Abstract
Onset of psychosis may be associated with abnormal adolescent neurodevelopment. Here we examined the neurocognitive profile of first-episode, adolescent onset psychosis (AOP) as compared to typically developing adolescents, and asked whether neurocognitive performance varied differentially as a function of age in the cases compared with controls. A comprehensive neuropsychological battery was administered to 35 patients experiencing a first-episode of a DSM-IV psychotic disorder and to 31 matched controls. Clinicians also rated subjects’ social and role functioning, both at the time of neuropsychological assessment and 1 year later. Although patients displayed a wide range of impairments relative to controls, their most pronounced deficits included verbal memory, sensorimotor dexterity and cognitive processing speed. Among these, only processing speed showed a significant group-by-age interaction, consistent with an aberrant developmental course among AOP patients. Processing speed also accounted for substantial variance in other areas of deficit, and predicted social functioning 1 year later. AOP patients fail to show normal age-related increases in processing speed, which in turn predicts poorer functional outcomes. This pattern is consistent with the view that adolescent brain developmental processes, such as myelination, may be disrupted in these patients.
Keywords: Schizophrenia, Adolescence, Neurodevelopment, Outcome, Cognitive processing speed
Introduction
Epidemiological investigation has estimated that approximately 18% of adult schizophrenia patients experience initial onset of psychosis prior to 18 years of age (Hafner et al. 1993; Schimmelmann et al. 2007). Relative to patients with onset during adulthood, these early-onset psychosis patients exhibit increased neuroanatomic abnormality (DeLisi 1992; Rajji et al. 2009), greater cognitive impairment (Rajji et al. 2009), poorer medication response (Meltzer et al. 1997), and worse long-term outcome (Ballageer et al. 2005). Despite our knowledge of the tremendous burden early-onset psychosis inflicts, exactly why earlier onset predicts greater impairment remains unknown.
One possibility is that, when assessed later in adulthood, early-onset patients have had relatively longer exposure to factors associated with chronic illness. However, prospective study of early-onset psychosis and adult first-episode patients has demonstrated that these increased levels of impairment are evident from the earliest stages of illness (White et al. 2006). An alternative hypothesis is that the emergence of psychosis, which is associated with degraded cognitive functioning in adults (Woodberry et al. 2008), may reflect an even more severe pathophysiological processes when it occurs in younger patients (Shaw et al. 2010). Importantly, several neurodevelopmental processes are known to persist into late adolescence, including accelerated pruning of synaptic connections and myelination of tracts linking prefrontal cortex to more posterior regions (Karlsgodt et al. 2008a; Pantelis et al. 2009).
Concomitant improvement in several neuropsychological domains over this time period is also evident (Waber et al. 2007). Although diverse, these later-maturing abilities (e.g., verbal memory recall, sensorimotor dexterity) share at least three key qualities. First, they all place substantial demand on the maturing white matter tracts linking prefrontal and parietal or prefrontal and temporal cortices (Karlsgodt et al. 2008b; Nestor et al. 2008; Turken et al. 2008). Second, they are all relatively complex cognitive capacities that benefit from efficient basic information processing, suggesting that cognitive processing speed may serve as a rate-limiting factor potentially constraining complex abilities (Fry and Hale 2000). Third, these laterdeveloping capacities are among those most severely and reliably compromised in schizophrenia, showing particularly striking deficits among adolescent-onset psychosis (AOP) patients (Rajji et al. 2009).
Together, these findings suggest that AOP patients suffer from a particularly severe disruption in the neurodevelopmental maturation processes that subserve the efficient coordination of prefrontal-posterior neural networks (Davenport et al. 2011). Nevertheless, even evidence that aberrant adolescent neurodevelopment accounts for poorer outcome in AOP patients would involve demonstration of a divergent developmental trajectory in AOP patients relative to typically developing adolescents, with the patients’ developmental course predicting their real-world functioning at a later time point. Two studies (Frangou et al. 2008; Øie et al. 2010) have affirmed that AOP patients fail to make typical gains in neurocognitive performance during adolescence. Verbal memory and cognitive processing speed appear particularly sensitive to psychosis in adolescence. To our knowledge, only one published report examines the relationship between cognitive impairments and real-world functioning among AOP patients (Cervellione et al. 2007). However, in that case, age effects on performance were not examined, leaving unexamined any abnormal developmental trajectory in specific cognitive domains.
The present study aimed to identify more definitive cognitive intermediate phenotypes of aberrant adolescent neurodevelopment in AOP patients, and to assess whether abnormal development predicts later functional disability. By intermediate phenotype, we are referring to a quantitative phenotype that is not obvious to the unaided eye, is heritable, and is thought to represent more elementary phenomena than those involved in complex psychiatric diagnosis (Bearden and Freimer 2006). We examined the effects of an initial episode of psychosis during adolescence by administering a comprehensive neuropsychological battery to adolescent patients with recent-onset psychosis and demographically matched, typically developing controls. Social and role functioning were rated at the time of cognitive assessment, using measures developed specifically for adolescents, and then again 1 year later. Specifically, we aimed:
To examine AOP patients’ neuropsychological performance, with the prediction that relatively greater impairment would be seen in the domains of cognitive processing speed, divided attention, delayed recall of verbal memory, and vocabulary (Rajji et al. 2009);
To test for a significant Diagnostic Group-by-Age interaction, whereby AOP patients exhibit a deviant developmental course characterized by attenuated growth in performance across the age span assessed;
To determine whether impairment in one of these key areas might account for variability in related cognitive domains, suggesting constraint on cognitive performance by a more narrowly defined capacity (e.g., cognitive processing speed, Rodriguez-Sanchez et al. 2007); and
To assess whether any impairment nominated by the earlier steps might uniquely predict change in patients’ level of functional impairment over the course of 1 year.
Method
Participants
AOP patients (N=35) and community comparison subjects (N=31) were recruited as part of an ongoing study of psychosis risk at UCLA. All subjects were 12–20 years of age at the time of testing, and patients were within 2 years of psychosis onset. Informed consent was provided by all participants, and for participants under 18 years of age, by their parents as well, using procedures approved by UCLA’s Institutional Review Board. Mean values for age, level of education, and parental education achieved did not differ between the groups, nor did gender, handedness, and race/ethnicity distributions (P-value=0.05); see Table 1 for demographic information.
Table 1.
Demographic information characterizing study sample
| Controls (N=31) | AOP Patients (N= 35) | |
|---|---|---|
| Mean age, years (+/−s.d.) | 16.88(2.17) | 16.38(2.06) |
| Number female (%) | 16(52) | 15(43) |
| Number left-hand dominant (%) | 3(10) | 4(11) |
| Mean patient education, years (+/−s.d.) | 10.80(2.12) | 10.06(1.95) |
| Mean parental education, years (+/−s.d.) | 12.74(2.90) | 12.11(2.58) |
| Race/Ethnicity (%) | ||
| Caucasian, Non-Hispanic | 19(61) | 17(49) |
| Caucasian, Hispanic | 5(16) | 8(23) |
| African-American | 2(6) | 4(11) |
| Asian-American/Pacific Islander | 2(6) | 3(8) |
| Native American | 1(3) | 0(0) |
| Other/Declined to State | 2(6) | 3(8) |
Table 1 provides demographic information. Mean values for each of the variables were tested for group differences at the univariate level. Using a P-value of 0.05, no significant differences were detected. Gender, handedness, and race/ethnicity distributions were tested with Chi-squared analyses, again using a P-value=0.05; no group differences were detected in these distributions.
At baseline assessment, diagnoses for all patients’ were determined using the Structured Clinical Interview for DSM-IV Axis I diagnoses (SCID; Spitzer et al. 1992) and review of medical records; final diagnoses required consensus among supervising clinical psychologists. Thirteen patients were diagnosed with schizophrenia, and 10 were diagnosed with schizoaffective disorder (5 bipolar subtype and 5 depressive subtype). The remaining 12 patients were given a diagnosis of psychotic disorder NOS. Diagnoses comorbid with patients’ psychotic disorders are listed in Supplementary Information. The primary statistical analyses outlined below in Steps 1–4 were performed both with and without the psychosis NOS sub-group included in the patient sample; although the results changed quantitatively (due to the change in sample size), the pattern or direction of differences did not. Additionally, examination of scatter plots showed no clear differentiation between these diagnosis-specific groups on cognitive performance variables. Therefore, all results are reported for analyses including the psychosis NOS subgroup in the patient sample.
Patients with past substance abuse diagnoses were permitted to participate if they were free of substance abuse for the preceding 6 months (two patients and no controls met abuse criteria more than 6 months earlier); patients with substance dependence diagnoses were excluded. Control subjects were screened for Axis I disorders using the SCID, and for history of schizophrenia-spectrum disorder among first-degree relatives using the Family Interview for Genetic Studies (FIGS, Maxwell 1992). All assessments were administered by clinicians trained to a standard reliability criterion (Ventura et al. 1998).
The 24-Item, Extended Brief Psychiatric Rating Scale (BPRS, Lukoff et al. 1986) was administered to assess symptom severity in the AOP group, and all participants completed the Beck Depression Inventory-II (BDI-II; Beck et al. 1996). In light of their appropriateness for adolescents, the clinician-rated Global Functioning: Social and Role scales (Cornblatt et al. 2007) were used to assess interpersonal and occupational functioning. These scales have shown 12-month test-retest reliability in both young people at risk for developing schizophrenia and those already diagnosed with schizophrenia that is adequate to support longitudinal analysis (Cornblatt et al. 2007; Piskulic et al. 2011). The Global Assessment of Functioning (GAF) was also rated, using a set of refined, reliable anchors (Miller et al. 2002).
A convenience sample of 17 of the initial 35 patients returned an average of 13.74 (s.e.=1.72) months after baseline assessment for repeated administration of the functioning measures. The subset of patients who returned for follow-up assessment did not differ significantly from those who did not on any of the clinical or neuropsychological summary measures (P>0.05). Neuropsychological measures were not re-administered during this follow-up session.
Medication information was gathered by patient and parent/guardian report and medical record review. Twenty-three patients were taking atypical antipsychotic medication at the time of the baseline assessment, 4 were taking typical antipsychotics, and 8 were taking no antipsychotic medication. Three patients reported taking anticholinergic medication on a regular basis. Ten patients were also taking mood stabilizing medication, and 6 were taking an SSRI or SNRI. Patients reported a mean of 92.9 (s.e.=13.1) days on antipsychotic medication and a mean of 120.4 (s.e.=13.3) days on other psychoactive medications. Medication exposure did not correlate significantly (P>0.05) with any of the neuropsychological variables.
Neuropsychological Assessment
Study participants were administered a comprehensive battery of tests taking approximately 3.5 h to complete. The battery included the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 1999), and the Computerized Category Test from the Halstead-Reitan battery (Berger et al. 1997); total score was computed for the former two subtests, and total number of errors was computed for the latter. The Digit Symbol-Coding (DSC) and Digit Span subtests of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Wechsler 1999) were administered, as was the Spatial Span subtest of the Wechsler Memory Scale-Third Edition (WMS-III; Wechsler 1997); total score was calculated for each. Verbal fluency was assessed using the Controlled Oral Word Association Test (FAS; Benton and Hamsher 1976) and the Category Fluency test (Animal Naming; Lezak 1995); total correct was calculated for each. The Trail Making Test (A and B; Reitan 1985) was administered, and time to completion for parts A and B was recorded. Participants completed the Visual Memory subtest of the WMS-III, and total scores were determined for immediate recall (Part I) and delayed recall (Part II). Participants ages 12.0–15.9 years were also administered the Stories subtest of the Children’s Memory Scale (Cohen 1997), while participants 16 years and older were administered the Logical Memory test from the WMS-III. Again, for both tests, total scores were determined for immediate recall (Part I) and for delayed recall (Part II). In light of the difference in tests used on the different age groups, for the story memory domain only, group differences were tested on Z-score values determined separately for each individual, using sample values, rather than being tested on raw scores. Verbal list memory was also evaluated using the California Verbal Learning Test-Second Edition (CVLT-II; Delis et al. 2000); primary dependent measures were total correct responses during immediate recall of Trials 1–5, and Short and Long Delay Free and Cued Recall total scores. Finally, motor speed and dexterity were assessed with the Purdue Pegboard test and the Halstead Finger Tapping Test; total score for dominant hand was scored for each. We tested each measure separately, and employed the conservative Bonferroni correction to address the multiple comparison issue.
Data Analysis
Planned analyses involved 4 steps. Unless specified, all scores are raw scores, so that effects of age could be examined. All P-values reported are two-tailed.
-
Step 1
Group Differences: Each neuropsychological test’s summary score (specified above) was subjected to univariate ANOVA, with Group entered as a fixed effect. Bonferroni correction was applied, requiring effects to surpass a critical P-value of 0.0024 (0.05/21) for significance. Effect sizes were quantified using the partial η2 statistic, which provides a measure of effect size for group differences (Ferguson 2009; Olejnik and Algina 2003).
-
Step 2
Group-by-Age Interaction: Any neuropsychological scores showing a significant Group effect in Step 1 were then tested in a linear regression using Group, Age, and their interaction as predictors. A significant Group-by-Age interaction, coupled with a post hoc comparison showing a significant group difference at higher age values, can be interpreted as evidence suggesting a divergent trajectory of cognitive maturation in the AOP group.
-
Step 3
ANCOVA: Neuropsychological variables showing a significant effect of Group in Step 1, as well as a Group-by-Age interaction in Step 2, were then entered in a set of ANCOVAs to determine whether they account for variability in other neuropsychological measures that also demonstrated a group difference. This procedure involved rerunning the univariate ANOVAs from Step 1, but with the neuropsychological variable(s) identified in Step 2 entered as covariates, to assess whether the identified variable shares substantial variance with other measures (Brebion et al. 1998; Rodriguez-Sanchez et al. 2007).
-
Step 4
Predicting Functional Impairment: Variables showing a significant Group-by-Age interaction were then entered into a linear regression to determine whether they would predict change in global functioning between baseline and follow-up assessment, when accounting for baseline functioning. Specifically, Global Functioning: Social score at baseline was entered into the step-wise regression, followed by the identified neuropsychological measure; Global Functioning: Social score at 1-year follow-up was entered as the dependent variable. The process was then repeated for Global Functioning: Role scores.
Results
-
Step 1
Group Differences: Results of the univariate ANOVAs testing the effect of group on neuropsychological performance (Table 2) include 11 of 21 variables were significant at a P-value<0.0024. Tests resulting in significant group differences involved measures of immediate and delayed verbal memory recall, cognitive processing speed, semantic verbal fluency, sensorimotor speed and dexterity, and verbal working memory. Z-score indices reveal that AOP patients’ performance fell more than one standard deviation below control subjects’ performance on 10 of the 11 significant measures. All 11 measures involved a group difference in the “large effect” range (Partial η2>0.14; Stevens 2002). Performance on neuropsychological measures was not significantly correlated with medication exposure (P>0.05 for all comparisons), and did not differ between patients diagnosed with schizophrenia and schizoaffective disorder and patients diagnosed with psychotic disorder NOS (P>0.50). Group differences in all working and delayed memory, and in semantic verbal fluency, remained significant when Finger Tapping Test score was entered as a covariate to control for pure motor speed’s potential contribution to overall cognitive slowing. Additionally, BDI-II total score did not correlate significantly (P<0.05) with any of the neuropsychological summary values, or with Global Functioning Scales rated at baseline or at follow-up.
-
Step 2
Group-by-Age Interaction: Since several of the neuropsychological measures found to be significant in Step 1 involved delayed verbal memory recall, the two verbal memory measures with the largest group difference effect sizes were selected (Long Delay Free Recall, and Logical Memory II/ Stories II) were selected, in order to limit the overall number of comparisons. Regression analyses indicated a significant Diagnostic Group-by-Age interaction only for DSC performance (df=1, Wald X2=4.108, P=0.043). In particular, controls showed a highly significant age-associated increase in cognitive processing speed during the course of adolescence; whereas AOP patients show no significant age-associated increase (Fig. 1a). Trend level Group-by-Age interactions were also observed for delayed verbal memory recall (Long Delay Free Recall; df=1, Wald X2= 3.427, P=0.064) and sensorimotor speed and dexterity (Purdue Pegboard; df=1, Wald X2 = 3.265, P=0.071; Fig. 1b).
-
Step 3
ANCOVA: As DSC was the only neurocognitive measures showing a significant diagnostic group x age interaction, univariate ANOVA’s from Step 1 were repeated with DSC performance as a covariate. As shown in Table 2, the effect of diagnostic group on cognitive performance no longer reached the corrected significance threshold when covarying for DSC. In fact, only the complex verbal memory recall measure (Logical Memory II/Stories II) retained a significant effect of Diagnostic Group (F [1,62]=10.493, P<0.002, Partial η2=0.145).
-
Step 4
Predicting Functional Impairment: Baseline DSC performance in the patient group was entered into a step-wise linear regression analysis (after baseline Global Functioning Scale: Social was entered to control for initial social functioning). When measured concurrently, DSC performance correlated moderately with Global Functioning Scale: Social rating (r=0.353, P=0.005) and more so with Global Functioning Scale: Role score (r= 0.542, P<0.001). Baseline DSC performance was a significant predictor of change in social functioning over one year, with lower baseline DSC performance predicting less improvement in social functioning (F [1,14]=5.467, P=0.035, R2=0.281; Fig. 2). However, when the same procedure was repeated for the Global Functioning Scale: Role score, DSC performance did not predict later role functioning, after controlling for baseline role functioning (F [1,14]=2.487, P= 0.137, R2=0.151).
Table 2.
Group differences across neuropsychological measures
| Test | z | No Covariate F | No Covariate Partial η2 | DSC Covaried F | DSC Covaried Partial η2 |
|---|---|---|---|---|---|
| WMS-III Logical Memory II/CMS Stories Del. | −1.61 | 20.918 | 0.249*** | 9.820 | 0.137* |
| Purdue Pegboard Dom. Hand | −1.31 | 20.166 | 0.240*** | 8.642 | 0.122* |
| Animal Naming | −1.11 | 19.758 | 0.236*** | 9.167 | 0.129* |
| CVLT-II Long Delay Free Recall a | −1.41 | 18.608 | 0.234*** | 8.627 | 0.128* |
| WMS-III Logical Memory I/CMS Stories Imm. | −1.45 | 18.601 | 0.228*** | 10.493 | 0.145*** |
| CVLT-II Trials 1-5 Imm. Recall | −1.19 | 14.253 | 0.182*** | 5.643 | 0.083* |
| WAIS-III Digit Symbol-Coding | −1.02 | 13.910 | 0.181*** | − | − |
| CVLT-II Long Delay Cued Recall | −1.15 | 13.153 | 0.170*** | 4.868 | 0.073* |
| CVLT-II Short Delay Free Recall | −1.13 | 12.825 | 0.167*** | 4.192 | 0.063* |
| CVLT-II Short Delay Cued Recall | −1.01 | 11.327 | 0.152*** | 4.000 | 0.062 |
| WAIS-III Digit Span | −0.84 | 10.963 | 0.146*** | 3.503 | 0.053 |
| WMS-III Spatial Span | −0.85 | 10.078 | 0.138* | 2.890 | 0.045 |
| WAIS-III Vocabulary | −0.98 | 9.606 | 0.131* | 2.112 | 0.033 |
| Trail Making Test Part B | −0.92 | 9.077 | 0.124* | 2.032 | 0.032 |
| COWAT (FAS) | −0.75 | 7.477 | 0.105* | 1.737 | 0.027 |
| Computerized H-R Category Test (errors) b | −0.78 | 5.594 | 0.099* | 0.909 | 0.018 |
| WMS-III Visual Memory II | −0.83 | 5.620 | 0.081* | 0.833 | 0.013 |
| Trail Making Test Part A | −0.47 | 3.221 | 0.048 | 0.020 | 0.000 |
| WAIS-III Matrix Reasoning | −0.67 | 3.129 | 0.047 | 0.358 | 0.006 |
| WMS-III Visual Memory I | −0.52 | 1.913 | 0.029 | 0.129 | 0.002 |
| Finger Tapping Dom. Hand | −0.18 | 0.349 | 0.005 | 1.487 | 0.023 |
ANOVA carried out with d.f.=1,61, and ANCOVA carried out with d.f.=1,59.
ANOVA carried out with d.f.=1,51, and ANCOVA carried out with d.f.=1,50.
P<0.05, indicating significance at a conventional level
P<0.0024, indicating significance after Bonferroni correction
Table 2 includes AOP patients’ z-scores, standardized to the control group’s values; negative z-scores indicate worse performance. The results of the univariate ANOVAs, with Group as a between-subjects factor, are denoted under “No Covariate.” The ANCOVA results, with WAIS-III Digit Symbol-Coding (DSC) score entered as a covariate to control for the influence of cognitive processing speed are denoted as “DSC Covaried.” Bonferroni correction required effects to surpass a critical P-value of 0.0024 for significance. Partial η2 provides a measure of effect size for group differences; values over 0.14 are considered large effects. Tests are listed in order of decreasing effect size for the ANOVAS. The results of the ANCOVAs demonstrate that when DSC performance is controlled, only patients’ complex verbal learning and memory impairment remains significant. All other previously significant effects disappear.
Fig. 1.
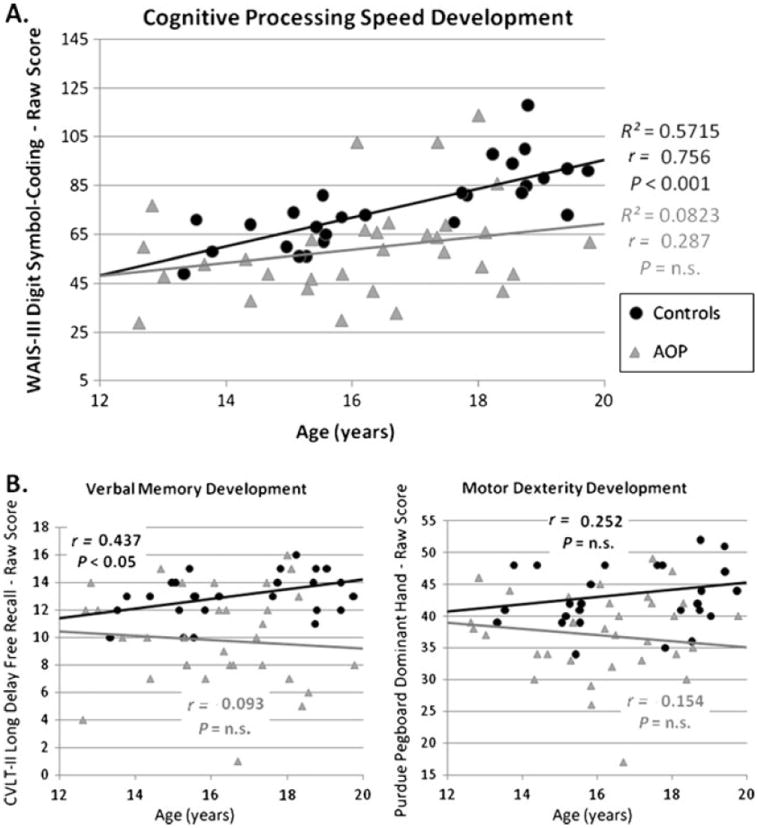
Psychosis-specific neurocognitive impairments over the course of adolescence. Neuropsychological tests showing significant group differences were examined for a Group-by-Age interaction, suggestive of a divergent course of development among AOP patients. a The interaction was significant for WAIS-III Digit Symbol-Coding (DSC), P= 0.043, with controls displaying a strong correlation between age and processing speed (r=0.756, P<0.001) and patients showing a less reliable association (r = 0.287, P>0.10). b The Group-by-Age interaction reached a trend level of significance for delayed verbal memory recall (P=0.064) and motor coordination (P=0.071)
Fig. 2.
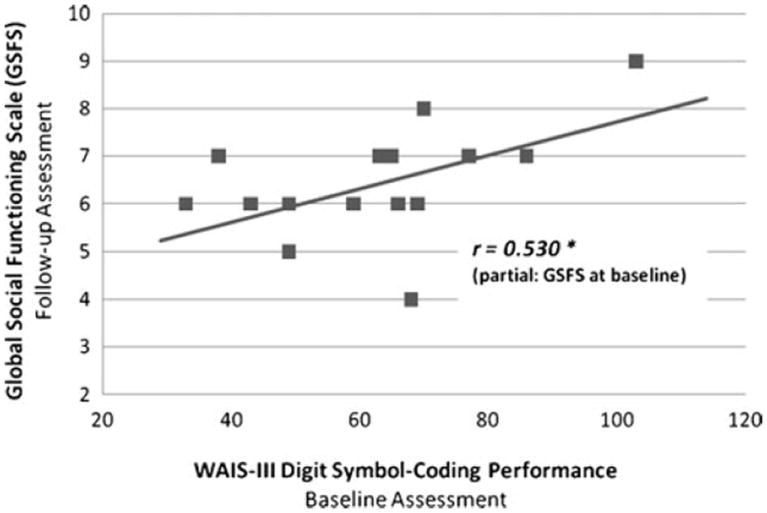
Cognitive processing speed predicts later social functioning. Results of regression analysis demonstrate that, among AOP patients, baseline cognitive processing speed predicts social functioning 1 year later (P=0.035), even after controlling for baseline social functioning
Discussion
The present study aimed to characterize how psychosis affects neuropsychological performance over the course of adolescence - a critical, initial step in determining how normal developmental processes interact with latent vulnerability in the pathogenesis of schizophrenia-spectrum disorders. We predicted that against a background of general cognitive deficit, specific impairments would stand out by demonstrating: 1) an especially large illness-related decrement, 2) a divergent developmental course in the AOP group relative to typically developing controls, and 3) the capacity to account for group effects in other cognitive measures. Cognitive processing speed, as measured by DSC, not only fulfilled these criteria, but it also predicted improvement in real-world social functioning one the course of 1 year. It therefore stands as a promising potential intermediate phenotype for characterizing the interaction between emergent psychosis and typical development. In addition to cognitive processing speed, measures of verbal memory, semantic fluency, and motor speed and dexterity were among the domains showing the greatest magnitude of impairment in the AOP group. However, only cognitive processing speed showed evidence of a significant developmental effect, in that typically developing adolescents displayed a normative age-associated increase in cognitive processing speed, whereas AOP patients did not show the expected pattern of age-related improvement. Therefore, since the group difference continues to increase as long as performance on the DSC test continues to mature, earlier deviation away from typical development is associated with greater impairment.
In addition, DSC performance displayed a capacity to account for group differences in a range of other cognitive abilities, including measures of working and verbal memory, as well as verbal fluency. We take this result as consistent with the prediction that cognitive slowing may constrain other, more complex cognitive processes (Brebion et al. 1998; Rodriguez-Sanchez et al. 2007). Nevertheless, it is possible that DSC performance’s ability to account for group differences in performance on other neuropsychological tests may be related to DSC’s sensitivity to schizophrenia patients’ substantial generalized cognitive deficit. To our knowledge, only a factor analytic approach could measure this sensitivity accurately, and unfortunately, the present study lack the sample size to support that approach. The coincidence of this overlapping variance among tasks and DSC performance’s unique developmental course, however, suggest that the neurocognitive mechanisms supporting DSC performance do play a central role in the early expression of AOP. Furthermore, DSC’s strong association with a generalized, latent impairment speaks to the potential of the measure to help elucidate the widespread network-level neural pathology that likely underlies schizophrenia (Dickinson 2008).
The finding that cognitive processing speed predicted patients’ global social functioning 1 year after baseline assessment suggests that performance on this test reflects variability in an underlying neurocognitive mechanism that may also account for AOP patients’ severely impaired community functioning. For instance, since successful social functioning requires nearly instantaneous facial affect recognition, detection of prosody in speech, and processing of a range of other subject social cues, it is not surprising that slowed information processing would impair one’s ability to efficiently integrate these cues into valid representations of interpersonal situations and to use these representations to select behaviors most appropriate for the social context.
The chief effects we report remain after controlling for patients’ slowed pure motor speed and are unrelated to exposure to antipsychotic or other psychoactive medication, or by factors secondary to illness chronicity, as all psychosis patients had experienced a recent onset of illness. The degree of cognitive slowing we observed in AOP patients is consistent with the extent of cognitive slowing among AOP patients reported in a recent meta-analysis (Rajji et al. 2009), and the DSC performance of typically developing adolescents in our sample closely matches the trajectory of improving processing speed reported by Waber and colleagues (Waber et al. 2007) in a large, typically developing sample of children and adolescents.
The significant diagnostic group-by-age interaction, whereby AOP patients fail to show the normative increase in processing efficiency, agrees with the findings of two earlier longitudinal investigations (Cervellione et al. 2007; Frangou et al. 2008; Øie et al. 2010) that tested adolescentonset psychosis patients at a first-episode baseline session, and then again at least 1 year later. The agreement of the present results with the findings of Øie et al. (2010) and Frangou et al. (2008) - the latter study having used a range of speeded cognitive tasks to create a cognitive efficiency composite - argues against group differences in developmental course resulting from DSC’s psychometric properties (e.g., reliability). One study (Cervellione et al. 2007) failed to detect evidence that the trajectory of AOP patients’ coding performance deviates from controls’ over the course of adolescence. Additional work will be needed to determine the source of heterogeneity in these findings. Nevertheless, the preponderance of evidence points to DSC performance reflecting aberrant neurodevelopment.
Prior to the present investigation, only one study examined cognitive predictors of functional outcome among adolescent first-episode patients (Cervellione et al. 2007). The present findings build on those results by adding substantial specificity to the neurocognitive predictors involved, and by mapping the course by which neurodevelopment leads to this functional impairment. They also complement our earlier demonstration that improvement in processing speed, as well as visual learning and memory, is associated with improvements in social and role functioning among adolescents and young adults at clinical high risk for developing psychosis (Niendam et al. 2007) by showing that this relationship persists during the period immediately after psychosis onset as well.
The present study is therefore the first systematic investigation of cognitive indices that are neurodevelopmentally informative, that account for a substantial portion of patients’ widespread information-processing impairments, and that predict deleterious consequences for psychotic disorder patients’ real-world functioning. In showing that cognitive processing speed meets all three criteria—in the same set of first-episode, AOP patients—the present findings provide the strongest findings to date that a neurodevelopmentally aberrant failure to make typical adolescent gains in cognitive processing efficiency provides the mechanism underlying the well-known association between earlier psychosis onset and poorer long-term outcome.
In 2007, Dickinson and colleagues published an influential meta-analysis demonstrating that DSC performance represents one the most reliable, robust measures of cognitive deficit in adult schizophrenia patients, sparking interest in understanding how the neural substrate of DSC performance overlaps with the pathophysiology of schizophrenia. A great deal of work remains in specifying how the brain supports DSC performance, but the present results build upon Dickinson and colleagues’ work by employing a neurodevelopmental framework to suggest that the neural infrastructure that matures late in adolescence may indeed be the critical neural network that links DSC performance and psychosis.
Members of our group recently reported that adolescents and young adults at elevated clinical risk for developing psychosis fail to show typical age-related increases in white matter integrity in tracts linking prefrontal and posterior cortex, and this aberrant developmental trajectory predicts later functional impairment (Karlsgodt et al. 2009). These neuroimaging findings provide empirical support for the suggestion that the large white matter tracts linking prefrontal and other areas of the cortex are particularly sensitive to the neurodevelopmental processes that occur during late adolescence, and contribute to this period’s status as a highly sensitive period for the expression of psychosis (Paus et al. 2008).
These same neural assemblies undergoing maturation during late adolescence are the systems that support complex, distributed cognitive functions such as those recruited during efficient DSC performance (Rypma and Prabhakaran 2009; Turken et al. 2008). The task’s complex information processing demands (requiring efficient coordination of visual scanning, memory retrieval, and psychomotor tasks, among others; Bachman et al. 2010), as well as the need to meet these demands as quickly as possible, may tax this neural infrastructure so heavily that any compromise is directly reflected in behavioral impairment—making DSC performance uniquely sensitive to the emerging dysconnectivity associated with psychosis (Lisman et al. 2008; Stephan et al. 2006).
The chief limitation of the present study is that the influence of age on the trajectory of neurocognitive development was assessed cross-sectionally, rather than as a longitudinal design. A within-subject prospective design may reduce overall variability, and the resulting increase in power may have allowed the age by group interactions for verbal memory and motor coordination to reach significance. Nevertheless, the fact that the cognitive processing speed interaction was significant in this between-subjects design speaks to the robustness of the effect. Future study will be necessary to identify more precisely the physiological basis for this aberrant trajectory of cognitive processing speed development observed in adolescent-onset psychosis.
As clinical research strives to identify intermediate phenotypes reflecting the pathogenesis of psychotic symptoms and functional impairment, the present results nominate cognitive processing speed as playing a critical role in explaining the long-known but poorly understood association between earlier onset of psychosis and worse long-term outcome. Our findings suggest that psychosis arrests typical developmental increases in neurocognitive efficiency, so that earlier onset is associated with a greater deviation from adult levels of cognitive performance.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10802-011-9592-5) contains supplementary material, which is available to authorized users.
Contributor Information
Peter Bachman, Email: bachman@psych.ucla.edu, Semel Institute of Neuroscience & Human Behavior, Department of Psychiatry & Biobehavioral Sciences, University of California, Los Angeles, BOX 951759, 760 Westwood Plaza, Los Angeles, CA 90095-1759, USA.
Tara A. Niendam, Department of Psychiatry and Behavioral Sciences, University of California - Davis, Sacramento, CA, USA
Maria Jalbrzikowkski, Department of Psychology, University of California - Los Angeles, Los Angeles, CA, USA.
Chan Y. Park, Department of Psychology, University of California - Los Angeles, Los Angeles, CA, USA
Melita Daley, Semel Institute of Neuroscience & Human Behavior, Department of Psychiatry & Biobehavioral Sciences, University of California, Los Angeles, BOX 951759, 760 Westwood Plaza, Los Angeles, CA 90095-1759, USA.
Tyrone D. Cannon, Semel Institute of Neuroscience & Human Behavior, Department of Psychiatry & Biobehavioral Sciences, University of California, Los Angeles, BOX 951759, 760 Westwood Plaza, Los Angeles, CA 90095-1759, USA Department of Psychology, University of California - Los Angeles, Los Angeles, CA, USA.
Carrie E. Bearden, Semel Institute of Neuroscience & Human Behavior, Department of Psychiatry & Biobehavioral Sciences, University of California, Los Angeles, BOX 951759, 760 Westwood Plaza, Los Angeles, CA 90095-1759, USA Department of Psychology, University of California - Los Angeles, Los Angeles, CA, USA.
References
- Bachman P, Reichenberg A, Rice P, Woolsey M, Chaves O, Martinez D, et al. Deconstructing processing speed deficits in schizophrenia: application of a parametric digit symbol coding test. Schizophrenia Research. 2010;118(1–3):6–11. doi: 10.1016/j.schres.2010.02.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballageer T, Malla A, Manchanda R, Takhar J, Haricharan R. Is adolescent-onset first-episode psychosis different from adult onset? Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(8):782–789. doi: 10.1097/01.chi.0000164591.55942.ea. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Freimer NB. Endophenotypes for psychiatric disorders: ready for primetime? Trends in Genetics. 2006;22(6):306–313. doi: 10.1016/j.tig.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory—II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Benton AL, Hamsher K. Multilingual aphasia examination. Iowa City, IA: AJA; 1976. [Google Scholar]
- Berger SG, Chibnall JT, Gfeller JD. Construct validity of the computerized version of the category test. Journal of Clinical Psychology. 1997;53(7):723–726. doi: 10.1002/(sici)1097-4679(199711)53:7<723::aid-jclp9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Brebion G, Amador X, Smith MJ, Gorman JM. Memory impairment and schizophrenia: the role of processing speed. Schizophrenia Research. 1998;30(1):31–39. doi: 10.1016/s0920-9964(97)00123-0. [DOI] [PubMed] [Google Scholar]
- Cervellione KL, Burdick KE, Cottone JG, Rhinewine JP, Kumra S. Neurocognitive deficits in adolescents with schizophrenia: longitudinal stability and predictive utility for short-term functional outcome. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(7):867–878. doi: 10.1097/chi.0b013e318054678d. [DOI] [PubMed] [Google Scholar]
- Cohen MJ. Children’s memory scale. San Antonio, TX: Psychological Corporation, Harcourt, Brace & Co.; 1997. [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia Bulletin. 2007;33(3):688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatric Research. 2011;181(3):193–198. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test. 2. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- DeLisi LE. The significance of age of onset for schizophrenia. Schizophrenia Bulletin. 1992;18(2):209–215. doi: 10.1093/schbul/18.2.209. [DOI] [PubMed] [Google Scholar]
- Dickinson D. Digit symbol coding and general cognitive ability in schizophrenia: worth another look? British Journal of Psychiatry. 2008;193(5):354–356. doi: 10.1192/bjp.bp.108.049387. [DOI] [PubMed] [Google Scholar]
- Ferguson CJ. An effect size primer: a guide for clinicians and researchers. Professional Psychology: Research and Practice. 2009;40(5):532–538. [Google Scholar]
- Frangou S, Hadjulis M, Vourdas A. The Maudsley early onset schizophrenia study: cognitive function over a 4-year follow-up period. Schizophrenia Bulletin. 2008;34(1):52–59. doi: 10.1093/schbul/sbm124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biological Psychology. 2000;54(1–3):1–34. doi: 10.1016/s0301-0511(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Hafner H, Maurer K, Loffler W, Riecher-Rossler A. The influence of age and sex on the onset and early course of schizophrenia. The British Journal of Psychiatry. 1993;162:80–86. doi: 10.1192/bjp.162.1.80. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG, et al. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Development and Psychopathology. 2008a;20(4):1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biological Psychiatry. 2008b;63(5):512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biological Psychiatry. 2009;66(6):562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. New York, NY: Oxford Univeristy Press; 1995. [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in Neurosciences. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoff D, Liberman RP, Nuechterlein KH. Symptom monitoring in the rehabilitation of schizophrenic patients. Schizophrenia Bulletin. 1986;12(4):578–602. doi: 10.1093/schbul/12.4.578. [DOI] [PubMed] [Google Scholar]
- Maxwell ME. Maual for the family interview for genetic studies (FIGS) Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health; 1992. [Google Scholar]
- Meltzer HY, Rabinowitz J, Lee MA, Cola PA, Ranjan R, Findling RL, et al. Age at onset and gender of schizophrenic patients in relation to neuroleptic resistance. The American Journal of Psychiatry. 1997;154(4):475–482. doi: 10.1176/ajp.154.4.475. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. The American Journal of Psychiatry. 2002;159(5):863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology. 2008;22(2):246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Zinberg J, Johnson JK, O’Brien M, Cannon TD. The course of neurocognition and social functioning in individuals at ultra high risk for psychosis. Schizophrenia Bulletin. 2007;33(3):772–781. doi: 10.1093/schbul/sbm020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øie M, Sundet K, Rund BR. Neurocognitive decline in early-onset schizophrenia compared with ADHD and normal controls: evidence from a 13-year follow-up study. Schizophrenia Bulletin. 2010;36(3):557–565. doi: 10.1093/schbul/sbn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejnik S, Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychological Methods. 2003;8(4):434–447. doi: 10.1037/1082-989X.8.4.434. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Bora E, Fornito A, Testa R, Brewer WJ, et al. Neurobiological markers of illness onset in psychosis and schizophrenia: the search for a moving target. Neuropsychology Review. 2009;19(3):385–398. doi: 10.1007/s11065-009-9114-1. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskulic D, Addington J, Auther A, Cornblatt BA. Using the global functioning social and role scales in a first-episode sample. Early Intervention Psychiatry. 2011 doi: 10.1111/j.1751-7893.2011.00263.x. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. The British Journal of Psychiatry. 2009;195(4):286–293. doi: 10.1192/bjp.bp.108.060723. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Relationships between measures of brain functions and general intelligence. Journal of Clinical Psychology. 1985;41(2):245–253. doi: 10.1002/1097-4679(198503)41:2<245::aid-jclp2270410219>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sanchez JM, Crespo-Facorro B, Gonzalez-Blanch C, Perez-Iglesias R, Vazquez-Barquero JL. Cognitive dysfunction in first-episode psychosis: the processing speed hypothesis. The British Journal of Psychiatry. Supplement. 2007;51:s107–s110. doi: 10.1192/bjp.191.51.s107. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: the mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37(2):207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmelmann BG, Conus P, Cotton S, McGorry PD, Lambert M. Pre-treatment, baseline, and outcome differences between early-onset and adult-onset psychosis in an epidemiological cohort of 636 first-episode patients. Schizophrenia Research. 2007;95(1–3):1–8. doi: 10.1016/j.schres.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Shaw P, Gogtay N, Rapoport J. Childhood psychiatric disorders as anomalies in neurodevelopmental trajectories. Human Brain Mapping. 2010;31(6):917–925. doi: 10.1002/hbm.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID). I: history, rationale, and description. Archives of General Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biological Psychiatry. 2006;59(10):929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Stevens JP. Applied multivariate statistics for the social sciences. Mahwah, New Jersey: Lawrence Erlbaum Associate; 2002. [Google Scholar]
- Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. NeuroImage. 2008;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79(2):163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Waber DP, De Moor C, Forbes PW, Almli CR, Botteron KN, Leonard G, et al. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. Journal of the International Neuropsychological Society. 2007;13(5):729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale-third edition-(WMS-III) San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- White T, Ho BC, Ward J, O’Leary D, Andreasen NC. Neuropsychological performance in first-episode adolescents with schizophrenia: a comparison with first-episode adults and adolescent control subjects. Biological Psychiatry. 2006;60(5):463–471. doi: 10.1016/j.biopsych.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. The American Journal of Psychiatry. 2008;165(5):579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


