoma cell invasion and migration. Med Oncol 2011;28:1469–1474. [DOI] [PubMed] [Google Scholar]
- Song B Wang Y Titmus MA Botchkina G Formentini A Kornmann M et al. Molecular mechanism of chemoresistance by miR-215 in osteosarcoma and colon cancer cells. Mol Cancer 2010;9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B Wang Y Xi Y Kudo K Bruheim S Botchkina GI et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene 2009;28:4065–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougelet A Pissaloux D Besse A Perez J Duc A Dutour A et al. Micro-RNA profiles in osteosarcoma as a predictive tool for ifosfamide response. Int J Cancer 2011;129:680–690. [DOI] [PubMed] [Google Scholar]
- Zhou Y Huang Z Wu S Zang X Liu M Shi J. miR-33a is up-regulated in chemoresistant osteosarcoma and promotes osteosarcoma cell resistance to cisplatin by down-regulating TWIST. J Exp Clin Cancer Res 2014;33:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YK Wang H Leng Y Li ZL Yang YF Xiao FJ et al. Overexpression of microRNA-29b induces apoptosis of multiple myeloma cells through down regulating Mcl-1. Biochem Biophys Res Commun 2011;414:233–239. [DOI] [PubMed] [Google Scholar]
- Amodio N Leotta M Bellizzi D Di Martino MT D'Aquila P Lionetti M et al. DNA-demethylating and anti-tumor activity of synthetic miR-29b mimics in multiple myeloma. Oncotarget 2012;3:1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio N Bellizzi D Leotta M Raimondi L Biamonte L D'Aquila P et al. miR-29b induces SOCS-1 expression by promoter demethylation and negatively regulates migration of multiple myeloma and endothelial cells. Cell Cycle 2013;12:3650–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino MT Leone E Amodio N Foresta U Lionetti M Pitari MR et al. Synthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: in vitro and in vivo evidence. Clin Cancer Res 2012;18:6260–6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccaro AM Sacco A Thompson B Leleu X Azab AK Azab F et al. MicroRNAs 15a and 16 regulate tumor proliferation in multiple myeloma. Blood 2009;113:6669–6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CY She XM Qin Y Chu ZB Chen L Ai LS et al. miR-15a and miR-16 affect the angiogenesis of multiple myeloma by targeting VEGF. Carcinogenesis 2013;34:426–435. [DOI] [PubMed] [Google Scholar]
- Di Martino MT Gulla A Cantafio ME Lionetti M Leone E Amodio N et al. In vitro and in vivo anti-tumor activity of miR-221/222 inhibitors in multiple myeloma. Oncotarget 2013;4:242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi N Kang Y. Dysregulation of developmental pathways in bone metastasis. Bone 2011;48:16–22. [DOI] [PubMed] [Google Scholar]
- Pencheva N Tavazoie SF. Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol 2013;15:546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi-Garimella S Yun J Eves EM Newman M Erkeland SJ Hammond SM et al. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J 2009;28:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S Goldstein RH Scepansky EM Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res 2009;69:8742–8751. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF Alarcon C Oskarsson T Padua D Wang Q Bos PD et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008;451:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M Ma J Guillemette R Zhou M Yang Y Yang Y et al. miR-335 inhibits small cell lung cancer bone metastases via IGF-IR and RANKL pathways. Mol Cancer Res 2014;12:101–110. [DOI] [PubMed] [Google Scholar]
- Hassan MQ Maeda Y Taipaleenmaki H Zhang W Jafferji M Gordon JA et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem 2012;287:42084–42092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollari S Leivonen SK Perala M Fey V Kakonen SM Kallioniemi O. Identification of microRNAs inhibiting TGF-beta-induced IL-11 production in bone metastatic breast cancer cells. PLoS ONE 2012;7:e37361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y He W Tulley S Gupta GP Serganova I Chen CR et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA 2005;102:13909–13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Png KJ Halberg N Yoshida M Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 2012;481:190–194. [DOI] [PubMed] [Google Scholar]
- Valastyan S Chang A Benaich N Reinhardt F Weinberg RA. Activation of miR-31 function in already-established metastases elicits metastatic regression. Genes Dev 2011;25:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Saini S Majid S Yamamura S Tabatabai L Suh SO Shahryari V et al. Regulatory role of mir-203 in prostate cancer progression and metastasis. Clin Cancer Res 2011;17:5287–5298. [DOI] [PubMed] [Google Scholar]
- Takeshita F Patrawala L Osaki M Takahashi RU Yamamoto Y Kosaka N et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther 2010;18:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL Chiang A Chang D Ying SY. Loss of mir-146a function in hormone-refractory prostate cancer. RNA 2008;14:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W Ren D Chen X Tu X Huang S Wang M et al. HEF1 promotes epithelial mesenchymal transition and bone invasion in prostate cancer under the regulation of microRNA-145. J Cell Biochem 2013;114:1606–1615. [DOI] [PubMed] [Google Scholar]
- Peng X Guo W Liu T Wang X Tu X Xiong D et al. Identification of miRs-143 and -145 that is associated with bone metastasis of prostate cancer and involved in the regulation of EMT. PLoS One 2011;6:e20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ell B Mercatali L Ibrahim T Campbell N Schwarzenbach H Pantel K et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 2013;24:542–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo PL Liao SH Hung JY Huang MS Hsu YL. MicroRNA-33a functions as a bone metastasis suppressor in lung cancer by targeting parathyroid hormone related protein. Biochim Biophys Acta 2013;1830:3756–3766. [DOI] [PubMed] [Google Scholar]
- Sung SY Liao CH Wu HP Hsiao WC Wu IH Jinpu Yu et al. Loss of let-7 microRNA upregulates IL-6 in bone marrow-derived mesenchymal stem cells triggering a reactive stromal response to prostate cancer. PLoS ONE 2013;8:e71637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PK Bliss SA Patel SA Taborga M Dave MA Gregory LA et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res 2011;71:1550–1560. [DOI] [PubMed] [Google Scholar]
- Valadi H Ekstrom K Bossios A Sjostrand M Lee JJ Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654–659. [DOI] [PubMed] [Google Scholar]
- Montecalvo A Larregina AT Shufesky WJ Stolz DB Sullivan ML Karlsson JM et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012;119:756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M Gutierrez-Vazquez C Villarroya-Beltri C Gonzalez S Sanchez-Cabo F Gonzalez MA et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2011;2:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia K Luis-Ravelo D Bovy N Anton I Martinez-Canarias S Zandueta C et al. miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol Oncol 2014;8:689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H Aleckovic M Lavotshkin S Matei I Costa-Silva B Moreno-Bueno G et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012;18:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N Iguchi H Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 2010;101:2087–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J Cui X Jiang Z Sun J. MicroRNA-23a modulates tumor necrosis factor-alpha-induced osteoblasts apoptosis by directly targeting Fas. J Cell Biochem 2013;114:2738–2745. [DOI] [PubMed] [Google Scholar]
- Wu T Zhou H Hong Y Li J Jiang X Huang H. miR-30 family members negatively regulate osteoblast differentiation. J Biol Chem 2012;287:7503–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L Holmstrom K Qiu W Ditzel N Shi K Hokland L et al. MicroRNA-34a inhibits osteoblast differentiation and in vivo bone formation of human stromal stem cells. Stem Cells 2014;32:902–912. [DOI] [PubMed] [Google Scholar]
- Wang X Guo B Li Q Peng J Yang Z Wang A et al. miR-214 targets ATF4 to inhibit bone formation. Nat Med 2013;19:93–100. [DOI] [PubMed] [Google Scholar]
- Bhushan R Grunhagen J Becker J Robinson PN Ott CE Knaus P. miR-181a promotes osteoblastic differentiation through repression of TGF-beta signaling molecules. Int J Biochem Cell Biol 2013;45:696–705. [DOI] [PubMed] [Google Scholar]
- Kureel J Dixit M Tyagi AM Mansoori MN Srivastava K Raghuvanshi A et al. miR-542-3p suppresses osteoblast cell proliferation and differentiation, targets BMP-7 signaling and inhibits bone formation. Cell Death Dis 2014;5:e1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P Chen C He HB Hu R Zhou HD Xie H et al. miR-148a regulates osteoclastogenesis by targeting V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. J Bone Miner Res 2013;28:1180–1190. [DOI] [PubMed] [Google Scholar]
- Nakasa T Shibuya H Nagata Y Niimoto T Ochi M. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum 2011;63:1582–1590. [DOI] [PubMed] [Google Scholar]
- Zhang JF Fu WM He ML Xie WD Lv Q Wan G et al. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biol 2011;8:829–838. [DOI] [PubMed] [Google Scholar]
- Zhang J Tu Q Bonewald LF He X Stein G Lian J et al. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res 2011;26:1953–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimalraj S Partridge NC Selvamurugan N. A positive role of microRNA-15b on regulation of osteoblast differentiation. J Cell Physiol 2014;229:1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M Ma J Chen S Chen X Yu X. MicroRNA-17-92 cluster regulates osteoblast proliferation and differentiation. Endocrine 2014;45:302–310. [DOI] [PubMed] [Google Scholar]
- Gamez B Rodriguez-Carballo E Bartrons R Rosa JL Ventura F. MicroRNA-322 (miR-322) and its target protein Tob2 modulate Osterix (Osx) mRNA stability. J Biol Chem 2013;288:14264–14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z Hassan MQ Jafferji M Aqeilan RI Garzon R Croce CM et al. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 2009;284:15676–15684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinas K Kessler C Ricks T Gronowicz G Delany AM. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J Biol Chem 2010;285:25221–25231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E Zhang J Yuan T Ma B. miR-143 suppresses osteogenic differentiation by targeting Osterix. Mol Cell Biochem 2014;390:69–74. [DOI] [PubMed] [Google Scholar]
- Wu T Xie M Wang X Jiang X Li J Huang H. miR-155 modulates TNF-alpha-inhibited osteogenic differentiation by targeting SOCS1 expression. Bone 2012;51:498–505. [DOI] [PubMed] [Google Scholar]
- Yang L Cheng P Chen C He HB Xie GQ Zhou HD et al. miR-93/Sp7 function loop mediates osteoblast mineralization. J Bone Miner Res 2012;27:1598–1606. [DOI] [PubMed] [Google Scholar]
- Kim KM Park SJ Jung SH Kim EJ Jogeswar G Ajita J et al. miR-182 is a negative regulator of osteoblast proliferation, differentiation, and skeletogenesis through targeting FoxO1. J Bone Miner Res 2012;27:1669–1679. [DOI] [PubMed] [Google Scholar]
- Guo J Ren F Wang Y Li S Gao Z Wang X et al. miR-764-5p promotes osteoblast differentiation through inhibition of CHIP/STUB1 expression. J Bone Miner Res 2012;27:1607–1618. [DOI] [PubMed] [Google Scholar]
- Itoh T Takeda S Akao Y. MicroRNA-208 modulates BMP-2-stimulated mouse preosteoblast differentiation by directly targeting V-ets erythroblastosis virus E26 oncogene homolog 1. J Biol Chem 2010;285:27745–27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K Lu J Zhao Y Wang L Li J Qi B et al. MicroRNA-214 suppresses osteogenic differentiation of C2C12 myoblast cells by targeting Osterix. Bone 2013;55:487–494. [DOI] [PubMed] [Google Scholar]
- Itoh T Nozawa Y Akao Y. MicroRNA-141 and -200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. J Biol Chem 2009;284:19272–19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y Qu X Li H Huang S Wang S Xu Q et al. MicroRNA-100 regulates osteogenic differentiation of human adipose-derived mesenchymal stem cells by targeting BMPR2. FEBS Lett 2012;586:2375–2381. [DOI] [PubMed] [Google Scholar]
- Li H Li T Wang S Wei J Fan J Li J et al. miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res 2013;10:313–324. [DOI] [PubMed] [Google Scholar]
- Kim D Song J Kim S Park HM Chun CH Sonn J et al. MicroRNA-34a modulates cytoskeletal dynamics through regulating RhoA/Rac1 cross-talk in chondroblasts. J Biol Chem 2012;287:12501–12509. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Abouheif MM Nakasa T Shibuya H Niimoto T Kongcharoensombat W Ochi M. Silencing microRNA-34a inhibits chondrocyte apoptosis in a rat osteoarthritis model in vitro. Rheumatology (Oxford) 2010;49:2054–2060. [DOI] [PubMed] [Google Scholar]