Abstract
Adaptive immunity to self-antigens causes autoimmune disorders, such as multiple sclerosis, psoriasis and type 1 diabetes; paradoxically, T- and B-cell responses to amyloid-β (Aβ) reduce Alzheimer's disease (AD)-associated pathology and cognitive impairment in mouse models of the disease. The manipulation of adaptive immunity has been a promising therapeutic approach for the treatment of AD, although vaccine and anti-Aβ antibody approaches have proven difficult in patients, thus far. CD4+ T cells have a central role in regulating adaptive immune responses to antigens, and Aβ-specific CD4+ T cells have been shown to reduce AD pathology in mouse models. As these cells may facilitate endogenous mechanisms that counter AD, an evaluation of their abundance before and during AD could provide important insights. Aβ-CD4see is a new assay developed to quantify Aβ-specific CD4+ T cells in human blood, using dendritic cells derived from human pluripotent stem cells. In tests of >50 human subjects Aβ-CD4see showed an age-dependent decline of Aβ-specific CD4+ T cells, which occurs earlier in women than men. In aggregate, men showed a 50% decline in these cells by the age of 70 years, but women reached the same level before the age of 60 years. Notably, women who carried the AD risk marker apolipoproteinE-ɛ4 (ApoE4) showed the earliest decline, with a precipitous drop between 45 and 52 years, when menopause typically begins. Aβ-CD4see requires a standard blood draw and provides a minimally invasive approach for assessing changes in Aβ biology that may reveal AD-related changes in physiology by a decade. Furthermore, CD4see probes can be modified to target any peptide, providing a powerful new tool to isolate antigen-specific CD4+ T cells from human subjects.
Introduction
Alzheimer's disease (AD) is the most common cause of dementia in the elderly, afflicting >5.2 million people in the USA and >30 million worldwide.1,2 In spite of considerable research over the past 25 years, no therapies are available to slow or halt AD.3, 4, 5 The greatest risk factor is increasing age, with ~1% AD risk at age 60 that roughly doubles every 5 years, exceeding 35% by age 85. The ApoE4 allele of a cholesterol transporter further increases lifetime risk to 20% for a single copy and 80% for two copies.6,7 Importantly, women account for 60% of AD cases and have a higher lifetime risk, even after adjusting for longevity differences.8,9
Clinical AD begins with amnestic memory problems that coincide with neuritic plaque and neurofibrillary tangle formation in the medial temporal lobe.10 Neuritic plaques contain insoluble deposits of amyloid-β (Aβ)11 surrounded by dystrophic neurites, reactive astrocytes and activated microglia.12 As the disease progresses, this pathology spreads to other neocortical areas with corresponding aphasia, apraxia, dementia, loss of personality and eventually death.13 In the late 1990s it was discovered that Aβ vaccination alleviates pathological and behavioral features of AD mouse models.14, 15, 16 Subsequent studies established that the benefits of Aβ vaccines could be transferred from mouse to mouse with Aβ-specific CD4+ T cells.17,18 Although a clinical trial of the Aβ vaccine AN1792 was halted in phase II, due to aseptic meningoencephalitis in some subjects,19 adaptive immune responses to Aβ remain a highly promising avenue for AD therapies. Since AN1792, passive immunity has been pursued with anti-Aβ antibodies,20 although no phase III trials have demonstrated sufficient efficacy for US Food and Drug Administration approval.19, 20, 21 Follow-up studies continue with anti-Aβ antibodies as prophylactics that might delay or prevent AD onset in high-risk individuals who carry familial AD mutations associated with early onset AD, such as Presenilin-1 (PSEN1).22, 23, 24 Nonetheless, the translation of any successful therapies from those trials into noncarriers (of familial AD mutations) will require the development of new biomarkers that indicate the earliest possible stage(s) of preclinical AD.25
A key step in immune responses elicited by Aβ vaccination is the proliferation of Aβ-specific CD4+ T cells.17,18 Importantly, proinflammatory CD4+ T cells (TH1) are not necessary as Aβ-specific CD4+ cells with a noninflammatory TH2 profile accounted for most of the beneficial effects in adoptive transfer studies.17,18 Ironically, adjuvant used in the AN1792 trial triggers TH1 responses, and the infiltration of TH1 cells into the brain meninges of a minority of subjects forced the trial to be stopped.19 Nevertheless, AN1792 established that Aβ-specific CD4+ T cells are carried in the immune repertoire of most people, which may facilitate endogenous mechanisms that counter AD. The influence of T cells on cognition is a complex process, but recent studies have suggested several possible mechanisms26, 27, 28 that are consistent with the beneficial effects of Aβ-specific CD4+ T cells in AD in mouse models.17,18 Therefore, assessing Aβ-specific CD4+ T-cell prevalence could provide valuable insights of disease etiology during preclinical AD.
A clinical test for Aβ-specific CD4+ T cells must overcome heterogeneity of human HLADRB alleles and millions of potential T-cell receptor combinations29 to identify a relatively rare population of cells. Briefly, thymic development produces a repertoire of ~50 million different CD4+ T cells, which collectively respond to every possible antigen sequence.30,31 Naive CD4+ T cells circulate through vascular and lymphatic systems in a quiescent state until they encounter dendritic cells (DCs) or other antigen-presenting cells that display their cognate antigen within the context of class II histocompatibility (HLA class II) complexes.32 After an immunological synapse forms with the DC, the T cell begins to proliferate, leading to clonal expansion.33 Newly produced antigen-specific CD4+ T cells leave secondary lymphoid organs and circulate throughout the body, accumulating at sites where the antigen is most abundant.34 Vaccines trigger clonal expansion of CD4+ T cells specific for the inoculated antigen, but aside from post-vaccine periods or during acute infections the proportion of CD4+ T cells specific for any particular antigen represent a small fraction of the entire T-cell repertoire. To increase assay sensitivity for Aβ-specific CD4+ T cells, we developed a new platform, called Aβ-CD4see (Supplementary Figure S1), and used it to evaluate subjects from 35 to 95 years of age, including AD patients. We found that the cells are abundant until middle age and decline thereafter at rates that depend on sex and ApoE4 genotype. Men showed a 50% decline by the age of 70, but women showed an earlier decline, reaching 50% before the age of 60. The presence of an ApoE4 allele accelerated the decline with women carriers showing a precipitous loss of Aβ-specific CD4+ T cells between 45 and 52 years of age, when menopause typically starts.
Materials and methods
Stem cells and reagents
H9 (National Stem Cell Bank code WA09, passage 23) human embryonic stem cell (hESC) lines were maintained in mTeSR media with 5 × supplement (Stem Cell Technologies, Vancouver, BC, Canada), supplemented with additional basic fibroblast growth factor (4 μg ml−1, Life Technologies, Carlsbad, CA, USA). Bone marrow stromal cells OP9 (ATCC, Manassas, VA, USA) were maintained in gelatinized (G1393, Sigma, St Louis, MO, USA) T75 flasks in the OP9 growth medium (OP9M: α-MEM (Life Technologies) with 20% fetal bovine serum (FBS; Hyclone, Logan, UT, USA). Hematopoietic stem cell (HSC) differentiation medium (HDM: α-MEM, 10% FBS, 100 μM monothioglycerol) was used to induce initial hematopoietic differentiation. Myeloid and dendritic cells were maintained in pHEMA-coated (Sigma) T25 flasks. Media used were as follows: myeloid differentiation medium (α-MEM, 10% FBS, 100 ng ml−1 ganulocyte-macrophage colony-stimulating factor (GM-CSF), 100 μM monothioglycerol) was also used to expand myeloid cell numbers; DC differentiation medium (DDM: Stem Span SFEM medium (Stem Cell Technologies), Excyte growth supplement (Millipore, Temecula, CA, USA), 100 ng ml−1 GM-CSF, 100 ng ml−1 interleukin (IL)4 (Endogen, Waltham, MA, USA); DC maturation was induced using DDM supplemented with 100 ng ml−1 tumor necrosis factor (TNF)-α (PeproTech, Rocky Hill, NJ, USA) and 250 ng ml−1 lipopolysaccharides (LPS; Sigma); IL2 (Life Technologies), influenza HA peptide (amino acids 126–138, H-HNTNGVTAACSHE-OH; Anaspec, San Francisco, CA, USA) and Aβ1-42 (BioMer Technology, San Francisco, CA, USA) were used as indicated. Most chemicals used were from Sigma, except type IV collagenase and trypsin-EDTA (Life Technologies). Magnetic beads and reagents were from Miltenyi (San Diego, CA, USA).
Antibodies for the following human antigens were used for flow cytometry: Non conjugated SSEA4 (Abcam, Cambridge, MA, USA), CD34-PE, CD45-FITC CD14-FITC, DCsign-PE, CD80-FITC, CD4-Alexafluro647 (BD Biosciences, San Jose, CA, USA) and DCsign-APC, CD86-PE, HLA-DR-PECy7, CD43-APC, CD11b-APC (eBioscience, San Diego, CA, USA); isotype control antibodies were obtained from the same company as the primary antibody. T cells and DC-labeling reagents were: CFDA/CFSE, CM-Dil, Vybrant DiO (Life Technologies). Phagocytic activity was assessed with fluorescent latex beads (Sigma) that were coated with human AB serum (Sigma).
hESC maintenance
Pluripotent H9 cells were maintained in as colonies in matrigel-coated T25 flasks. H9 cells were fed with complete mTeSR medium, supplemented with basic fibroblast growth factor (20 μg ml−1), every other day. Colonies were picked for maintenance and differentiation 5–7 days after plating.
HSC differentiation of H9 cells
The differentiation of hESC into HSCs was induced by coculturing them with OP9 stromal cells.35 Briefly, undifferentiated hESCs were harvested by gentle scrapping and collected them into 15 ml tube followed by the centrifugation with 300 g for 5 min. The pellet was resuspended in HDM. The optimal confluence of OP9 cells was found to be ~70%, and not over-confluent as reported by others.35 Before harvesting H9 cells for differentiation, flasks of OP9 cells were rinsed and pre-incubated with HDM. HDM containing hESC clumps were added directly to the flasks. On day 4, all HDM was replaced with fresh HDM, and on days 6 and 8 half of the HDM was replaced with fresh HDM.
Myeloid expansion
HSC-containing colonies were harvested by the treatment with collagenase IV (1 mg ml−1) in KnockOut DMEM/F-12 (Life Technologies) for 20 min at 37 °C, followed by treatment with 0.05% trypsin-EDTA for 15 min at 37 °C. Trypsin activity was quenched with 10% FBS, pelleted (300 g), resuspended in myeloid differentiation medium and plated in pHEMA-coated flasks. Myeloid expansion was carried out for 10–12 days.
DC culture
For generating myeloid DCs after myeloid expansion, cells were filtered using a 70-μm strainer and fractioned with 25% Percoll to remove dead cells and cell aggregates. Cells were rinsed with 5% FBS in phosphate-buffered saline (PBS) and replated in DDM for 10 days. Half the media volume was replaced every 4 days with fresh DDM. To expand the number of immature DCs (iDCs), they were split and maintained under the same conditions. DC maturation was induced in DDM supplemented with TNF-α (100 ng ml−1) and LPS (250 ng ml−1) for 3 days.
Phenotypic analysis of iDCs and mature DCs
Hematoxylin and eosin (H&E) staining was done using fixed cells (2% paraformaldehyde) that were spread onto glass slides by cytospin. Slides with iDCs and mature DCs were covered by H&E solution and incubated for 15 min, followed by treatment with acid and bluing solutions (Vector Laboratories, Burlingame, CA, USA). Slides were rinsed with water, run through and alcohol series (50%, 70%, 95% and 100% ethanol) and coverslips were mounted with Permount (Fisher, Waltham, MA, USA). Images of H&E staining were captured with a color charge-coupled device camera mounted on a Nikon microscope (Nikon, Melville, NY, USA).
Phagocytic activity of iDC
Carboxylate-modified red fluorescent latex beads with a mean diameter of 2 μm (L3030; Sigma) were opsonized in 50% human AB serum with PBS for 30 min at 37 °C. After incubation, these opsonized latex beads were added to the DC cultures and incubated with gentle shaking for 30 min at 37 °C. An identical control was incubated at 4 °C. After 30 min, phagocytosis was halted by adding 2 ml of ice cold PBS, followed by washing the cells twice with ice cold PBS. DCs were resuspended in 1 ml of cold PBS and fixed by adding an equal volume of 4% paraformaldehyde, and kept in the dark at 4 °C until imaging.
Proportional balancing studies of CD14:DCsign
For proportional balancing studies, iDCs were purified using anti-CD14 or anti-DCsign magnetic beads. Briefly, DCs were filtered with a70-μm strainer to remove aggregates and purified with myeloid dendritic cell kits for separating CD14-expressing cells followed by the MACS column (Miltenyi) purification. Isolated cells were cultured for 4 weeks in DDM. To enrich for isolation of DCsignhi cells, we used a human DCsign kit (Miltenyi) to purify the cells and they were also expanded for 4 weeks in DDM.
Isolation and activation of CD4+ T cells
Whole blood (20 ml) was drawn from subjects in EDTA-containing blood tubes (#366643, BD Biosciences). Under sterile conditions the blood sample was diluted to 1:1 with PBS, layered (40 ml) on top of 30 ml of Ficoll-hypaque and spun at 400 g for 30 min. The peripheral blood lymphocytes layer was collected (Figure 4a) and diluted with PBS (1:7) followed by the centrifugation at 300 g for 10 min. Cells were rinsed twice more by resuspension in PBS. Viable cells were counted by Trypan blue exclusion. Adherent cells were removed to enrich for T cells with sterile nylon wool columns. Briefly, the nylon wool column was washed with T-cell medium (1640 RPMI with 10% FBS) and incubated at 37 °C for 1 h. Peripheral blood lymphocytes (107 cells ml−1) were added to the pre-warmed column and incubated for 1 h at 37 °C, after which non-adherent cells were collected by opening the column's stopcock. The column was washed twice with 5 ml of T-cell medium to collect total effluent. T-cell-enriched fractions were incubated with carboxyfluorescein diacetate succinimidyl ester (CFSE) solution (4 μM in PBS) for 8 min at 37 °C water bath. After incubation, the solution was diluted with 10 volumes of T-cell medium, pelleted by centrifugation (300 g, 10 min) and washed twice with PBS containing 0.1% bovine serum albumin. CFSE-labeled cells were resuspended, counted and a fraction was used to evaluate CFSE fluorescence by flow cytometry (Accuri division of BD Biosciences, San Jose, CA, USA). Prior to flow cytometry cells were immunostained with Alexa647-conjugated anti-CD4 antibody (BD Biosciences), to allow for analysis of CFSE fluorescence in the CD4+ population. Further flow cytometric data and histogram analyses were done using FCS express 4 flow cytometry software (De Novo Software, Los Angeles, CA, USA).
Imaging islands of activation
DCs were fluorescently labeled by incubating them in pre-warmed serum-free media containing Dil (2 μM, Life Technologies) for 20 min at 37 °C and isolated T cells were labeled with DiO (1 μM) in the same manner. After incubation, 10 × volumes of T-cell medium was added and cells were pelleted at 300 g for 5 min. Cells were then washed twice with 0.1% bovine serum albumin in PBS. Fluorescently labeled DCs (red) and T cells (green) were mixed and cultured in a pHEMA-coated flask overnight (16 h), then fixed by adding an equal volume of 4% paraformaldehyde and let to stand for 30 min. Nuclei were labeled with 4′,6-diamidino-2-phenylindole by adding it directly to the fixing solution (20 μM final). The cells were not washed as centrifugation and resuspension may disrupt the islands of activation, so islands of activation were picked with a microscope and placed in a cover glass bottomed chamber for imaging. Images of islands of activation were captured with a Nikon C1 confocal microscope (Nikon).
Human subjects
All procedures involving human subjects were performed under approved Institutional Review Board Protocols from the Western University of Health Sciences (Pomona, CA, USA), Arrowhead Regional Medical Center (Colton, CA, USA) and Casa Colina (Pomona, CA, USA). Informed consent forms were explained to all participants and signed by all subjects and/or legal guardians in the case of subjects with a presumptive diagnosis of AD. Family members of Subject D also signed an amendment to the informed consent stating agreeing to the presentation of age and sex data in Figure 7; as presented it may have allowed members of that family to determine the results of other family members, so they agreed to that possibility of disclosure.
ApoE genotyping
Genomic DNA was isolated from 0.2 ml of blood from each subject using DNeasy (Qiagen, Germantown, MD, USA). Real-time PCR was used to determine ApoE-ɛ4 genotype, as previously described.36 Briefly, three primer sets specific for ApoE-ɛ2, -ɛ3 and -ɛ4 were run with each sample, and genotype was determined by threshold (Ct). That is a perfect nucleotide sequence match with the oligos decreases Ct and indicated the presence of the corresponding allele.
Influenza study
Volunteers with the intention of receiving an influenza vaccine were recruited to the study and signed informed consent forms. All procedures were done with an approved Institutional Review Board protocol. Each subject has previously been vaccinated for influenza >2 years before this study. Subjects agreed to have whole blood drawn (20 ml) before receiving the influenza vaccine (one adult dose of FLULAVAL, GSK, Brentford, UK) by intramuscular injection. A second sample of blood was drawn (20 ml) from each subject 1 week later. Whole blood isolated from all subjects was processed immediately, as described above. DCs were preloaded with 10 mg ml−1 of an immunogenic peptide from influenza HA (amino acids 126–138, H-HNTNGVTAACSHE-OH; Anaspec) for 2–3 days. Isolated T cells were cocultured with mature DCs that had been preloaded with influenza peptide37 as iDCs, control DCs, were not loaded with peptide. CFSE analysis of CD4+ T-cell proliferation was done as described above.
Alzheimer's study
Subjects with a presumptive diagnosis of AD were recruited from WesternU families, outpatients at Casa Colina Rehabilitation Hospital and inpatients at Arrowhead Regional Medical Center. Control subjects, with no signs of dementia or memory impairment were recruited from the WesternU community. Whole blood (20 ml) was drawn and processed as above to yield T cells for assay. T cells were cocultured with lentivirus-infected DCs, and CD4+ proliferation was assessed as described above.
Results
The Aβ-CD4see method stimulates the proliferation of Aβ-specific CD4+ T cells by coculture with hESC-derived DCs. To provide a consistent source of potent DCs, hESCs were differentiated into HSCs, common myeloid progenitors and immature DCs, which were then modified to express Aβ/HLADRA fusion proteins. The proliferation of primary CD4+ T cells was monitored with flow cytometry to evaluate Aβ-specific responses in healthy subjects and AD patients.
DC differentiation
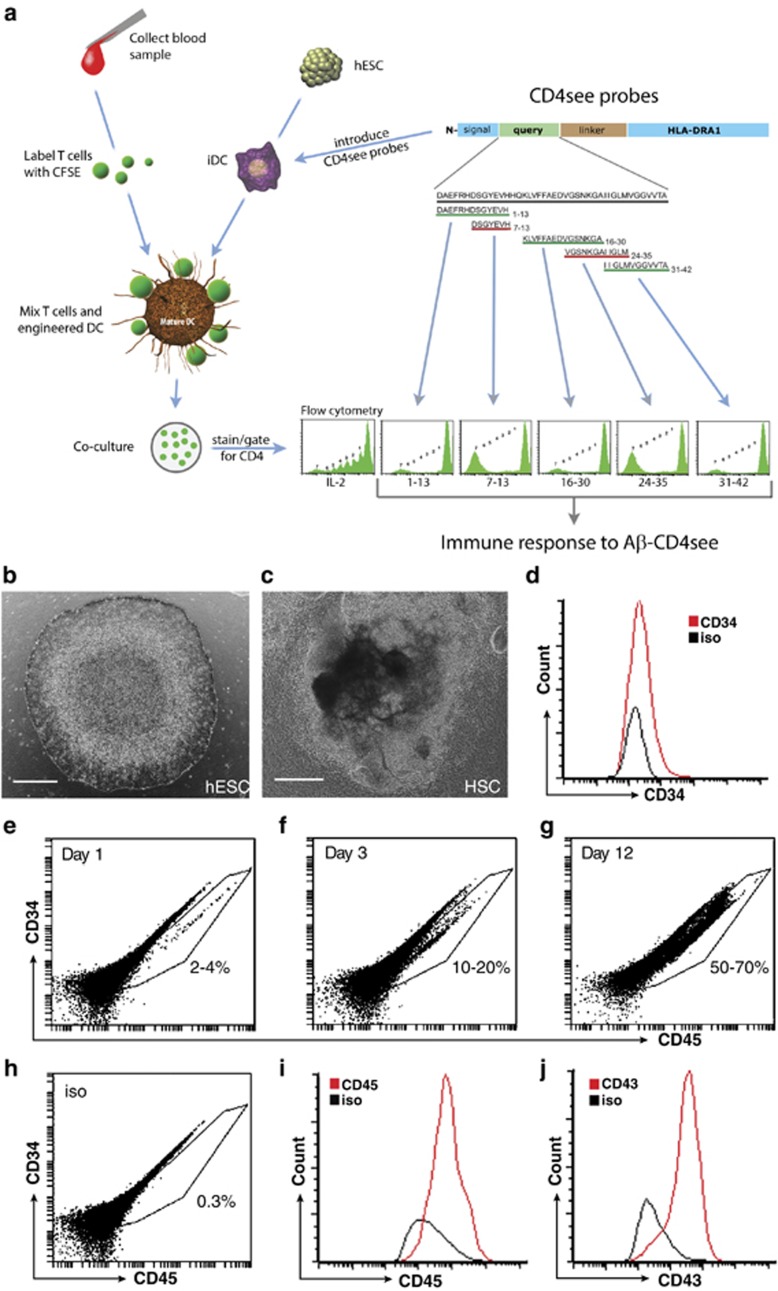
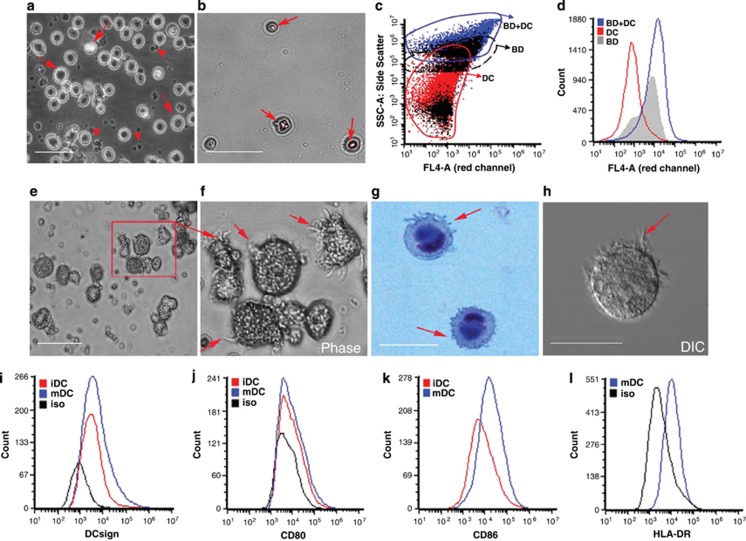
Myeloid DCs were differentiated from hESCs with a multistep process that took ~7 weeks. Pluripotent H9 hESCs (Figure 1a) were scraped into clumps and cocultured with OP9 stromal cells for 10–12 days to yield HSCs. After 8 days, colony morphologies were distinctly different from the smooth margins of pluripotent hESC colonies (Figure 1b). Expression analysis of cells isolated from HSC colonies confirmed CD34 expression (Figure 1c). Common myeloid progenitors (CMPs) were differentiated from HSCs by culturing cell suspensions with GM-CSF. Surface expression of HSC markers, such as CD34, decreased while CMP markers increased with time, including CD45 (Figures 1d–h), CD43 (Figure 1i), CD14 and CD11b (Supplementary Figure S1). iDCs were generated by culturing CMPs with GM-CSF and IL4. iDCs had wrinkled morphologies (Figure 2a), and expressed of DCsign, CD80 and CD86 (Figures 2i–k). An important behavior of iDCs is the phagocytosis of opsonized particles, which we tested in hESC-derived DCs with human Ig-coated fluorescent latex beads (Figures 2b–d).
Figure 1.
Differentiation of human embryonic stem cells (hESCs) into common myeloid progenitors (CMPs). (a) Photomicrograph of a pluripotent hESC (H9) colony with smooth margins. (b) Hematopoietic stem cell (HSC)-containing colony after 8 days of coculture. (c) Flow cytometry showing CD34+ cells (red) isolated from colonies after 8 days of coculture, isotype control is black. (d–f) Flow cytometry dot plots of CD34 and CD45 expression in HSC cultured with ganulocyte-macrophage colony-stimulating factor after 1, 3 and 12 days. (d) The CD34lo/CD45hi population increased from 2–4% on day 1 to (e) 10–30% by day 3 and (f) 50–70% by day 12. (g) Example of isotype control for d–f. (h) Dot plot and (i) histogram of CD45+ staining in CMPs after 12 days. (j) Histogram of CD43+ immunostaining in CMPs after 12 days. Scale bars, 100 μm. Aβ, amyloid-β CFSE, carboxyfluorescein diacetate succinimidyl ester; DC, dendritic cell; IL, interleukin.
Figure 2.
Morphology and functional activity of human embryonic stem cell-derived dendritic cells (DCs). (a) After 10 days of culture with ganulocyte-macrophage colony-stimulating factor and interleukin (IL)4 a significant proportion of cells differentiated into immature DCs (iDCs), which were larger and brighter (arrows) than undifferentiated CMP (arrowheads). Scale bar, 25 μm for a, b and e. (b) Photomicrographs showing phagocytosis of carboxylate-modified red fluorescent latex beads (2 μm size) by iDC. Fluorescent overlay of bright field image showing fluorescent beads within iDC (arrows). (c) Dot plot of FSC/SSC showing iDC with internalized beads. (d) Histogram of flow cytometry showing red fluorescent beads internalized by iDC (FL4 channel). (e) Image of iDC that were stimulated with TNF-α and LPS for 72 h. (f) Inset of cells boxed in b, arrows indicate long processes and mature DC (mDC) morphology. (g) DIC image of mature DC shown using Hoffman optics. (h) Hematoxylin staining of mature DC showing long dendritic processes (arrows) and dark purple nuclei. Scale bar, 10 μm. (i) Flow cytometry showing DCsign immunostaining of iDC with (red) and without (blue) tumor necrosis factor (TNF)-α- and lipopolysaccharide (LPS)-induced maturation. Scale bar, 10 μm. (j) Flow cytometry of showing increased CD80+ immunostaining following DC maturation. (k) CD86+ immunostaining after TNF-α and LPS induced maturation. (l) Surface expression of HLA-DR on mature DCs.
Upon stimulation with TNF-α and LPS, iDCs developed branched dendritic processes—a sign of DC maturation (Figures 2f–h). Flow cytometry confirmed that both iDCs and mature DCs expressed DCsign (Figure 2i), CD80 (Figure 2j), CD86 (Figure 2k) and CD11c. Surface expression of HLA class II increased with DC maturation (Figure 2l).
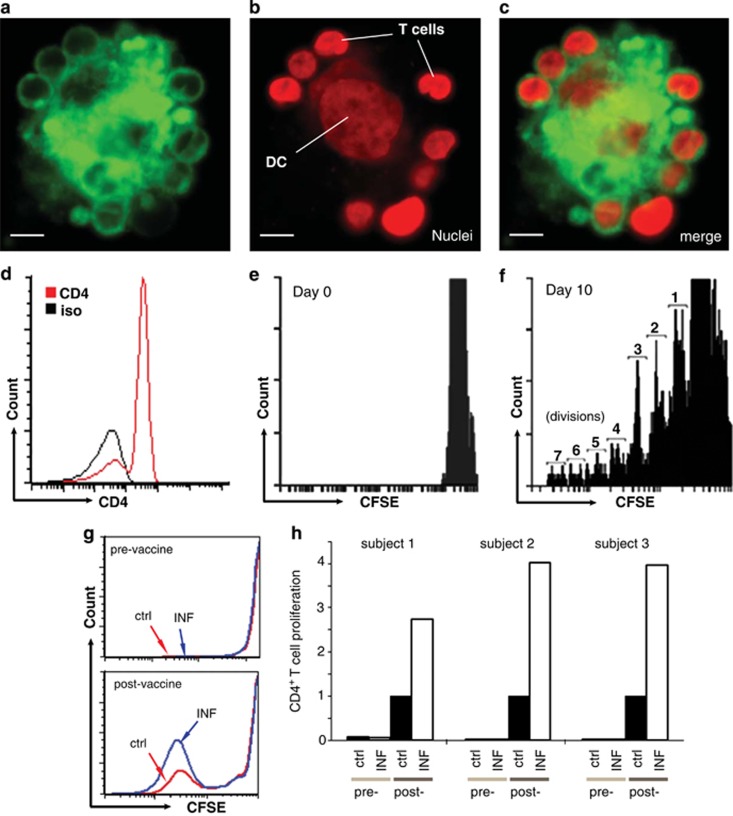
To establish whether hESC-derived DCs could stimulate antigen-specific CD4+ T-cell proliferation, we analyzed influenza-specific T cells in human subjects before and after they received a commercial influenza vaccine. T cells were isolated from whole blood, labeled with CFSE and cocultured with influenza peptide-loaded DCs37 that were then matured with TNF-α/LPS (Figures 3a–c). CD4+ T-cell proliferation was assessed by gating on CD4+ cells (Figure 3d) and assessing CFSE fluorescence to determine cell divisions (Figures 3d and f). Pre-vaccine T cells showed little proliferation in response to control or influenza-peptide-loaded DCs (Figure 3g). However, T cells isolated from subjects 1 week after vaccination showed robust proliferation in response to influenza-peptide-loaded DCs, which was more than that with control DCs (Figures 3g, h). The increase of CD4+ T-cell proliferation in influenza-peptide-loaded DCs over control DCs was consistent in three different subjects, indicating the proliferation of influenza-specific CD4+ T cells (Figure 3h). These results established the competence of hESC-derived DCs to stimulate primary CD4+ T cells in an antigen-specific manner.
Figure 3.
Coculture of human embryonic stem cell-derived dendritic cells (DCs) with primary T cells. (a) DiI-labeled T cells mixed surrounding a DC. (b) Nuclear staining of cells with 4′,6-diamidino-2-phenylindole, pseudo-colored red for contrast, showing large DC nucleus in the center surrounded by smaller nuclei of T cells around the perimeter. (c) Merged image of a and b. Scale bar, 20 μm. (d) Flow cytometry histogram of CD4+ population before coculture. (e) Histogram of carboxyfluorescein diacetate succinimidyl ester (CSFE) staining of CD4+ T cells before coculture. (f) Histogram of CFSE intensity in CD4+ T-cell population 10 days after coculture with control DCs and IL2. This condition was used as a positive control to establish CFSE fluorescence levels for 1–7 cell divisions in each assay, as indicated. (g) Histograms of CFSE fluorescence in CD4+ T cells after coculture with control (red) or influenza-peptide-preloaded (blue) DC. Top shows CFSE responses in T cells before vaccination, and bottom shows proliferation of T cells isolated from the same subject 1 week after receiving an influenza vaccine. (h) Pre- and post-vaccine responses of CD4+ T cells in three subjects, before and 1week after receiving an influenza vaccine, after coculture with control (ctrl, black) or influenza-peptide-preloaded (white) DCs. The y axis indicates normalized values of proliferating CD4+ T cells that divided five or more times. Values for each subject were normalized to post-vaccine CD4+ T cells cocultured with control DCs.
Aβ-CD4see probes: development and testing
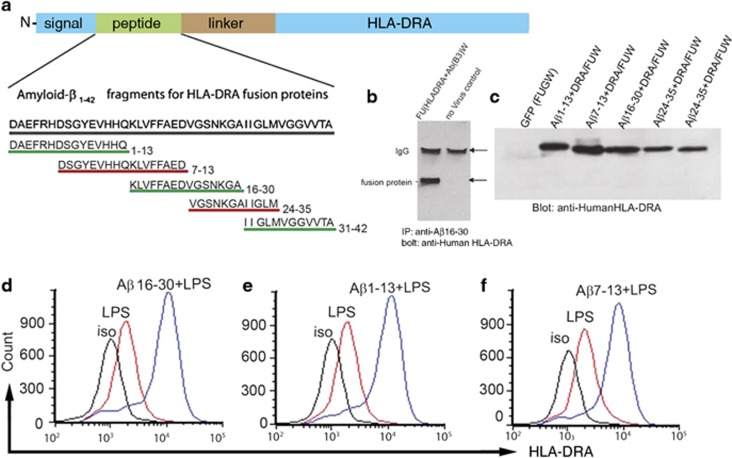
The proportion of Aβ-specific CD4+ T cells in most people is a vanishingly small proportion of the immune repertoire, so to enhance the proportion of class II complexes loaded with fragments of Aβ we developed recombinant probes (Figure 4a). Fragments of Aβ (7–16 amino acids long) were linked to the α-chain of human class II (HLADRA) by a serine/glycine linker, to generate Aβ-CD4see probes. The HLADRA allele used is carried by most of the human population.29
Figure 4.
Assembly and testing of CD4see probes. (a) Schematic illustration of amyloid-β (Aβ)-CD4see probes design. The base construct consisted of the signal peptide from HLADRA1, a query peptide site, a glysine/serine linker and the remainder of the HLA-DRA1 coding sequence. Query peptides for Aβ consisted of 5 fragments from human Aβ or human Aβ1-42 as indicated. (b) Whole cells lysates from 293 cells were subjected to immunoprecipitation with anti-Aβ peptide specific for residues 16–30, followed by immunoblotting with anti-HLADRA antibody. On the left cells were infected with lentivirus containing Aβ16-30/HLADRA fusion protein, the lower band is the size expected for the fusion protein. On the right control cells were processed as a negative control. (c) Anti-HLARDA immunoblot of lysates from 293 cells infected with lentiviruses containing fusion proteins indicated. (d–f) Representative flow cytometry histograms of HLADRA immunostaining in KG1 cells infected with lentiviruses that contained (d) Aβ16-30/HLADRA, (e) Aβ1-13/HLADRA, or (f) Aβ7-13/HLADRA fusion proteins. Uninfected KG1 cells stimulated with LPS are shown in red, fusion protein infected KG1 cells stimulated with lipopolysaccharides (LPS) are shown in blue.
To confirm that fusion proteins contained epitopes from both Aβ and HLADRA, Aβ16-30/HLADRA was expressed in HEK293 cells and whole-cell lysates were immunoprecipitated with anti-Aβ antibody, followed by immunoblotting with anti-HLADRA antibody (Figure 4b). Anti-HLADRA immunoblots confirmed that all Aβ-CD4see probes were expressed in human cells with molecular weights that corresponded to the Aβ fragments inserted (Figure 4c). Aβ-CD4see probes were packaged in a lentiviral vector (FUW)38 and delivered to an immortalized human DC line (KG1); flow cytometry confirmed that all probes were presented on the surface of LPS-activated KG1 (Figures 4d–f). KG1 infected with Aβ-CD4see probes showed a fourfold increase in HLADRA immunostaining, indicating that up to 80% of the class II complexes contained fusion protein.
Testing of Aβ-CD4see with clinical samples
hESC-derived DCs were infected with lentiviruses containing one of five Aβ-CD4see probes, matured with LPS/TNF-α and cocultured with T cells isolated from freshly drawn blood. Primary T cells were preloaded with CFSE to assess cell divisions in the CD4+ population using flow cytometry, as above. This procedure was used to assess the proliferation of Aβ-specific CD4+ T cells in human subjects from 35 to 95 years of age, including several AD patients.
Aβ-specific CD4+ T cells decline with age and AD
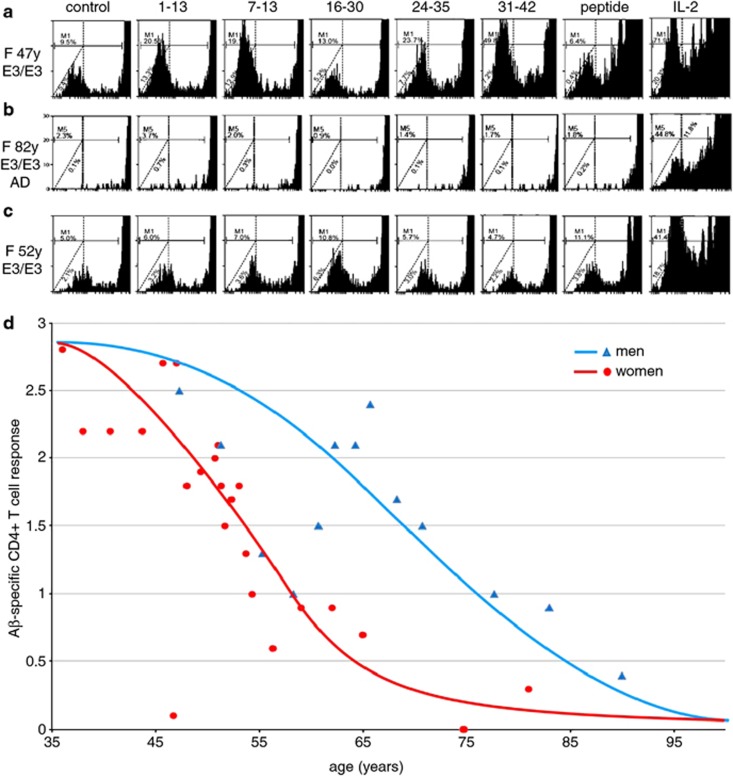
Overall, young and middle-age subjects showed robust CD4+ T-cell responses to Aβ-CD4see probes (Figure 5a). In contrast, subjects with a presumptive diagnosis of AD had undetectable levels of Aβ-specific CD4+ T cells (Figure 5b). Men showed a decline in the number of these cells reaching about 50% of the levels in 35-year-old men by the age of 70 (Figure 5d). Interestingly, women showed an earlier decline, reaching 50% reduction before the age of 60 (Figure 5c).
Figure 5.
Amyloid-β (Aβ)-CD4see responses in men and women. (a) Example of CD4+ T-cell proliferation in a middle-age women, 47 years old, ApoE3/3. Cells were cocultured with dendritic cells (DCs; left to right) that were control, infected with fusion proteins containing Aβ1-13, Aβ7-13, Aβ16-30, Aβ24-35, Aβ31-42, preloaded with human Aβ1-42 peptide, or cultured with control DCs and interleukin (IL)2. Note proliferation in response to probes 1–13, 7–13, 24–35 and 31–42. (b) The same assay in an 82-year-old woman with Alzheimer's disease, ApoE3/3, showed little T-cell response. (c) The same assay in a 52-year-old woman, ApoE3/3, showed diminished responses compared to the younger woman in a, but more stronger responses compared with the older woman in b. (d) Scatter plot of age versus Mean Aβ-CD4+ T-cell responses in men (triangles) and women (circles) from 35 to 95 years of age. Markers represent mean age and mean CD4see score of three subjects in the same group. The blue trend line indicates the curve of best fit for men (n=15 subjects) and the red line indicates the curve of best fit for women (n=32 subjects).
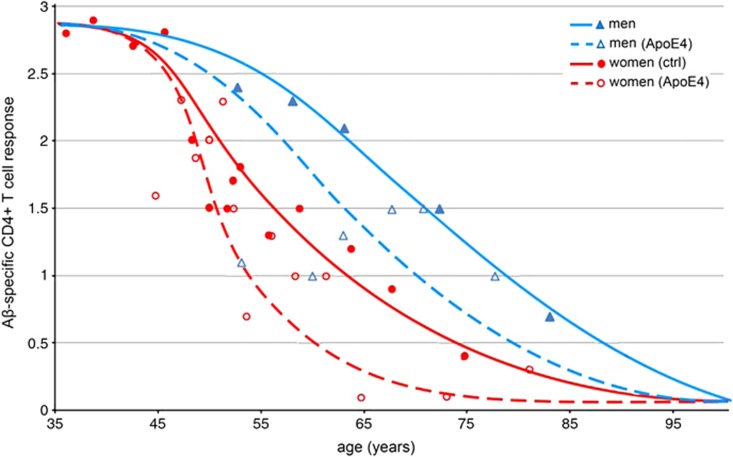
ApoE4 accelerates the decline in Aβ-specific CD4+ T cells
We tested 32 women over the age of 35 years, including 16 ApoE4 carriers and 16 noncarriers. Trend lines for women based on ApoE4 genotype revealed a 50% decline in the number of Aβ-specific CD4+ T cells by the age of 51 for carriers and 58 for noncarriers (Figure 6). A sharp decline was seen in women who carried ApoE4 in the mid-40s to early-50s, an age that is notable for the commencement of menopause. It is worth noting that the loss of estrogen, associated with menopause, has been suggested to elevate AD risk in women.8,9 This early decline in women, and particularly in perimenopausal women who carried ApoE4, is consistent with epidemiological evidence showing that postmenopausal women with ApoE4 are the largest high-risk group in the population. Our analysis also included 15 men over the age for 40, including seven ApoE-ɛ4 carriers. Men with ApoE4 also showed an earlier decline in Aβ-specific CD4+ T cells than noncarriers. Substantially, more variability in the Aβ-CD4see responses was seen in ApoE4 carriers of both sexes compared with noncarriers of either sex. These results show an age-dependent decline of Aβ-specific CD4+ T cells that is influenced by sex and ApoE genotype. Diminishing numbers of Aβ-specific CD4+ T cells may be an early event in preclinical AD that reflects the changing Aβ physiology and could be useful as a diagnostic.
Figure 6.
Impact of ApoE4 on declining amyloid-β (Aβ)-specific CD4+ T cell responses. Scatter plot of age versus Aβ-CD4+ T-cell response in men and women, with ApoE4 carrier status indicated. Women who carried an ApoE4 allele (hollow circles and dashed red trend line) showed the earliest decline in Aβ-specific CD4+ T cells. Women who did not carry the allele (solid circles and red trend line) showed a more gradual decline than women carriers. Values for men who carried at least one copy of ApoE4 (hollow triangles and dashed blue trend line) were below noncarrier men (solid triangles and solid blue trend line), with outliers that had much lower responses.
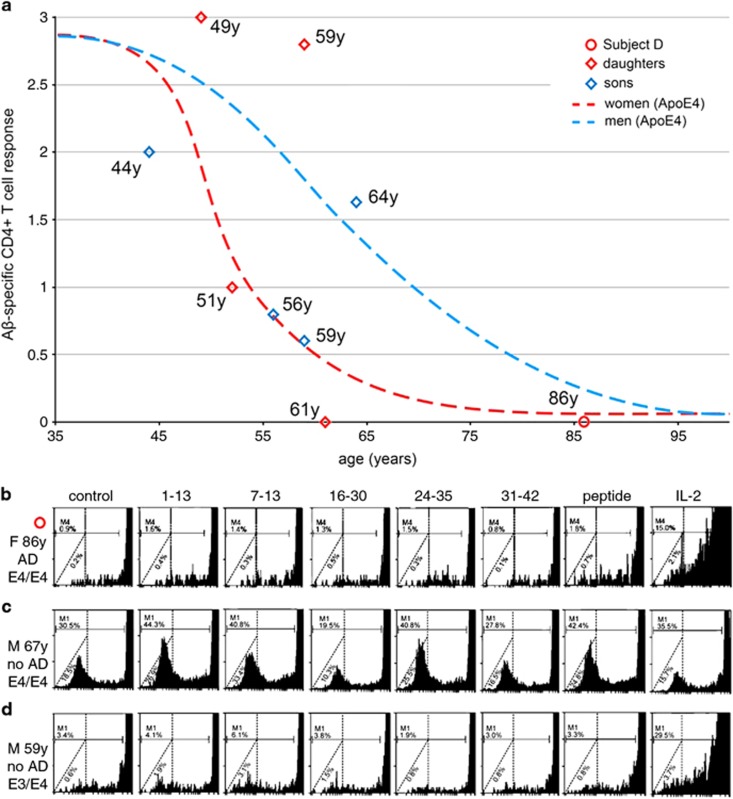
Aβ-CD4see evaluation of individual subjects
Our study included a family of nine subjects who all carried at least one ApoE4 allele. The 86-year-old matriarch (Subject D) began showing clinical signs of AD in her early 70s and was in the late stages of the disease at the time of testing. Subject D showed no detectable Aβ-specific CD4+ T-cell response (Figures 7a and b). She carried two copies of ApoE4, and each of her four adult daughters and four adult sons carried a single copy. Two daughters (aged 51 and 61 years) and three sons (aged 44, 56 and 59 years) showed Aβ-CD4see responses near or below the trend line for women with ApoE4, and well below the trend line for men with ApoE4. The eldest sibling, a 64-year-old man, had an Aβ-CD4see response near the trend line for men of his age, with a response that was above his younger brothers (for their respective ages); notably, this man had suffered a heart attack 4 years earlier (age 60) and was taking a blood thinner (Plavix) and statins, which are a class of cholesterol-lowering drugs that has been suggested to decrease AD risk.39, 40, 41, 42 Two sisters, aged 49 and 59, also showed strong Aβ-CD4see responses that were above trend lines for both women and men, at those ages (Figure 7a). In summary, two of Subject D's eight adult children had Aβ-CD4see responses that were above trend lines for their respective age and sex. One son had was near the trend line for men with ApoE4, and the remaining five siblings had Aβ-specific CD4+ T-cell levels near or below the trend line for women who carried ApoE4. Given the potential benefits of Aβ-specific CD4+ T cells in countering AD pathology, individuals with higher levels of these cells may have a greater resistance to AD, especially those who carry ApoE4. Although this allele correlates with higher AD risk, ApoE4 is not in itself a reliable predictor of AD onset, and we observed considerable variability in the abundance of Aβ-specific CD4+ T cells between carriers and noncarriers. For example, our study included a 67-year-old man with two copies of ApoE4, who held a highly technical position and showed no signs of memory impairment at the time of testing (Figure 7c). By way of contrast, a 59-year-old woman with one copy of ApoE4 and no signs of memory impairment, showed low levels of Aβ-specific CD4+ T cells, which may be an indication of higher risk for preclinical AD (Figure 7d). Aβ-CD4see analysis could provide an indicator of immunological changes that reflect preclinical AD, particularly for carriers of the ApoE4 allele. Five-year follow-ups are planned for study participants.
Figure 7.
Comparison of individual amyloid-β (Aβ)-CD4see responses in an Alzheimer's disease (AD) affected family. (a) Aβ-specific CD4+ T-cell responses for an 86-year-old woman with AD (Subject D, red circle) and her eight adult children (hollow diamonds). Trend lines for ApoE4-positive men and women are shown for reference. The daughters (red hollow diamonds) were 49, 51, 59 and 61 years old at the time of testing. The sons (blue hollow diamonds) were 44, 56, 59 and 64 years old when tested. None of Subject D's children showed signs of dementia or memory impairment when evaluated. (b) CD4+ proliferation in subject D. (c) Aβ-CD4see responses in a healthy 67-year-old man, unrelated to Subject D, who carried two copies of ApoE4. (d) A 52-year-old woman, unrelated to subject D, who had one copy of ApoE4, and a diminished response to Aβ-CD4see probes.
Discussion
We have described a protocol to assess the abundance of Aβ-specific CD4+ T cells using a modest volume of whole blood. The combination of hESC-derived DC and Aβ-CD4see probes is sufficiently sensitive to detect these cells in human subjects without prior Aβ vaccination. Mouse studies have established that Aβ-specific CD4+ T cells can attenuate AD progression and pathology, but the assessment of these cells in patients has lagged due to the lack of a sufficiently sensitive clinical assay. Aβ-CD4see provides this tool and in our evaluation of >50 subjects we have found that these cells are abundant up to middle age, and decline thereafter. Age-associated declines in immune function are well known, but the relative impact of diminished DC and T-cell function on that decline has remained unclear.43,44 We used hESC-derived DCs as a consistent source of potent antigen-presenting cells, so the decline we observed was due to fewer Aβ-specific CD4+ T cells in older subjects and not DC insufficiency.45 The timing and rate of decline was sex dependent with women reaching a 50% reduction before the age of 60, whereas men did not reach that level until 70 years of age. These findings are consistent with a substantial body of prior work showing that women have a higher overall risk for AD than men, even after accounting for longevity differences.8,9 Both mouse and human studies have implicated menopause-related hormone deficiencies in this elevated risk profile.46,47 Specifically, menopause causes rapid and significant decline in estrogen and progesterone, which could contribute to AD pathology;48 further, estrogen levels significantly impact adaptive immunity.49 Indeed, the earliest decline of Aβ-specific CD4+ T cells occurred in women who carried ApoE4, reaching 50% of maximal around the age of 50, within the perimenopausal period for most women. ApoE alleles vary by arginine and cysteine residues at positions 112 and 158, which impact ApoE-mediated Aβ clearance from the brain,50, 51, 52, 53 although it is also possible that indirect effects on hormone levels and/or immune responses could be involved. ApoE4 carriers are the largest at-risk group for AD, with >7 million women over the age of 50 who carry the allele, in the USA alone. This correlation between loss of Aβ-specific CD4+ T cells and the perimenopausal period warrants further investigation.
An evaluation of Aβ-specific CD4+ T-cell abundance may provide a diagnostic indicator of immune changes that correlate with preclinical AD. Although this study was comprised of a modest sample size, it was sufficient to discern trend lines for the age-dependent decline of Aβ-specific CD4+ T cells in men and women, with and without ApoE4. Evaluating the responses of individual subjects in relation to those trend lines established that Aβ-specific CD4+ T-cell abundance varies between individuals, even within the same family. Our study benefited from the participation of an 86-year-old woman with AD, and eight of her adult children between the ages of 44 and 64. All of the siblings were ApoE3/4, but the decline in Aβ-specific CD4+ T cells varied by individual. Although the responses of most siblings plotted closely to the trend line for women with ApoE4, the eldest brother and two sisters showed higher levels of Aβ-specific CD4+ T cells. In light of that brother's statin use, it would be informative to determine how those drugs affect the abundance of Aβ-specific CD4+ T cells in a larger sample of subjects.
CD4see is superior to current strategies for enumerating antigen-specific CD4+ T cells such as the use of MHC/peptide tetramers. Whereas tetramers mark antigen-specific CD4+ cells for detection with flow cytometry, CD4see sustains the proliferation of a discrete population of CD4+ T cells, resulting in more functional specificity and a lower signal-to-noise ratio. For example, quiescent CD4+ T cells that bear an Aβ-specific T-cell receptor might be detected and scored in a blood sample using a class II tetramer, but Aβ-CD4see will only score those cells if they have the capacity for sustained proliferation in response to the presentation of their cognate antigen by DCs. This point is particularly relevant in the assessment of aging populations as the proportion of quiescent T cells increases with age. Therefore, CD4see is superior to tetramers as it measures the responsiveness of antigen-specific CD4+ T cells and not simply their presence. Further, tetramers of class II complexes are technically more difficult to develop than class I probes due to heterogeneity at the HLA-DRB locus in human populations. CD4see probes are made on the backbone of a common α-chain (HLA-DRA1) that is present in ~98% of the human population. Therefore, CD4see-expressing DCs present the query peptide in a near-native class II complex.
Aβ-CD4see requires 20 ml of blood, which is similar to volumes taken for routine blood panels, so this test could be included with annual physicals to alert physicians of changes that might indicate an elevated risk for preclinical AD. Subjects with low numbers of Aβ-specific CD4+ T cells could be evaluated with more costly and invasive procedures, perhaps involving positron emission tomography imaging or the analysis of cerebrospinal fluid. Parsing subjects based on Aβ-CD4see may also benefit clinical trials for AD, as it would provide a way to subscribe studies with subjects that have a higher risk for AD, whether they carry familial AD mutations or not. Trials that might benefit from this measure include ongoing trials for the prophylactic use of anti-Aβ antibodies that target adaptive immune responses to Aβ. Importantly, Aβ-CD4see can be used to identify older subjects who retain strong Aβ-specific immunity in spite of advanced age, ApoE4 genotype or familial AD mutations. Careful analysis of genetic and epigenetic factors in these people may provide new insights into environmental, dietary or behavioral factors that reduce AD risk and/or progression.
Last, the CD4see platform can be adapted to any query peptide, allowing for the isolation of antigen-specific CD4+ T cells implicated in autoimmune disorders, and vaccine development. DCs produced with this protocol are capable of stimulating the proliferation of influenza-specific CD4+ T cells in blood samples taken from subjects 1 week after influenza vaccination. Vaccine efficacy is usually inferred from antibody titers taken weeks or months after inoculation, but with the methods described here can assess antigen-specific CD4+ T-cell responses within 2–3 weeks. Evaluating this arm of adaptive immunity in vitro could improve the development of vaccines for highly pathogenic organisms that often kill before antibody responses can be mounted (for example, Ebola or Marburg viruses). Furthermore, high-throughput screening with CD4see probes could determine the best possible epitope(s) to stimulate human CD4+ T-cell responses to any given antigen. Finally, CD4see can be used to isolate antigen-specific CD4+ T cells, providing T-cell receptor sequences that mediate pathogenic responses in autoimmune disorders such as type 1 diabetes, psoriasis or rheumatoid arthritis.
We have developed a new approach for assessing Aβ-specific CD4+ T cells in healthy subjects and AD patients. Our findings show that an age-dependent decline of these cells occurs after middle age, which is affected by sex and ApoE4 genotype. These results are consistent with prior studies showing that higher AD risk is associated with those factors, and provide a novel assay for antigen-specific CD4+ T-cell responses in diverse populations of human subjects.
Acknowledgments
We thank Dr Ruijun Su, Dr Xilinguli Wushouer and other members of the Ethell lab for technical assistance with this project. Thanks also to Dr Michel Baudry and Dr Iryna Ethell for careful reading of the manuscript. We also acknowledge Veronica Hazen, Tatyana Jones, Dr Dan Miulli, Dr Cesar Ochoa and Dr Trena Rich for help with blood draws. We are grateful to the research subjects and their families for participating in this study. Funding was provided by a grant from the California Institute for Regenerative Medicine to DWE (RN1-00538).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L. 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ.Resolving controversies on the path to Alzheimer's therapeutics Nature Med 2011171060–1065.Erratum in: Nature Med2011; 17: 1693. Nature Med 2011; 17: 1521. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Mandelkow E, Selkoe DJ. Alzheimer disease in 2020. Cold Spring Harb Perspect Med. 2012;2:11. doi: 10.1101/cshperspect.a011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savonenko AV, Melnikova T, Hiatt A, Li T, Worley PF, Troncoso JC, et al. Alzheimer's therapeutics: translation of preclinical science to clinical drug development. Neuropsychopharmacology. 2012;37:261–277. doi: 10.1038/npp.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Estrogen, cognition, and a woman's risk for Alzheimer's disease. Am J Med. 1997;103:11S–18S. doi: 10.1016/s0002-9343(97)00261-1. [DOI] [PubMed] [Google Scholar]
- Bretsky PM, Buckwalter JG, Seeman TE, Miller CA, Poirier J, Schellenberg GD, et al. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13:216–221. doi: 10.1097/00002093-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease Nature 2000408982–985.Erratum in: Nature 2001; 412: 660. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nature Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Ethell DW, Shippy D, Cao C, Cracchiolo JR, Runfeldt M, Blake B, et al. Aβ-specific T cells reverse cognitive decline and synaptic loss in Alzheimer's mice. Neurobiol Dis. 2006;23:351–361. doi: 10.1016/j.nbd.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Cao C, Arendash GW, Dickson A, Mamcarz MB, Lin X, Ethell DW. Aβ-specific T cells are sufficient for beneficial effects in the APP+PS1 mouse model for Alzheimer's disease. Neurobiol Dis. 2009;34:63–70. doi: 10.1016/j.nbd.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extance A. Alzheimer's failure raises questions about disease-modifying strategies. Nat Rev Drug Discov. 2010;9:749–751. doi: 10.1038/nrd3288. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Perry G. Pathogenesis and disease-modifying therapy in Alzheimer's disease: the flat line of progress. Arch Med Res. 2012;43:694–698. doi: 10.1016/j.arcmed.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Colombia at the centre of preclinical AD research. Lancet Neurol. 2012;11:567. doi: 10.1016/S1474-4422(12)70144-8. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Trojanowski JQ. Primary or secondary prevention for AD: who cares. Lancet Neurol. 2012;11:661–662. doi: 10.1016/S1474-4422(12)70143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen PS, Vellas B. Passive immunotherapy for Alzheimer's disease: What have we learned, and where are we headed. J Nutr Health Aging. 2013;17:49–50. doi: 10.1007/s12603-013-0001-3. [DOI] [PubMed] [Google Scholar]
- Oh ES, Troncoso JC, Fangmark Tucker SM. Maximizing the potential of plasma amyloid-beta as a diagnostic biomarker for Alzheimer's disease. Neuromolecular Med. 2008;10:195–207. doi: 10.1007/s12017-008-8035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J. A conceptual revolution in the relationships between the brain and immunity. Brain Behav Immun. 2011;25:817–819. doi: 10.1016/j.bbi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nat Rev Immunol. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J, Knight JC. Major histocompability complex genomics and human disease. Ann Rev. Genomics Human Genet. 2013;24:301–323. doi: 10.1146/annurev-genom-091212-153455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haars R, Kroneneberg M, Gallatin WM, Weissman IL, Owen FL, Hood L. Rearrangement and expression of T cell antigen receptors and gamma genes during thymic development. J Exp Med. 1986;164:1–24. doi: 10.1084/jem.164.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch U, Radtke F. Mechanisms of T cell development and transformation. Ann Rev Cell Dev Biol. 2011;27:539–562. doi: 10.1146/annurev-cellbio-092910-154008. [DOI] [PubMed] [Google Scholar]
- Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, et al. Functional anatomy of T cell activation and synapse formation. Ann Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Ann Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Chen K, Kolls JK. T cell–mediated host immune defenses in the lung. Ann Rev Immunol. 2013;31:605–633. doi: 10.1146/annurev-immunol-032712-100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodynik MA, Sluvkin II. Directed differentiation of human embryonic stem cells to dendritic cells. Methods Mol Biol. 2007;407:275–293. doi: 10.1007/978-1-59745-536-7_19. [DOI] [PubMed] [Google Scholar]
- Calero O, Hortigüela R, Buillido MJ, Calero M. Apolipoprotein E genotyping method by real time PCR, a fast and cost-effective alternative to the TaqMan and FRET assays. J Neurosci Methods. 2009;183:238–240. doi: 10.1016/j.jneumeth.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Le Nouën C, Hillyer P, Munir S, Winter CC, McCarty T, Bukreyev A, et al. Effects of human respiratory syncytial virus, metapneumovirus, parainfluenza virus 3 and influenza virus on CD4+ T cell activation by dendritic cells. PLoS One. 2010;5:e15017. doi: 10.1371/journal.pone.0015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, et al. Simvastatin strongly reduces levels of Alzheimer's disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Arch Neurol. 2001;68:1239–1244. doi: 10.1001/archneurol.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamboli IY, Barth E, Christian L, Siepmann M, Kumar S, Singh S, et al. Statins promote the degradation of extracellular amyloid {beta}-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J Biol Chem. 2010;2010;285:37405–37414. doi: 10.1074/jbc.M110.149468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Wong C, Goldstein DR. Impact of aging on antigen presentation cell function of dendritic cells. Curr Opin Immunol. 2013;25:535–541. doi: 10.1016/j.coi.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LF, de Souza AP, Borges TJ, Bonorino C. Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: decreased stimulation by aged dendritic cells. Mech Ageing Dev. 2011;132:187–194. doi: 10.1016/j.mad.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Baron AM, Pike CJ. Sex hormones, aging and Alzheimer's disease. Front Biosci. 2012;4:976–997. doi: 10.2741/e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer's disease risk and therapy. Prog Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER. The potential use of hormone-based therapeutics for the treatment of Alzheimer's disease. Curr Alzheimer Res. 2012;9:18–34. doi: 10.2174/156720512799015109. [DOI] [PubMed] [Google Scholar]
- Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9:56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- Wisniewski T, Golabek A, Matsubara E, Ghiso J, Frangione B. Apolipoprotein E: binding to soluble Alzheimer's beta-amyloid. Biochem Biophys Res Commun. 1993;192:359–365. doi: 10.1006/bbrc.1993.1423. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, et al. ApoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline A. Apolipoprotein E, amyloid-β clearance and therapeutic opportunities in Alzheimer's disease. Alzheimers Res Ther. 2012;4:32. doi: 10.1186/alzrt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.