Abstract
Fracture repair is a complex process that involves the interaction of numerous molecular factors, cell lineages and tissue types. These biological processes allow for an impressive feat of engineering: an elastic soft callus is progressively replaced by a more rigid and mineralized callus. During this reparative phase, the healing bone is exposed to a risk of re-fracture. Bone volume and bone quality are the two major factors determining the strength of the callus. Although both factors are important, often only bone volume is analyzed and reported in preclinical studies. Recent developments in techniques for examining bone quality in the callus will enable the rapid and detailed analysis of its material properties and its microstructure. This review aims to give an overview of the methods available for quantitatively phenotyping the bone callus in preclinical studies such as Raman spectroscopy, nanoindentation, scanning acoustic microscopy, in vivo micro-computed tomography (micro-CT) and high-resolution micro-CT. Consolidated and emerging experimental methods are described with a focus on their applicability, and with examples of their utilization.
Introduction
Fracture risk is increasing worldwide with the aging population.1 High-trauma fractures and fractures in the elderly, mainly due to osteoporosis, are challenging to manage. Bone nonunion and re-fracture are the most common postfracture problems. Where early bridging does occur, it is of critical importance to establish sufficient bone stability and prevent infection. In addition, interventions aimed at increasing bone formation and decreasing excessive or premature resorption have a crucial role for daily clinical management.
Currently, there are two major approaches for augmenting fracture repair: biophysical or pharmaceutical. Biophysical strategies point toward increasing bone anabolism (bone formation) using exogenous, nonchemical stimuli such as ultrasound, shockwaves, low-magnitude high-frequency vibration and electromagnetic field stimulation.2 Although these strategies have shown promise in preclinical models and individual clinical studies, they have not been widely adopted as frontline treatments for the majority of fracture cases. On the other hand, the general strategy of pharmaceutical approaches is to increase bone tissue quantity either by shortening the time for union or by increasing the strength of union through modulation of bone formation or resorption. For both approaches, it is essential to examine the underlying anabolic and catabolic (bone resorbing) processes, which influence bone repair capacity.3
The efficacy of the treatments for augmenting fracture repair and their effect on bone quantity is usually tested in preclinical studies on animal models. However, in many of these experiments, only a limited number of bone calluses per group and/or per time point are usually analyzed, and the biological intersample variance makes the analysis of the differences in the resistance to re-fracture challenging. Therefore, toward a complete characterization of the bone callus, methods to systematically assess the changes in the structure and the material properties of the regenerating bone tissue are needed. This will help to better understand the effects of a particular treatment strategy supporting bone fracture repair.
Besides helping to characterize the resistance to re-fracture, these methods already told us much about how the callus develops in terms of collagen organization, material properties, micromechanical properties and architecture. As new treatments for fracture repair sometimes involve unknown biological changes, a deeper analysis of the structure could also help in better understanding the working principles.
For instance, collagen organization was found to be different from lamellar bone and to gradually develop toward a more organized pattern.4,5 Material properties of the newly generated bone tissue were also found to be different from lamellar bone, as the callus is usually less mineralized and the mineral particle size seems to increase during fracture healing, and then decrease again at the late stage of bone remodeling.4 The micromechanical properties of the callus gradually improve during the remodeling phase.6 Moreover, the mineralized struts architecture was found to largely change during the development, and the trabeculae forming the callus greatly change in number and shape during the whole reparative process.7 A brief description of the bone healing process will be presented in the following section. Subsequently, the techniques to assess fracture repair quality will be reviewed.
Biology and biomechanics of fracture repair
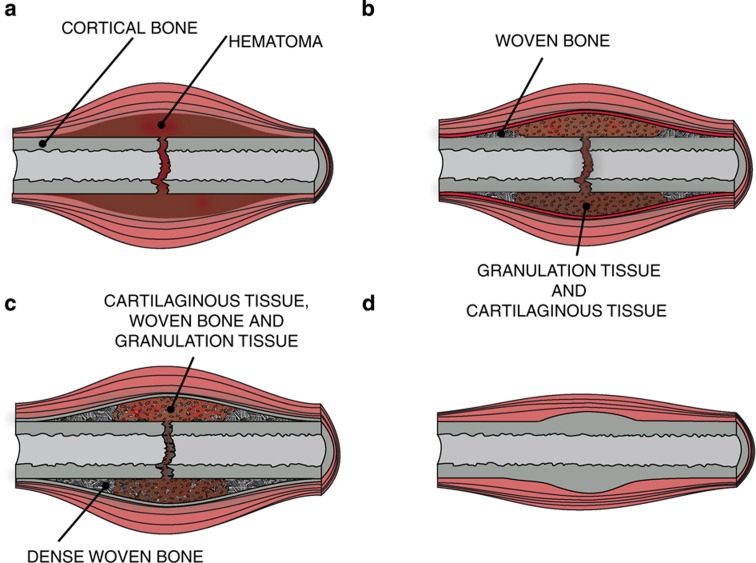
Fracture healing is a sophisticated process that progresses along both the spatial and the temporal axis. Microfractures or rigidly fixated opposing bone surfaces can heal by primary or direct bone healing, which involves remodeling without any external tissue formation.8 In contrast, many clinically managed fractures of long bones heal by endochondral ossification.1 After the creation of a fracture hematoma and a reparative inflammatory phase, a cartilaginous soft callus is formed. The soft, cartilaginous callus is then gradually replaced by woven bone of the developing hard callus. This structure will be eventually resorbed, while lamellar bone is built up, such that the architecture and the overall shape of the original long bone is restored (Figure 1).9
Figure 1.
A sequential schematic of four classical stages of fracture healing. (a) After inflammation a hematoma is generated. (b) In the first stage of the reparative phase, the initial fibrin is gradually replaced by cartilaginous tissue and woven bone starts to form. (c) In a later stage of the reparative phase, the cartilaginous tissue mineralizes, more bone is formed and the volume of granulation tissue substantially decreases. (d) Eventually, once the bone is bridged, remodeling restores the original cortex.
Biomechanically, the properties of a bone fracture site evolve as the stages of fracture healing progress. During the early inflammatory phase, the hematoma or fibrin clot provides little mechanical stability. At this stage, the callus is mechanically weak and the structure is chiefly supported by the muscles surrounding the bone, which will contract in an effort to stabilize the fracture gap. Formation of the soft callus provides some additional strength, but the structure is largely flexible and it is incapable of carrying any significant load. As endochondral ossification proceeds, the hard callus forms and this is progressively transformed into a hard and resistant structure. During this transformation, it passes through the formation of woven bone, which is the earliest form of bone tissue with correspondingly low strength, to lamellar bone with superior mechanical characteristics.
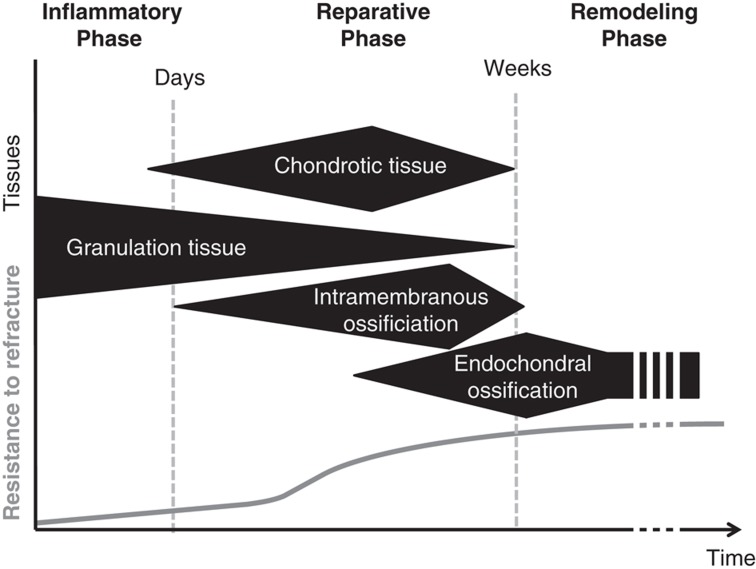
During fracture healing, the relative proportions of granulation tissue, cartilaginous tissue, as well as woven and lamellar bone, progressively shift. These tissues are of different quality, that is, they have varying structural and material properties. Together with their extension, the quality of these developing tissues ultimately determines the strength of the callus and the repaired bone tissue (Figure 2).
Figure 2.
Qualitative scheme of the tissue evolution during bone fracture repair. Granulation tissue is the only tissue present in the fracture gap shortly after fracture. After a few days, the fibroblasts in the granulation tissue make way for chondrocytes producing cartilaginous tissue. Bone formed by intramembranous ossification will appear within the first few days after fracture. About 2 weeks later, the cartilage formed in the fracture gap will start mineralizing to be converted into lamellar bone by endochondral ossification.1
Much of the insight into the biology and biomechanics of fracture repair has emerged from animal experiments. Rodent models are of prime importance as they are inexpensive, easy to breed and because a relatively high number of animals can be bred concurrently.10 Such rodent models allow inducing reproducible fracture patterns or bone defects in a manner that is not achievable in a clinical setting, where injuries are highly heterogeneous. Mouse models, in particular, can be used for gene targeting technologies and antibody-mediated suppression of protein function,10 which are crucial for investigating the genetic fingerprint of bone diseases and their underlying molecular pathways.
Measurement of bone quality
The examination of bone properties can be performed at a variety of hierarchical levels, from the macroscale down to the nanoscale. One concept that is particularly pertinent to characterize bone competence is that of bone quality, which originates from the field of osteoporosis research. It was noted that although the quantity or density of bone can be highly predictive for fracture risk,11,12 other factors likely influence bone strength.13 It is recognized that changes in bone architecture and material properties at different hierarchical levels can negatively influence bone quality, leading to increased bone fragility.14 It is important to notice that there is a discrepancy in the literature regarding the use of the term bone quality. Some researchers define bone quality as the combination of all parameters determining whole bone strength, including bone mass. In this review, a clear distinction is made between bone mass and bone quality, which is determined by distribution of bone mass (architecture/geometry/dimensions) and bone material properties (for example, mineralization, collagen properties, microdamage).
Different techniques are used to describe bone mass and bone quality or a composite of these two factors, which influence bone competence. For example, for whole bone mechanical testing (for example, four-point bending), both bone quantity and quality factors are important, whereas nanoindentation mainly sheds light on bone quality. The most common approaches to quantify the different determinants of bone competence encompass bone imaging and mechanical testing modalities.
In clinical practice, bone fracture risk is usually monitored with radiographic scanning techniques such as dual-energy X-ray absorptiometry, where bone mineral density is assessed. Other techniques that allow assessing bone quality in patients, such as high-resolution peripheral quantitative computed tomography measuring bone microarchitecture or reference point indentation examining in vivo tissue mechanical properties, are under ongoing development.15,16
In case of fracture, it is important to ensure that the bone is properly healing. Radiography can help diagnosing whether the bone has a problem to reunite (nonunion), which is a failure of healing that affects around 10% of people experiencing a bone fracture.17 When the bone achieves to bridge, bone volume is a commonly adopted criteria for monitoring the healing response.
In animal studies, other measures that are critical for bone competence are often evaluated along with bone mass. To measure the amount of calcified tissue at a certain skeletal site, micro-computed tomography (micro-CT) has emerged as a key technology.18 In these studies, micro-CT allows rapid measurement of specimens varying in size (a few millimeters up to a few centimeters), providing information regarding bone mass (distribution), three-dimensional (3D) microarchitecture and local mineralization in terms of hydroxyapatite content. Other two-dimensional (2D) methods, chiefly histology and light microscopy, are used to examine other tissue types including granulation tissue or cartilage present at the early stages of bone repair.19 Histology is a destructive and often labor-intensive process, which yields data that lack a 3D context.20 Thus, a combination of histological and CT approaches can be used in concert to describe a specimen in a more integral manner.
Finally, mechanical testing is important to interpolate how bone quantity and quality account for bone competence. In other words, mechanical measures describe bone competence, which is determined by a combination of factors such as bone mass, architecture/geometry/dimensions and material properties. The maximal force that a sample can bear, the energy to failure or the information on strength and stiffness are common outputs from whole-bone mechanical testing. One challenge in the field remains a lack of standardization regarding the mechanical tests that are all routinely used to assess long bone strength,10 for instance by three-point, four-point and torsional testing. Although torsional testing might be ideal for whole bones, after fracture, the callus can be stronger than the surrounding bone. If this is the case, failure occurs outside the callus and the data recorded do not relate to the callus. Three-point and preferably four-point bending isolate the callus as the region being tested, which is an advantage over torsion. However, the disadvantage is that the test relates to only one randomly selected plane. As every mechanical test has its pros and cons, it is nearly impossible to find a universally accepted consensus.
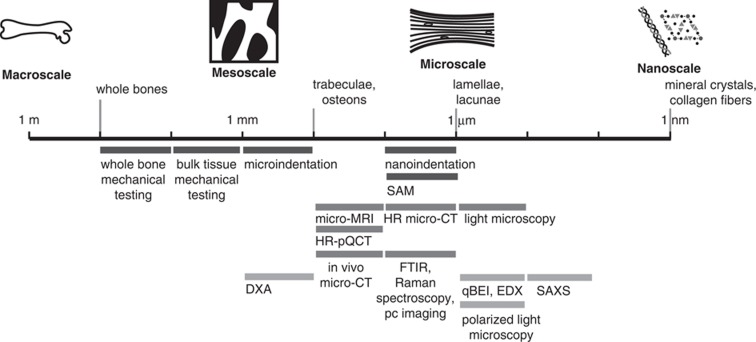
A range of alternative or more advanced methodologies have emerged to examine bone quality in preclinical animal experiments in the past decades. These techniques improved the investigation of the microarchitecture (for example, in vivo micro-CT), the material properties (for example, Raman spectroscopy) or the mechanical bone properties (for example, nanoindentation). These methods will be subsequently reviewed in greater detail. A summary of the techniques mentioned in this review is illustrated in Figure 3.
Figure 3.
Techniques for the assessment of bone quality and bone competence. Mechanical tests (dark gray bars), imaging techniques to study geometry and microarchitecture (medium gray bars) and techniques to analyze tissue composition (light gray bars) are indicated in logarithmic scale, according to their spatial resolution. The techniques include dual energy X-ray absorptiometry (DXA), energy-dispersive X-ray spectroscopy (EDX), Fourier transform infrared imaging (FTIR), high-resolution micro-computed tomography (HR micro-CT), high-resolution peripheral quantitative computed tomography (HR-pQCT), in vivo micro-computed tomography (in vivo micro-CT), micro-magnetic resonance imaging (micro-MRI), phase contrast-based computed tomography (pc imaging), quantitative backscattered electron imaging (qBEI), scanning acoustic microscopy (SAM) and small-angle X-ray scattering (SAXS). Adapted from Donnelly29 with kind permission.
Assessment of fracture callus quality
Some of the techniques that are used to investigate bone quality have recently been applied to regenerating bone. Unfortunately, literature discussing the assessment of callus quality in clinical scenarios is still very scarce. On the other hand, techniques for determining callus quality in preclinical studies are emerging. As this is a field of recent development, currently there is no established strategy to assess and describe the quality of the callus. In particular, there is a lack of direct comparisons as to how fracture callus quality—in terms of microstructure and material properties—can influence or correlate with whole bone strength. We will briefly discuss the current status regarding assessment of fracture callus quality, followed by an outlook on promising techniques and methods, which will help describe the properties of the callus that are (potentially) important for the competence of the whole bone.
Current status for the assessment of fracture callus quality
The assessment of the quality of the healing bone tissue, and especially the quality of the developing callus, represents a challenging task because we are confronted with a complex mix of tissues, which are rapidly evolving over time. Therefore, a thorough examination of the fracture healing process in terms of callus quality must not be focused on static measures only, but should include observations at the different stages during fracture healing (Figures 1 and 2).
Different studies on fracture calluses have revealed a correlation between local material or mechanical properties and resistance to re-fracture.18,21 These discoveries are not only important for deepening our understanding of the role of bone callus quality, but are also important for avoiding an erroneous overestimation of the influence of bone callus quantity on resistance to re-fracture. For example, treatment with a bisphosphonate that increased bone mineral content and bone volume did not correlate in a linear manner with whole-bone mechanical strength with particular dosages and posology.22,23,24 This suggests that specific interventions can increase bone quantity, but generate bone of inferior quality. However, to date, there is no established strategy to assess and describe the quality of the callus. In particular, there is a lack of direct comparisons as to how fracture callus quality—in terms of microstructure and material properties—can influence or correlate with whole bone strength.
Few indicators of bone quality are regularly considered in preclinical studies. The moment of inertia and the polar moment of inertia are possibly the only bone quality parameters that are frequently assessed. These descriptors are usually easily extrapolated from the micro-CT analysis of the healing bone together with the callus mass information. However, their value on the description of bone quality is still controversial, with studies reporting an absence of correlation with resistance to re-fracture18,25 and a recent study by O'Neill et al.26 showing a significant correlation.
In the following section, methods for assessing bone quality will be reviewed. These may give important insight into the mechanisms underlying regenerating bone competence, and may help guide future interventions to improve bone repair. An overview of the techniques for investigating bone callus mass and callus quality in preclinical studies is presented in Table 1.
Table 1. Techniques for the assessment of bone quality and bone competence adopted in preclinical studies.
| Tissue property | Technique | Object of investigation | Quantitative measure(s) | Major limitations/disadvantages | Application to callus | Correlation to resistance of re-fracture |
|---|---|---|---|---|---|---|
| Bone mass | Micro-CT/in vivo micro-CT | Bone volume, bone mineral density | TV, BV, BV/TV, BS, BS/BV, BS/TV, BMD | In vivo micro-CT: lower resolution (∼ 10 μm) than HR micro-CT (1–10 μm) | Yes (standard measure) | Morgan et al.18O'Neill et al.26 |
| Architecture (microstructure) | HR micro-CT | Mineralized struts | Tb.N, Tb.Th, Tb.Sp, Tb.Th.SD, Tb.Sp.SD, DA, Conn.D, SMI | Long acquisition times (hours) | Gerstenfeld et al.56 | Mehta et al.7 |
| Micro-MRI | Mineralized struts, cartilaginous and granulation tissue | Tb.N, Tb.Th, Tb.Sp, Tb.Th.SD, Tb.Sp.SD, DA, Conn.D, SMI | Axial resolution (∼ 100 μm) lower than micro-CT, strong magnetic fields | − | − | |
| Phase contrast-based CT | Mineralized struts, cartilaginous and granulation tissue | Conn.D, SMI, Tb.N, Tb.Th, Tb.Sp, Tb.Th.SD, Tb.Sp.SD, DA | Not commercial yet, longer scanning time than micro-CT (hours to days) | − | − | |
| Architecture (ultrastructure) | Polarized light microscopy | Orientation of collagen fibers | Change of image intensity depending on orientation | 2D, only visual | Tonna.57 | − |
| Raman imaging | Orientation of collagen fibers | Variation of peak intensities within Raman spectrum (Amide I, PO43-, C-O-C, etc.) | 2D, long acquisition times (hours) | Galvis et al.5 | − | |
| SAXS | Orientation of collagen fibers | Shape and intensity of diffuse scattering in diffraction pattern | 2D, advanced imaging equipment/limited access to SAXS at synchrotrons | Liu et al.39 | − | |
| Material properties | FTIR | Local compositional properties | Mineral-to-matrix ratio, carbonate-to-phosphate ratio, collagen quality, crystallinity | No absolute quantity measureable | Yang et al.58 | − |
| Raman spectroscopy | Local compositional properties | Mineral-to-matrix ratio, carbonate-to-phosphate ratio, collagen quality, crystallinity | No absolute quantity measurable, high variance | Meganck et al.45 | − | |
| qBEI | Local mineral composition | Distribution of mineral content | Destructive sample preparation (coating) | Manjubala et al.48 | − | |
| EDX | Local material composition | Atomic composition (for example, quantify Ca, C, H2, etc.) | No molecular quantification | Brüel et al.49 | − | |
| Mechanical properties | Nanoindentation | Local mechanical properties | Reduced elastic modulus, hardness | High variance depending on sample preparation | Leong and Morgan6, Leong and Morgan52, Manjubala et al.48 | − |
| SAM | Local mechanical properties | Impedance, elastic modulus | Perfectly flat surface required | Preininger et al.55 | Hube et al.54 |
Abbreviations: BMD, bone mineral density; BS, bone surface; BS/BV, specific bone surface; BS/TV, bone surface density; BV, bone volume; BV/TV, bone volume fraction; Conn.D, connectivity density; DA, degree of anisotropy; EDX, energy-dispersive X-ray spectroscopy; FTIR, Fourier transform infrared imaging; HR, high-resolution; micro-CT, micro-computed tomography; micro-MRI, micro-magnetic resonance imaging; qBEI, quantitative backscattered electron imaging; SAM, scanning acoustic microscopy; SAXS, small-angle X-ray scattering; 2D, two-dimensional; Tb.N, trabecular number; ; Tb.Sp, trabecular separation; Tb.Sp.SD, standard deviation of the trabecular separation; Tb Th.SD, standard deviation of trabecular thickness; TV, total volume; Tb.Th, trabecular thickness; SMI, structure model index.
Assessment of fracture callus structure
Assessment of the hard callus microstructure. The calculation of morphometric indices (called quantitative morphometry) is a standard method for describing trabecular bone microarchitecture.27 Indices such as trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), degree of anisotropy (DA), connectivity density (Conn.D), standard deviation of trabecular thickness (Tb.Th.SD) and of the trabecular separation (Tb.Sp.SD) have been shown to be predictors of the resistance to fracture of bone.27 Recently, these trabecular measures were used by Mehta et al.7 to evaluate the mineralized struts of the callus in the late reparative phase, which correlated with the resistance to re-fracture. Moreover, the authors showed that a combination of the morphometric measures mentioned beforehand predicted the resistance to fracture as good as basic bone quantity parameters such as bone volume fraction (BV/TV). These results suggest that in the analysis of the mechanical behavior of the callus, morphometric descriptors of the microstructure may enhance our capacity to predict callus strength.
Morphometric indices of trabecular structures can be assessed together with bone callus quantity parameters using micro-CT. In rodent models, the whole callus has to be scanned with a resolution below 10 μm to reliably image small trabeculae.28,29 To achieve this resolution, high-resolution micro-CT (HR micro-CT) is needed. HR micro-CT could also be used to assess whether complete bridging of mineralization across the callus or defect has occurred. This is an important issue that is usually overlooked in the analyses of the callus, where bone mass is used as an indicator of the healing progress. Algorithms for HR micro-CT that take into account the location and the extent of strut bridging across the callus will enable a better estimation of the resistance to re-fracture.
Assessment of soft tissue fraction of the fracture callus. The cartilaginous tissue present in the callus is pivotal in the first stage of the healing process, as it provides initial stability across the fracture gap.30 Therefore, a tool that is able to identify and quantify cartilage in the soft callus would provide benefits in the assessment of fracture repair. A promising imaging approach is micro-magnetic resonance imaging (micro-MRI). Micro-MRI provides good contrast for soft tissues, which are abundant in the young fracture callus. However, resolutions of micro-MRI measurements are in the order of 100 μm (in the longitudinal direction), which is rather low compared with desktop micro-CT in the micrometer regime.31 In addition, strong magnetic fields have been suspected of interfering with bone healing processes.32 Consequently, the application of micro-MRI for the assessment of bone repair is still limited in preclinical studies.
Micro-CT could be possibly used in the future for the evaluation of the soft tissues present in the callus. Recently, Hayward et al.33 developed a contrast-enhanced protocol for micro-CT imaging of the soft callus in an ex vivo setting. Alternatively, phase contrast-based CT might allow the assessment of soft tissues without the need to introduce contrast agents before scanning.34
In vivo assessment of fracture callus structure. An exciting advancement in bone research is the application of in vivo micro-CT scanners for animal models.35 In vivo micro-CT would allow tracking fracture callus structure in the same animal at multiple time points during the fracture healing progression. It could therefore substitute ex vivo micro-CT and reduce time, costs and number of animals in cross-sectional studies. The identification or development of appropriate biocompatible contrast agents for ex vivo micro-CT33 may allow for an improvement for in vivo micro-CT scanning of the fracture callus, similarly as gadolinium did for magnetic resonance angiography.
Although in vivo micro-CT allows for unique insights into the longitudinal assessment of bone repair, a number of limitations have hindered its widespread adoption. First, metal implants are often used in fixation, which can block/scatter X-rays and reduce or prevent high-quality image capture. Secondly, the resolution offered by in vivo micro-CT is lower than that for ex vivo micro-CT owing to X-ray dose considerations for in vivo measurements. Finally, frequent animal narcosis required to assess bone development in a time-lapsed manner can result in additional deaths/exclusions and may adversely affect bone healing.36
Assessment of collagen orientation in the callus. The direction of collagen fibers has been shown to contribute to bone quality.14 In the callus, this factor is likely to have a role in the resistance to re-fracture.5 Many techniques have been used to determine collagen fiber orientation in bone and cartilage, and they are potentially helpful for callus characterization. These include polarized light microscopy, Raman imaging and small-angle X-ray scattering (SAXS).
Polarized light microscopy uses polarized light, which enhances image contrast of birefringent systems. This technique gives access to the ultrastructural organization of biological tissues, such as the lamellar structure, owing to the collagen orientation in bone tissue.37 Raman imaging uses the scattering of monochromatic light—typically laser light—to extrapolate the orientation of collagen fibers,38 where different spectra are retrieved based on the collagen alignment.37 More recently, Raman imaging has been applied to analyze collagen orientation during the early stages of bone fracture healing in rats.5 High level of organization of the collagen fibers within the mineralized tissue was already found at early stages of fracture repair. Moreover, in the rodent model adopted, the fractured cortical bone showed signs of remodeling already three weeks after fracture. Finally, in SAXS, elastically scattered X-rays of the specimen provide local information about the periodicity and the orientation of the collagen fibers. SAXS can be challenging to adopt, as it requires an advanced laboratory SAXS instrument or access to a SAXS setup at a synchrotron light source. Recently, SAXS has been successfully applied to determine the collagen orientation in a sheep callus,39 where a progressive alignment of the collagen in the callus was found, parallel to the main direction of the long bone.
Assessment of fracture callus material properties
For characterization of the callus material properties, Raman spectroscopy, Fourier transform infrared imaging (FTIR), quantitative backscattered electron imaging (qBEI) and energy-dispersive X-ray spectroscopy (EDX) are potential candidates.40,41
Both Raman spectroscopy and FTIR measure partially overlapping subsets of the specimen's vibrational spectrum,42 and they give access to important bone quality factors by describing the quality of the newly formed bone tissue of the callus, such as the mineral-to-matrix ratio, the carbonate-to-phosphate ratio and collagen cross-linking.43 The mineral-to-matrix ratio could be used to determine the changes in mineralization in specific regions of the callus, and the carbonate-to-phosphate ratio could provide important insights into callus quality as it varies depending on the architecture of the collagen tissue, its age and its mineral crystallinity.41,44 Collagen can be considered to be the backbone of the bone's mineral structure, and it is therefore an important factor for assessing bone strength.14 Collagen cross-linking can be detected by changes in the amide I envelope of the spectra, which are strongly correlated to the bone collagen maturity.39 For the fracture callus, it means that non-completely mineralized areas present during the first stages of the reparative phase could be evaluated based on their collagen structure, which will show different levels of maturity. Recently, Meganck et al.45 applied Raman spectroscopy to calluses to analyze the effect of bisphosphonates on bone material properties for two different mouse models. The study showed that there was no significant change in the mineral-to-matrix ratio, but there were significant changes in crystallinity between the two mouse strains. Meganck et al. also noticed a high intragroup and intrasample variation in the calluses in mineral-to-matrix ratio, crystallinity and carbonate-to-phosphate ratio. Nonetheless, Raman spectroscopy has been shown to be a powerful tool to detect changes in material properties in murine bones interacting with bisphosphonates.46 Therefore, spectroscopic techniques have a potential application to assess the quality of healing bone tissue, which can help understanding how treatments influence its recovery.47
Backscattered microscopy or qBEI is a form of scanning electron microscopy where backscattering of incident electrons colliding with atoms of the sample is recorded, with the goal of estimating the local bone mineralization level.29 This technique was successfully applied for investigating the mineralization degree in the healing callus.48 EDX is a technique applied to study the atomic composition of a sample by analyzing the emission of characteristic X-rays through an energy-dispersive spectrometer.29 EDX can be particularly helpful to detect specific elements in the callus, which cannot be discerned by Raman spectroscopy or by FTIR. For example, Brüel et al.49 used EDX for investigating the presence of strontium ranelate in the callus after specific treatment with this drug. As EDX can detect the presence of specific atoms, it is particularly suited for studies involving treatments, which involve exogenous atoms (that is, strontium).
Assessment of callus biomechanics
There exist several widely used techniques for measuring local biomechanical properties of bone tissue. Probably the most common techniques are nanoindentation50 and scanning acoustic microscopy (SAM).51 For nanoindentation tests, a diamond tip penetrates the specimen up to a certain depth and the exerted forces are recorded to extrapolate the mechanical properties.50 Leong and Morgan52 investigated the local mechanical properties of rodent callus via nanoindentation, demonstrating the usefulness of this technique in characterizing the heterogeneous mixture of tissues present in the callus. In contrast, SAM uses measurements of a sample's reflection of acoustic waves to derive its stiffness.54 SAM was already successfully adopted for investigating the relationship between resistance to fracture and local mechanical properties in ovine calluses. A strong correlation between the two parameters was found.54 Both techniques provide the stiffness of the region under investigation at high spatial resolution. Compared with SAM, nanoindentation has the advantage to concomitantly extrapolate the elastic modulus and the hardness of the investigated tissue,55 which is not possible with SAM. Moreover, nanoindenters are more widely spread at universities and in research centers, and therefore more likely to be adopted in preclinical or clinical studies. This is supported by the much higher number of studies conducted using nanoindentation instead of SAM for retrieving bone stiffness.
Conclusions
This review has presented a range of different techniques used in the analysis of bone callus quality. In some cases, these methods have already been applied to the study of bone repair; for others, their application has been more limited in the research field of fracture healing.
Micro-CT and biomechanical testing are routinely applied for fracture studies; however, methods for structural analysis (for example, HR micro-CT) and for measuring material properties (for example, Raman spectroscopy or nanoindentation) are still uncommon. In the literature, there are often strong correlations described between the outcomes of different techniques, such as between structural data from micro-CT and material properties from mechanical tests or between elastic modulus from nanoindentation and tissue mineral density extrapolated from micro-CT measurements.6,7,18 The comparison of multiple testing modalities will be important to decide which methods are most appropriate to quantitatively describe the regenerating bone tissue.
Owing to biological variability, all calluses are to some extent at different healing points, even if the elapsed time after fracture is equal. Moreover, the callus can have local differences that vary depending on the tissue property considered. For this reasons, it is important to determine the impact of these differences on the variance of the results from the techniques describing callus quality. This will facilitate determining the correct number of samples and sampling size. For instance, assessment of the callus using Raman spectroscopy is subject to a high local variance within a specific sample, which is still not completely investigated.
The technologies for the investigation of bone tissue are rapidly evolving, and hence it is a challenge to establish a standard for the characterization of fracture healing. However, the high heterogeneity of the callus tissue has been shown to require more than merely mechanical testing and assessment of bone volume for estimating its quality and relevance for risk of bone re-fracture. On this account, we are convinced that in the next few years several of the methods mentioned in this review will be adopted in preclinical studies focused on fracture healing.
Footnotes
The authors declare no conflict of interest.
References
- Sfeir C, Ho L, Doll BA, Azari K, Hollinger JO. Fracture repair. In: Lieberman JR, Friedlaender GE (eds) Bone Regeneration and Repair: Biology and Clinical Applications Humana Press: Totowa, NJ, USA, 2005;21–44. [Google Scholar]
- Aaron RK, Ciombor DM, Wang S, Simon B. Clinical biophysics: the promotion of skeletal repair by physical forces. Ann N Y Acad Sci 2006; 1068:513–531. [DOI] [PubMed] [Google Scholar]
- Little DG, Ramachandran M, Schindeler A. The anabolic and catabolic responses in bone repair. J Bone Joint Surg Br 2007; 89:425–433. [DOI] [PubMed] [Google Scholar]
- Liu Y, Manjubala I, Schell H, Epari DR, Roschger P, Duda GN et al. Size and habit of mineral particles in bone and mineralized callus during bone healing in sheep. J Bone Miner Res 2010; 25:2029–2038. [DOI] [PubMed] [Google Scholar]
- Galvis L, Mehta M, Masic A, Dunlop JWC, Duda G, Fratzl P. Collagen orientation during early stages of bone fracture healing investigated by polarized raman imaging. In: Champion PM, Ziegler LD (eds) XXII International Conference on Raman Spectroscopy Amer Inst Physics: Boston, MA, USA, 2010;406–407. [Google Scholar]
- Leong PL, Morgan EF. Correlations between indentation modulus and mineral density in bone-fracture calluses. Integr Comp Biol 2009; 49:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta M, Heyland M, Toben D, Duda GN. Microstructure and homogeneity of distribution of mineralised struts determine callus strength. Eur Cell Mater 2013; 25:366–379. [DOI] [PubMed] [Google Scholar]
- Isaksson H. Recent advances in mechanobiological modeling of bone regeneration. Mech Res Commun 2012; 42:22–31. [Google Scholar]
- Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res 1998; 355:S7–S21. [DOI] [PubMed] [Google Scholar]
- Holstein JH, Garcia P, Histing T, Klein M, Becker SC, Menger MD et al. Mouse models for the study of fracture healing and bone regeneration. In: Duque G, Watanabe K (eds) Osteoporosis Research. Springer-Verlag: London, UK, 2011;175–191.
- Hui SL, Slemenda CW, Johnston CC. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 1988; 81:1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DM, Cummings SR, Genant HK, Nevitt MC, Palermo L, Browner W. Axial and appendicular bone density predict fractures in older women. J Bone Miner Res 1992; 7:633–638. [DOI] [PubMed] [Google Scholar]
- Nielsen SP. The fallacy of BMD: a critical review of the diagnostic use of dual X-ray absorptiometry. Clin Rheumatol 2000; 19:174–183. [DOI] [PubMed] [Google Scholar]
- Fratzl P, Gupta HS, Paschalis EP, Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem 2004; 14:2115–2123. [Google Scholar]
- Gallant MA, Brown DM, Organ JM, Allen MR, Burr DB. Reference-point indentation correlates with bone toughness assessed using whole-bone traditional mechanical testing. Bone 2013; 53:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 2005; 90:6508–6515. [DOI] [PubMed] [Google Scholar]
- Zimmermann G, Moghaddam A. Trauma: Non-Union: New Trends. In: Bentley G (ed) European Instructional Lectures 10 Springer: Berlin2010;15–19. [Google Scholar]
- Morgan EF, Mason ZD, Chien KB, Pfeiffer AJ, Barnes GL, Einhorn TA et al. Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone 2009; 44:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epari DR Duda GN Thompson MS. Mechanobiology of bone healing and regeneration: in vivo models. Proc Inst Mech Eng H 2010;224:1543–1553. [DOI] [PubMed] [Google Scholar]
- Hillman H. Limitations of clinical and biological histology. Med Hypotheses 2000; 54:553–564. [DOI] [PubMed] [Google Scholar]
- Harten RD, Lee FY, Zimmerman MC, Hurowitz E, Arakal R, Behrens FF. Regional and temporal changes in the acoustic properties of fracture callus in secondary bone healing. J Orthop Res 1997; 15:570–576. [DOI] [PubMed] [Google Scholar]
- McDonald MM, Dulai S, Godfrey C, Amanat N, Sztynda T, Little DG. Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone 2008; 43:653–662. [DOI] [PubMed] [Google Scholar]
- Li J, Mori S, Kaji Y, Mashiba T, Kawanishi J, Norimatsu H. Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. J Bone Miner Res 1999; 14:969–979. [DOI] [PubMed] [Google Scholar]
- Amanat N, McDonald M, Godfrey C, Bilston L, Little D. Optimal timing of a single dose of zoledronic acid to increase strength in rat fracture repair. J Bone Miner Res 2007; 22:867–876. [DOI] [PubMed] [Google Scholar]
- Shefelbine SJ, Simon U, Claes L, Gold A, Gabet Y, Bab I et al. Prediction of fracture callus mechanical properties using micro-CT images and voxel-based finite element analysis. Bone 2005; 36:480–488. [DOI] [PubMed] [Google Scholar]
- O'Neill KR, Stutz CM, Mignemi NA, Burns MC, Murry MR, Nyman JS et al. Micro-computed tomography assessment of the progression of fracture healing in mice. Bone 2012; 50:1357–1367. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 2010; 25:1468–1486. [DOI] [PubMed] [Google Scholar]
- Bart ZR, Wallace JM. Microcomputed tomography applications in bone and mineral research. Adv Computed Tomography 2013; 2:121. [Google Scholar]
- Donnelly E. Methods for assessing bone quality: a review. Clin Orthop Relat Res 2011; 469:2128–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AA W, Panjabi MM, Southwick WO. The four biomechanical stages of fracture repair. J Bone Joint Surg Am 1977; 59:188. [PubMed] [Google Scholar]
- Kim N, Lee JG, Song Y, Kim HJ, Yeom JS, Cho G. Evaluation of MRI resolution affecting trabecular bone parameters: determination of acceptable resolution. Magn Reson Med 2012; 67:218–225. [DOI] [PubMed] [Google Scholar]
- Nakahira A, Konishi S, Nishimura F, Iwasaka M, Ueno S. Effect of a high magnetic field on the bioactivity of apatite-based biomaterials. J Appl Phys 2003; 93:8513–8515. [Google Scholar]
- Hayward LNM, CM-J De Bakker, Lusic H, Gerstenfeld LC, Grinstaff MW, Morgan EF-I. MRT letter: contrast-enhanced computed tomographic imaging of soft callus formation in fracture healing. Microsc Res Tech 2012; 75:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Zhang K, Wang Z, Liu Y, Liu X, Wu Z et al. Low-dose, simple, and fast grating-based X-ray phase-contrast imaging. Proc Natl Acad Sci USA 2010; 107:13576–13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte FA, Ruffoni D, Lambers FM, Christen D, Webster DJ, Kuhn G et al. Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. PLoS One 2013; 8:e62172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laperre K, Depypere M, van Gastel N, Torrekens S, Moermans K, Bogaerts R et al. Development of micro-CT protocols for in vivo follow-up of mouse bone architecture without major radiation side effects. Bone 2011; 49:613–622. [DOI] [PubMed] [Google Scholar]
- Boyde A, Bianco P, Portigliatti Barbos M, Ascenzi A. Collagen orientation in compact bone: I. A new method for the determination of the proportion of collagen parallel to the plane of compact bone sections. Metab Bone Dis Relat Res 1984; 5:299–307. [DOI] [PubMed] [Google Scholar]
- Morris MD, Mandair GS. Raman assessment of bone quality. Clin Orthop Relat Res 2011; 469:2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YF, Manjubala I, Schell H, Epari DR, Roschger P, Duda GN et al. Size and habit of mineral particles in bone and mineralized callus during bone healing in sheep. J Bone Miner Res 2010; 25:2029–2038. [DOI] [PubMed] [Google Scholar]
- Pezzuti JA, Morris MD, Bonadio JF, Goldstein SK. Hyperspectral Raman imaging of bone growth and regrowth chemistry. In: Cogswell CJet al. (eds) Three-Dimensional and Multidimensional Microscopy: Image Acquisition and Processing V SPIE: Bellingham, 1998;270–276. [Google Scholar]
- Ouyang H, Sherman PJ, Paschalis EP, Boskey AL, Mendelsohn R. Fourier transform infrared microscopic imaging: effects of estrogen and estrogen deficiency on fracture healing in rat femurs. Appl Spectrosc 2004; 58:1–9. [DOI] [PubMed] [Google Scholar]
- Carden A, Morris MD. Application of vibrational spectroscopy to the study of mineralized tissues (review). J Biomed Opt 2000; 5:259–268. [DOI] [PubMed] [Google Scholar]
- Morris MD. Raman spectroscopy of bone and cartilage. In: Matousek P, Morris MD (eds). Emerging Raman Applications and Techniques in Biomedical and Pharmaceutical Fields Springer: Berlin2010;347–364. [Google Scholar]
- Mendelsohn R, Paschalis EP, Sherman PJ, Boskey AL. IR microscopic imaging of pathological states and fracture healing of bone. Appl Spectrosc 2000; 54:1183–1191. [DOI] [PubMed] [Google Scholar]
- Meganck J, Begun D, McElderry J, Swick A, Kozloff K, Goldstein S et al. Fracture healing with alendronate treatment in the Brtl/+ mouse model of osteogenesis imperfecta. Bone 2013; 56:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juillard A, Falgayrac G, Cortet B, Vieillard M-H, Azaroual N, Hornez J-C et al. Molecular interactions between zoledronic acid and bone: an in vitro Raman microspectroscopic study. Bone 2010; 47:895–904. [DOI] [PubMed] [Google Scholar]
- Brennan TC, Rizzoli R, Ammann P. Selective modification of bone quality by PTH, pamidronate, or raloxifene. J Bone Miner Res 2009; 24:800–808. [DOI] [PubMed] [Google Scholar]
- Manjubala I, Liu Y, Epari DR, Roschger P, Schell H, Fratzl P et al. Spatial and temporal variations of mechanical properties and mineral content of the external callus during bone healing. Bone 2009; 45:185–192. [DOI] [PubMed] [Google Scholar]
- Brüel A, Olsen J, Birkedal H, Risager M, Andreassen TT, Raffalt AC et al. Strontium is incorporated into the fracture callus but does not influence the mechanical strength of healing rat fractures. Calcif Tissue Int 2011; 88:142–152. [DOI] [PubMed] [Google Scholar]
- Oyen ML. Handbook of Nanoindentation: With Biological Applications Pan Stanford Publishing: Singapore, 2010;. [Google Scholar]
- Eckardt I, Hein HJ. Quantitative measurements of the mechanical properties of human bone tissues by scanning acoustic microscopy. Ann Biomed Eng 2001; 29:1043–1047. [DOI] [PubMed] [Google Scholar]
- Leong P, Morgan E. Measurement of fracture callus material properties via nanoindentation. Acta Biomater 2008; 4:1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raum K. Microelastic imaging of bone. IEEE Trans Ultrason Ferroelectr Freq Control 2008; 55:1417–1431. [DOI] [PubMed] [Google Scholar]
- Hube R, Mayr H, Hein W, Raum K. Prediction of biomechanical stability after callus distraction by high resolution scanning acoustic microscopy. Ultrasound Med Biol 2006; 32:1913–1921. [DOI] [PubMed] [Google Scholar]
- Preininger B, Checa S, Molnar FL, Fratzl P, Duda GN, Raum K. Spatial-temporal mapping of bone structural and elastic properties in a sheep model following osteotomy. Ultrasound Med Biol 2011; 37:474–483. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Sacks DJ, Pelis M, Mason ZD, Graves DT, Barrero M et al. Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res 2009; 24:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonna EA. Fracture callus formation in young and old mice observed with polarized light microscopy. Anatomical Record 1964; 150:349–361. [DOI] [PubMed] [Google Scholar]
- Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Camacho NP. Bostrom MPG. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 2007; 41:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]