Abstract
Zebrafish are capable of robust and spontaneous regeneration of injured retina. Constant intense light exposure to adult albino zebrafish specifically causes apoptosis of rod and cone photoreceptor cells and is an excellent model to study the molecular mechanisms underlying photoreceptor regeneration. However, this paradigm has only been applied to lesion zebrafish of the nonpigmented albino genetic background, which precludes the use of numerous transgenic reporter lines that are widely used to study regeneration. Here, we explored the effectiveness of constant intense light exposure in causing photoreceptor apoptosis and stimulating regeneration in normally pigmented zebrafish retinas. We show that constant intense light exposure causes widespread photoreceptor damage in the dorsal-central retinas of pigmented zebrafish. Photoreceptor loss triggers dedifferentiation and proliferation of Müller glia as well as progenitor cell proliferation. We also demonstrate that the timeline of regeneration response is comparable between the albino and the pigmented retinas.
Introduction
Photoreceptor loss is the leading cause of many human retina degenerative diseases. Retinal cell loss is irreversible in mammals, but the nonmammalian vertebrate zebrafish (Danio rerio) can spontaneously regenerate any lost retinal cell type. A range of retinal damage models have been employed to study regeneration in zebrafish, including light-induced damage [1-3], mechanical injury [4], chemical/toxin treatment [5,6], and laser ablation [7]. In all these cases, supportive retinal glial cells, called Müller glia (MG), dedifferentiate and proliferate to generate progenitor cells [8]. The progenitor cells rapidly proliferate and form progenitor clusters that migrate to the region of damage and differentiate into the lost retinal cell types [4,9].
Among the damage models, light-induced lesions are noninvasive and highly specific in that they cause synchronous photoreceptor apoptosis while leaving the rest of the retina intact, thereby allowing study of the molecular mechanisms underlying photoreceptor regeneration [10]. Light-induced photoreceptor apoptosis was discovered in rats, and the mechanisms involved have been characterized in rodent retinas [11]. Constant intense light exposure causes repetitive bleaching of the visual pigment rhodopsin, which results in accumulation of retinoid intermediates that ultimately induce apoptosis [10]. Activation of pro-apoptotic transcription factor activator protein 1 (AP-1) is also necessary for light-induced apoptosis, although the exact mechanisms remain unclear [12,13].
In zebrafish two main paradigms of light lesioning free-swimming fish have been described, although recently combinatory models and a third light lesioning model that utilizes focused retinal lesioning of immobilized fish have also been described [2,14,15]. Among the models used to lesion free-swimming zebrafish, the constant intense light paradigm uses exposure of dark-adapted albino fish to ~10,000 lux for 3–4 days [1]. This causes extensive photoreceptor apoptosis in the dorsal and central retina while leaving the ventral retina mostly undamaged. Although some rod photoreceptor loss was observed in the dorsal retinas of pigmented zebrafish, this light lesioning model does not elicit significant MG proliferation in pigmented zebrafish retinas [15]. The second model involves exposing normally pigmented free-swimming zebrafish to an ultra-high intensity wide-spectrum light source that includes ultraviolet (UV) light (~180,000 lux) for 30 min and causes photoreceptor apoptosis in the central retina with sporadic damage in the dorsal and ventral regions [3,14]. Extensive studies exploring the efficacy of each model, including the types of cells damaged and the initiation of regeneration response, have been undertaken [2,14,16]. Recently, combining the high-intensity wide-spectrum (UV) light exposure with 48 h of constant intense light exposure was shown to cause widespread photoreceptor apoptosis and trigger regeneration in pigmented zebrafish retinas [15]. However, it remains unknown if the timeline of regeneration is identical between pigmented and albino zebrafish.
In this study we sought to evaluate the utility of a modified constant intense light-exposure paradigm to damage normally pigmented, non-albino zebrafish retinas. We developed a protocol in which constant intense light triggers photoreceptor apoptosis and MG-induced regeneration response in the central-dorsal regions of the pigmented retinas. By performing parallel light lesioning experiments with albino and normally pigmented zebrafish, we demonstrate that the timeline of photoreceptor apoptosis and regeneration response is largely identical. These results are significant as they allow the use of existing pigmented transgenic reporter lines in the constant intense light lesion model without having to breed new albino lines. Additionally, this paradigm does not require the use of an expensive wide-spectrum (UV) light source, is relatively simple and cheap to set up, and regeneration in the central-dorsal retina can be compared between different zebrafish lines, irrespective of their pigmentation status.
Methods
Fish maintenance
Zebrafish (Danio rerio) were maintained in 14 h:10 h light-dark cycles at 28.5 °C. The following lines were used: fish line [1], tg:zop:nfsB:EGFP [6], tg:1016tuba1a:gfp [4], tg:gfap:gfp [17], and AB (wild-type). All experiments were performed with the approval of the Vanderbilt University Institutional Animal Care and Use Committee (Protocol # M/09/398).
Constant intense light lesioning
Adult zebrafish between 6- and 9-months old were used for the constant intense light lesioning. Light lesioning was performed as previously described, with a few modifications [1]. Briefly, adult zebrafish were dark adapted for 14 days, transferred to clear tanks placed between two fluorescent lights with light intensity at ~20,000 lux for 16 h to 4 days. The distance from the tank to each light source was ~10 cm. Each light treatment tank contained about 20 free-swimming zebrafish. An aquarium water heater (25 W) and one to two air bubblers were introduced into each tank to maintain the water temperature at 30–33 °C.
Immunohistochemistry and TUNEL assay
Adult zebrafish eyes were collected and fixed in 4% paraformaldehyde for 2–5 h at room temperature followed by cryoprotection in 30% sucrose/1X PBS recipe (136.9 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4; pH 7.4) and embedding in Shandon cryomatrix (Thermo Scientific, Waltham, MA). Eyes were cryosectioned at 10–12 µm and collected on charged Histobond slides (VWR, Radnor, PA). For immunohistochemistry (IHC), slides were rehydrated in 1X PBS and blocked with serum before overnight primary antibody incubation. Primary antibodies used were mouse anti-proliferating cell nuclear antigen (PCNA) monoclonal antibody (1:500; Sigma, St.Louis, MO), rabbit anti-green fluorescent protein (anti-GFP) polyclonal antiserum (1:1,000; Torrey Pines Biolabs, Secaucus, NJ), mouse anti-glutamine synthetase monoclonal antibody (1:200, Millipore, Billerica, MA), and mouse anti-zpr-1 monoclonal antibody (1:200; ZIRC, Eugene, OR). Following primary antibody incubation, sections were washed before secondary antibody and nuclear stain TOPRO 3 (1:1,000; Invitrogen, Carlsbad, CA) application. Secondary antibodies used were donkey anti-mouse AF488 (1:200), donkey anti-mouse Cy3 (1:100), donkey anti-mouse Cy5 (1:100), donkey anti-rabbit Cy3 (1:200), and donkey anti-rabbit AF488 (1:200; Jackson Immuno, West Grove, PA). Slides were washed, dried, and mounted with Vectashield (Vector Laboratories, Burlingame, CA). For PCNA IHC, antigen retrieval with 10 mM sodium citrate buffer containing 0.05% Tween-20 (pH 6) was performed before blocking. For terminal deoxynucleotidyl dUTP nick end labeling (TUNEL) assays, sections were processed as for IHC. TUNEL assays were performed using the TMR red in situ cell death detection kit (Roche, Indianapolis, IN).
Cell counts, retina morphometry, and statistical analyses
Confocal microscopy was performed using a Zeiss LSM 510 META inverted confocal microscope at the Vanderbilt Cell Imaging Shared Resource. Only retina sections containing optic nerves were used for IHC, retina morphometry, and quantifications. Unless otherwise noted, all quantifications were done in the central-dorsal retina at a linear distance of ~300 µm from the optic nerve. ImageJ (National Institutes of Health, Bethesda, MD) was used to measure the outer nuclear layer (ONL) thickness in the retina morphometric analyses. Five to seven retinas were used for each time point. For each retina, the average of three independent measurements of the ONL thickness across the central-dorsal retina was calculated. In all figures, data are represented as mean±standard deviation (SD). One-way analysis of variance (StatPlus software, Analystsoft) followed by Tukey’s honest significant difference post hoc test were used to calculate significance (p values). In all other figures, Student t tests were used to calculate significance.
Results
Constant intense light exposure of pigmented zebrafish destroys both rod and cone photoreceptors
To assess the effects of constant intense light exposure on pigmented zebrafish retinas, we subjected normally pigmented adult wild-type zebrafish (AB background) to constant intense light. Following a 2-week dark-adaptation period, AB fish were exposed to intense light for varying periods of time, after which retinas were dissected and probed using the Zpr1 antibody that recognizes red-green double cones. At the 0 h time point, before intense light exposure, we detected an ordered arrangement of zpr1+ double cones in the ONL with outer segments (OS) projecting beyond the ONL (Figure 1; arrowheads). However, by 24 h of intense light exposure, we detected disorganization and hypertrophy of the zpr1+ double cones in addition to thinning of the ONL across the central-dorsal retina (compare brackets in 0-h and 24-h panels), with sporadic damage in the ventral retina (data not shown). By 48 h of intense light exposure, the cone photoreceptor OS began to breakdown further and the cells appeared detached from the ONL. By 72 h, the ONL was thinner and the cone cells lost their projections entirely and appeared clumped or condensed (arrows).
Figure 1.
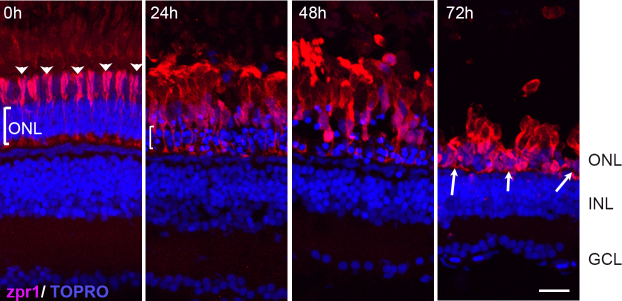
Effects of constant intense light exposure on cone cells of pigmented AB zebrafish. Retinas were collected from zebrafish before (0 h) and after the indicated length of light exposure. Retinas were processed for immunohistochemistry and stained with zpr1 antibody (red). Nuclei were counter-stained with TOPRO (blue). Dorsal retinas are shown. The arrowheads at 0 h indicate the ordered arrangement of the double cones; the bracket indicates ONL. White arrows show condensed zpr+ cells at 72 h of intense light. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar is 50 µm.
Intense light exposure induced thinning of the ONL, indicating loss of rod photoreceptors in addition to cones. To confirm rod loss, we used the pigmented zop:nfsB:EGFP transgenic zebrafish, which express enhanced green fluorescent protein (EGFP) specifically in rod photoreceptors [6], and subjected them to constant intense light lesioning. Retinas were collected at different time points before (0 h) and during intense light treatment (16 h, 35 h, 48 h, 72 h), processed for IHC and stained with zpr-1 and anti-GFP antibodies (Figure 2A). At 0 h, an intact array of GFP+ rod photoreceptors interspersed with zpr-1+ cones was detected in zop:nfsB:EGFP retinas. Rod OS were clearly seen projecting beyond the ONL. Disorganization of GFP+ rods and loss of rod OS were detected as early as 16 h of light exposure. By 35 h, there were fewer GFP+ and zpr-1+ cells in the ONL. By 72 h of light exposure, the ONL was much thinner with few GFP+ rod cells remaining. These cells looked rounded up and had lost their outer segments (arrows). The few zpr-1+ cones that persisted appeared condensed and had lost their projections.
Figure 2.
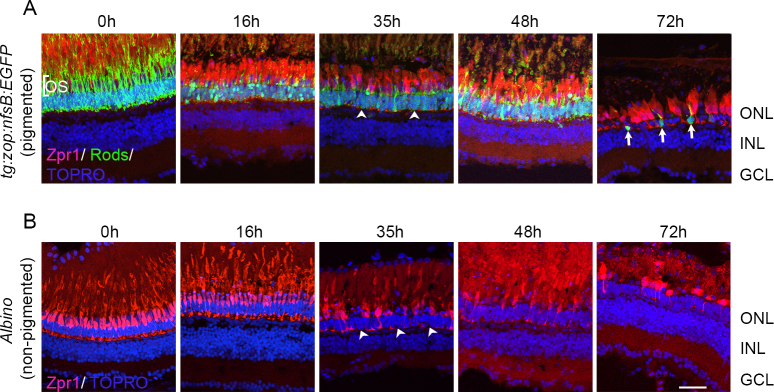
Photoreceptor loss in pigmented and nonpigmented retinas. Pigmented tg:zop:nfsB:EGFP zebrafish (A) or nonpigmented albino zebrafish (B) were subjected to intense light damage, and retinas were collected at the indicated time points and processed for immunohistochemistry. Retinas in (A) were stained with zpr-1 (red) and anti-green fluorescent protein (GFP; green) antibodies, while retinas in (B) were stained with zpr-1 (red) antibody; nuclei were counter-stained with TOPRO (blue). Dorsal retinas are shown. Arrowheads indicate regions of photoreceptor loss. Arrows denote rod photoreceptors that have rounded up and lost their outer segments. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; OS, outer segments. Scale bar is 50 µm.
Importantly, when we exposed fish line zebrafish to light treatment in parallel with the zop:nfsB:EGFP transgenic zebrafish, we observed that photoreceptor destruction occurred at comparable times in the pigmented and nonpigmented zebrafish retinas (Figure 2B). As in the pigmented retinas, by 35 h cone photoreceptor loss was observed in the albino retina (arrowheads), and by 72 h most cone photoreceptors were destroyed. Together, these studies show that the intense light paradigm used to lesion nonpigmented albino zebrafish was efficient in damaging normally pigmented retinas, and the time course of photoreceptor loss was identical between pigmented and nonpigmented retinas. Additionally, spatial loss of photoreceptors in the pigmented retinas was akin to that observed in albino retinas, with severe photoreceptor loss in the dorsal-central retina and patchy damage in the ventral retina.
Constant intense light exposure induces apoptosis of photoreceptors in pigmented zebrafish retinas
In albino retinas, intense light exposure induces photoreceptor apoptosis. The rounded and condensed photoreceptor morphology that we observed in the light-treated pigmented retinas was highly indicative of photoreceptors dying by apoptosis. To verify this, we performed TUNEL on sections from AB retinas exposed to constant intense light for different lengths of time (Figure 3). No TUNEL staining was detected before light exposure (0 h). However, as light treatment proceeded, several TUNEL+ nuclei were observed in the ONL, with TUNEL staining peaking at 24 h and attenuating at later time points.
Figure 3.
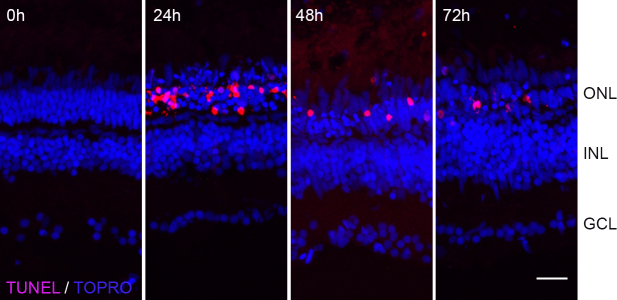
Terminal deoxynucleotidyl dUTP nick end labeling (TUNEL) labeling of light-treated pigmented retinas. Retinas from light-treated AB fish were collected at different times during light exposure and processed for TUNEL labeling (red). Nuclei were counterstained with TOPRO (blue). Scale bar is 50 µm.
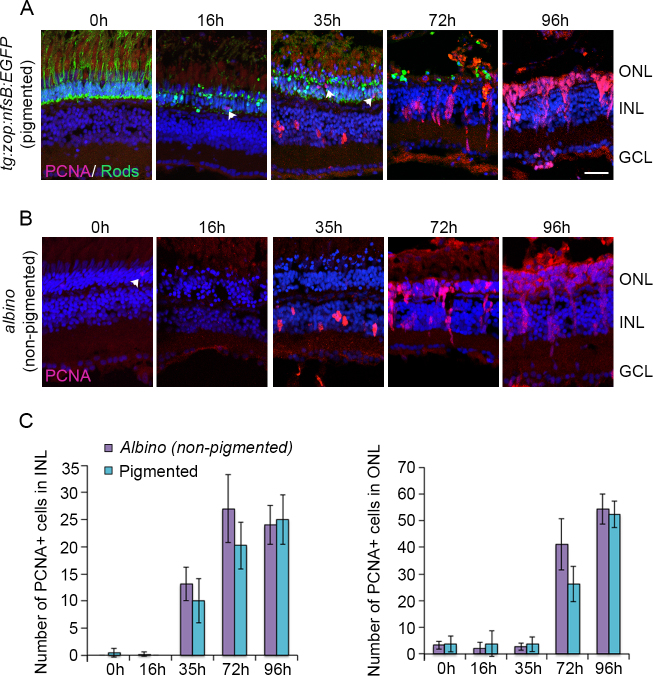
Previous studies in albino zebrafish established the peak of photoreceptor apoptosis at approximately 1 day of intense light exposure [1]. To directly compare the timeline of photoreceptor apoptosis in pigmented and nonpigmented retinas, we exposed fish line zebrafish to light damage concurrent with zop:nfsB:EGFP transgenic fish and quantified the number of TUNEL+ nuclei in the ONL. As shown in Figure 4A, TUNEL+ nuclei began to appear in the ONL of the tg:zop:nfsB:EGFP by 16 h of light exposure. Strikingly, the GFP+ rod photoreceptors lost their OS and became shortened. TUNEL labeling increased at 35 h of light exposure but was drastically reduced by 48 h in light and continued to decrease at 72 h and 96 h. By 72 h, few rods remained, and these cells looked condensed and lacked OS (arrowheads). When we compared the timeline of TUNEL labeling between the nonpigmented albino retinas and pigmented retinas, there were significantly fewer TUNEL+ nuclei in the pigmented retina ONL at 16 h compared to nonpigmented albino retinas (p<0.005; n = 5 retinas). However, by 35 h, TUNEL labeling was identical between the two groups and continued to taper off at later time points (Figure 4C). We also monitored changes in the thickness of the ONL across regeneration (Figure 4D). By 16 h of light damage, the ONL thickness was significantly reduced in both pigmented and albino zebrafish retinas (p<0.05; n = 5–7 retinas). However, we did not observe any differences in the ONL thickness between the pigmented and albino groups, indicating that the rate of photoreceptor loss was identical between both groups.
Figure 4.
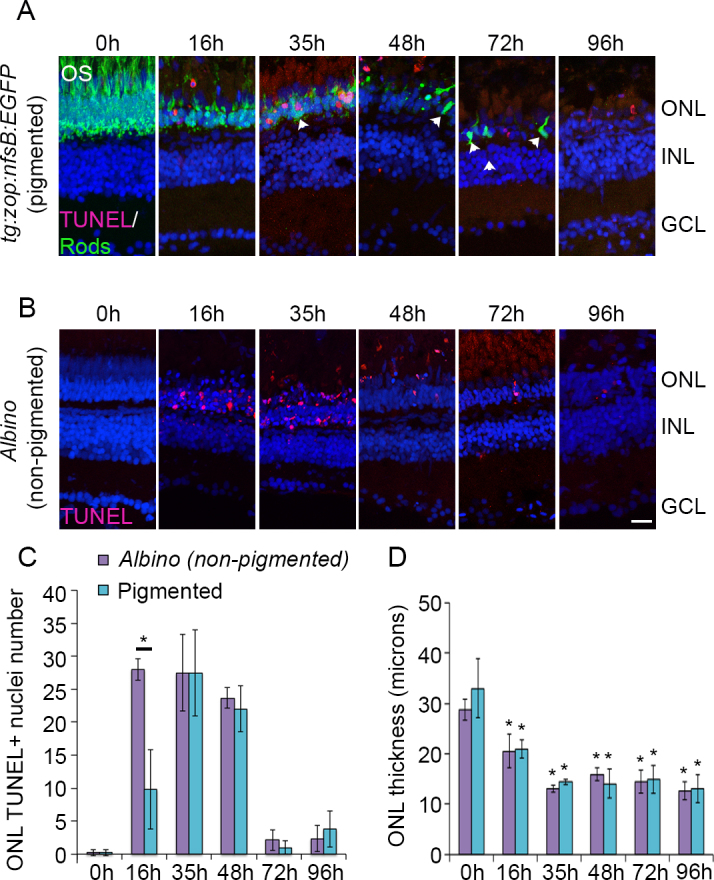
Time course of photoreceptor apoptosis in pigmented and nonpigmented retinas. Pigmented tg:zop:nfsB:EGFP zebrafish (A) or nonpigmented albino zebrafish (B) were subjected to intense light damage, and retinas were collected at the indicated time points and processed for terminal deoxynucleotidyl dUTP nick end labeling (TUNEL) labeling. A: Rods were stained with anti-green fluorescent protein (GFP; green) antibody; nuclei were counterstained with TOPRO (blue). Dorsal retinas are shown. Arrowheads indicate rods that appear condensed and lack OS. C: Quantification of TUNEL labeling for albino and pigmented zebrafish. Data represent mean±standard deviation (SD); *, p<0.005, n = 3–7 retinas for each time point. D: Quantification of the ONL thickness for albino and pigmented zebrafish. All comparisons were made at 0 h. Data represent mean±SD; *, p<0.05 using one-way analysis of variance with Tukey’s honest significant difference post hoc test; n = 5–7 retinas for each time point. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; OS, outer segments. Scale bar is 50 µm.
Constant intense light exposure triggers Müller glia cell cycle re-entry and progenitor cell proliferation in pigmented zebrafish retinas
In albino zebrafish, light-induced photoreceptor apoptosis triggers MG proliferation, which generates PCNA+ progenitor cells. These progenitors rapidly divide to form clusters of progenitors that migrate to the ONL and differentiate into photoreceptors [18]. We wanted to examine if light damage of pigmented zebrafish retinas stimulated the MG-induced regeneration response and if the timeline of the response corresponds to that in the albinos. For this, we exposed both tg:zop:nfsB:EGFP fish and albino fish to the intense light paradigm, collected retinas at various stages, and monitored progenitor generation and proliferation using PCNA IHC (Figure 5). We did not detect any statistically significant differences between the PCNA response in the pigmented tg:zop:nfsB:EGFP fish and the albino fish (Figure 5C). In both groups, PCNA+ cells began to appear in the inner nuclear layer (INL) around 35 h of light exposure. By 72 h, progenitor clusters have elaborated in the INL and the progenitor cells start migrating to the ONL. By 96 h, most of the PCNA+ progenitor cells have reached the ONL. In the tg:zop:nfsB:EGFP fish, the PCNA expression pattern was consistent across the dorsal-central retina but remained sporadic in the ventral retina.
Figure 5.
Proliferating cell nuclear antigen (PCNA) expression during intense light exposure. Pigmented tg:zop:nfsB:EGFP zebrafish (A) or nonpigmented albino zebrafish (B) were subjected to intense light damage, and retinas were collected at the indicated time points and processed for immunohistochemistry. Retina sections were stained with anti-green fluorescent protein (GFP; green in panel A) and anti-PCNA (panels A and B) antibodies; nuclei were counterstained with TOPRO (blue). Dorsal retinas are shown. Arrowheads indicate PCNA+ rod precursors in the ONL. C: Quantification of PCNA+ cells in the INL and ONL. Data represent mean±standard deviation; n = 3–7 retinas per time point. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar is 50 µm.
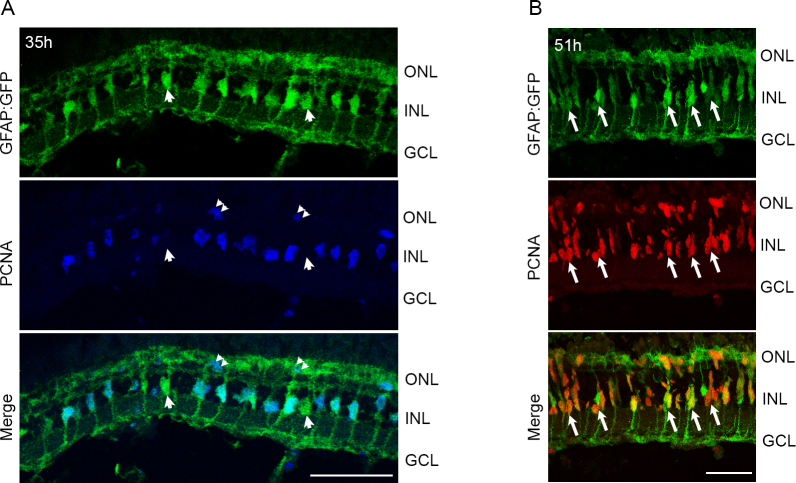
To confirm that the PCNA+ cells in the INL at 35 h of light were indeed MG, we used the pigmented tg:GFAP:GFP line [17], which expresses GFP under the glial-specific glial fibrillary acidic protein (GFAP) promoter. After 35 h of light exposure, numerous GFP+ MG in the dorsal-central retina expressed PCNA (Figure 6A). By 51 h of light exposure, ~85% of MG expressed PCNA or were associated with PCNA+ progenitor cells (85±7% of MG were PCNA+; n = 7 retinas; Figure 6B, arrows). Some progenitors were migrating to the ONL.
Figure 6.
Light treatment of pigmented retinas stimulates proliferation of MG and progenitor cells. tg:GFAP:GFP fish were exposed to intense light for 35 h (A) or 51 h (B), and retinas were processed for immunohistochemistry. A: Proliferating cell nuclear antigen (PCNA) immunohistochemistry (blue) reveals co-staining with GFP+ MG in the central-dorsal retina. Few MG do not express PCNA (arrowheads in A). PCNA also labels rod precursors in the ONL (double arrowheads). B: Most GFP+ MG continue to express PCNA. Arrows indicate MG associated with small PCNA+ progenitor clusters. Few PCNA+ cells are migrating to the ONL. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bars are 50 µm.
Together, these data demonstrated that intense light exposure triggered MG cell cycle re-entry, progenitor cell generation, and proliferation in the pigmented zebrafish. Importantly, the time line of the regeneration response was consistent with that in albino zebrafish.
Constant intense light exposure triggers tuba1a:green fluorescent protein transgene expression in dedifferentiated Müller glia and progenitor cells
MG dedifferentiation postretinal damage is one of the earliest steps in retina regeneration. In the transgenic pigmented zebrafish 1016:tuba1a:GFP [4], GFP expression is specifically driven in the dedifferentiated MG by activation of a 1.7-kb α1 tubulin promoter, and GFP persists in the MG-derived progenitor cells. Previously, this transgenic line has been used in a puncture injury model of retina regeneration to label dedifferentiated MG and progenitor cells [4,19].
To evaluate the applicability of this transgenic line to study light-damage-induced retina regeneration, we exposed these fish to the constant intense light paradigm and monitored GFP expression using IHC (Figure 7A). We did not detect any GFP expression before light damage (0 h). However, after 24 h of light exposure, we detected a few GFP+ cells in the INL of the dorsal retina (arrowheads in A). By 51 h, several GFP+ cells occupied the INL of the central-dorsal retina and most of the ventral retina (data not shown). GFP+ processes extended into the IPL, morphologically resembling MG processes. We also detected few GFP+ cells in the ONL. By 72 h, GFP+ cells existed in large cell clusters within the INL (double arrowheads) and staining persisted in the processes spanning the IPL, which was highly indicative of progenitor cell clusters associated with MG. We also detected a large number of GFP+ cells in the ONL. To verify that the tuba1a:GFP-expressing cells were indeed MG, we co-stained 51-h sections with an antibody against MG-specific glutamine synthetase (GS; Figure 7B). Approximately 80% of the GS+ cells expressed GFP, indicating that these were MG that had dedifferentiated post-light damage (arrows; 81±8%; n = 3 retinas).
Figure 7.
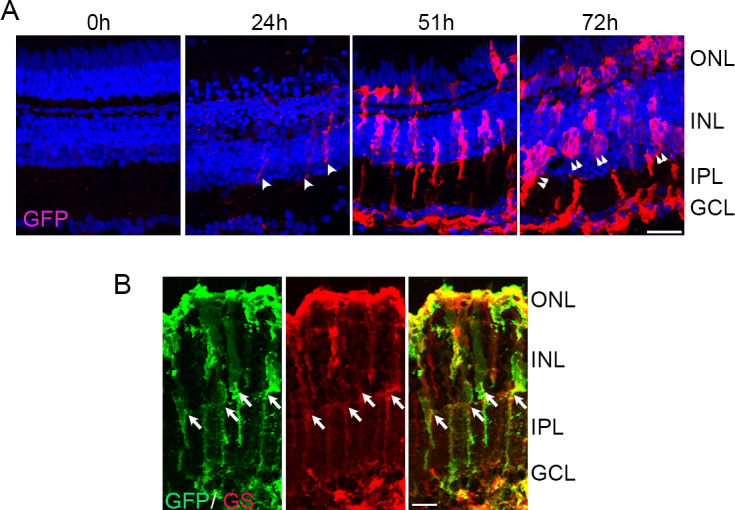
Light damage triggers tuba1a:GFP transgene expression in Müller glia (MG). A: tg:1016tuba1a:GFP zebrafish were exposed to the constant intense light paradigm, and retinas were collected at the indicated time points and assessed for GFP expression (red) by immunohistochemistry. GFP+ cells began to appear in the INL by 24 h (arrowheads). By 72 h, clusters of GFP+ cells were detected in the INL and ONL (double arrowheads). B: Immunohistochemistry for GFP and GS in 51-h light-exposed 1016tuba1a:GFP retinas. Arrows indicate GS+ and GFP+ MG. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; IPL, inner plexiform layer. Scale bars are 50 µm.
To confirm that the GFP+ cell clusters we observed at 72 h of light exposure were progenitor cell clusters that associate with MG, we co-stained the light-damaged retina sections with PCNA (Figure 8). We detected few PCNA+ rod precursors in the ONL of 0-h retinas (arrowheads). By 51 h, most of the INL was occupied by GFP+/PCNA+ cells and we detected small progenitor cell clusters containing two to four progenitor cells associating along a GFP+ MG process (double arrowheads). The large clusters of GFP+ cells at 72 h of light co-labeled with PCNA confirmed that these were indeed clusters of progenitor cells that were migrating to the ONL (arrows).
Figure 8.
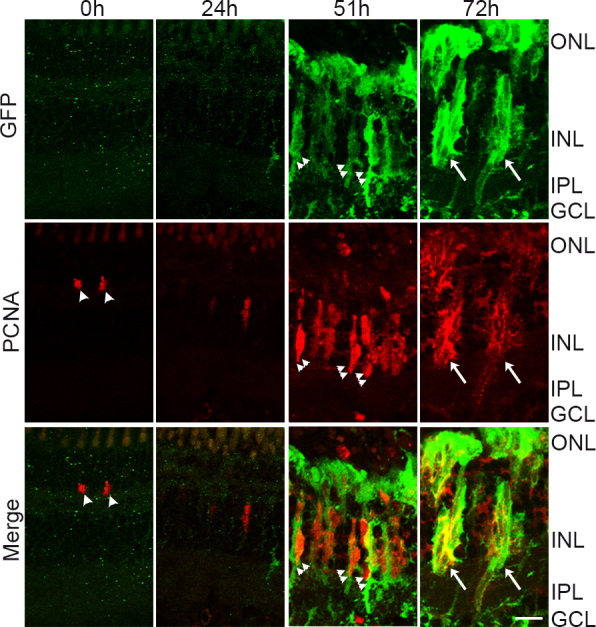
Proliferating progenitor cells express tuba1a:GFP transgene following light damage. tg:1016tuba1a:GFP zebrafish were exposed to the constant intense light paradigm, and retinas were collected at the indicated time points and assessed for green fluorescent protein (GFP, green) and proliferating cell nuclear antigen (PCNA, red) expression in the central-dorsal retina by immunohistochemistry. Small clusters of GFP+/PCNA+ cells associate with MG processes at 51 h (double arrowheads). Large clusters of GFP+/PCNA+ cells span in the INL at 72 h (arrows). Arrowheads indicate rod precursors in the ONL. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; IPL, inner plexiform layer. Scale bar is 50 µm.
Discussion
Here, we demonstrated that the constant intense light exposure model of lesioning retinal photoreceptors, which has been restricted to albino zebrafish, is also effective in lesioning pigmented retinas. Intense light exposure causes photoreceptor cell death within a day of exposure, prompts MG cell cycle re-entry, and induces progenitor cell production. The progenitor cells proliferate, form clusters associated with MG, and migrate to the ONL. Importantly, by performing simultaneous light lesioning, we showed that the timeline of initiation and progression of the regeneration response is identical in pigmented and albino fish.
There are two limitations to the extent of damage. First, photoreceptor apoptosis was extensive in the dorsal and central retinas, while damage was inconsistent and sparse in the ventral retina. However, this is also seen in albino zebrafish and rodents [16,20]. Although the exact causes for the regional difference remain unknown, possible explanations could be the presence of the retinal tapetum in the dorsal retina or the shorter rod outer segments found in the ventral retina. Ventral rod photoreceptors contain lesser amounts of rhodopsin, which limits constant intense light-induced damage [14]. Second, quantification revealed an early delay in the initiation of ONL apoptosis, although by 35 h, apoptosis looks identical to albino retinas (Figure 4C). In our analyses, we detected almost complete loss of rods and a large number of double cones by 3 days of light exposure (Figure 2). It is possible that intense light does not completely destroy other cone types in the pigmented retina or has a delayed effect on these cells. This, however, seems unlikely since others have reported that red-green double cones appear to be less susceptible to light-induced damage than UV and blue cones [2]. Probing UV cones and blue cones using specific antibodies will be important to address this issue. Melanin in the retinal pigment epithelium (RPE) of pigmented retinas also absorbs light, thus accounting for the delay in the onset of photoreceptor apoptosis compared to albino retinas. Reduced or delayed light-induced photoreceptor damage has been observed in fish and rats with pigmented retinas [2,11,20]. Albino retinas lack melanin, which allows for light to be scattered back to the photoreceptors, allowing more photoreceptor damage.
Regardless of these limitations, the extent of photoreceptor damage is sufficient to trigger a robust regeneration response in the dorsal-central retina of pigmented fish. By 51 h in light, ~85% of GFAP+ MG in the dorsal-central retina expressed PCNA and/or associated with PCNA+ progenitor cells (Figure 6) and ~80% of GS+ MG had dedifferentiated and expressed the tuba1a:GFP transgene (Figure 7). Additionally, progenitor cell proliferation and migration were identical to albino retinas (Figure 5), although it will be interesting to see if regeneration is completed at comparable times. Intriguingly, we detected tuba1a:GFP activation across most of the ventral retinas that we examined, although photoreceptor damage was sporadic in this region. Nevertheless, we rarely detected a proliferation response in the ventral retina. It is possible that extensive damage in the dorsal-central retina triggers MG dedifferentiation in ventral regions through a secreted or diffusible factor [21,22]. In the puncture model of retina damage, four to six “poke” lesions across the retina are sufficient to trigger MG dedifferentiation and proliferation response across the entire retina [4,19]. In our model, damage of the dorsal-central retina is not sufficient to trigger a proliferation response in the ventral retina.
Although constant intense light exposure can lesion pigmented zebrafish retinas, the regional differences in damage admittedly limit some experimental procedures. Examination of specific retinal regions using IHC or in situ hybridization should be reliable, whereas retina-wide gene, transcriptome, or protein profiling might be challenging. Nevertheless, this study demonstrates that both normally pigmented and albino zebrafish can be damaged using the constant intense light paradigm and the timing of initiation and progression of regeneration is identical irrespective of the pigmentation status of the zebrafish used. In addition to expanding the tools available to study molecular mechanisms underlying photoreceptor regeneration, this study allows direct comparisons of regeneration between different zebrafish lines.
Acknowledgments
We thank D. Hyde (University of Notre Dame) and D. Goldman (University of Michigan) for sharing zebrafish lines. We thank Qiang Guan for excellent zebrafish care at the Vanderbilt University Stevenson Center Fish Facility. This study was supported by a grant from the National Eye Institute–National Institutes of Health to J.G.P (R21EY019759). K.R. was supported in part by the Vanderbilt International Scholar Program and by the Gisela Mosig Fund in the Department of Biologic Sciences.
References
- 1.Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 2.Weber A, Hochmann S, Cimalla P, Gärtner M, Kuscha V, Hans S, Geffarth M, Kaslin J, Koch E, Brand M. Characterization of light lesion paradigms and optical coherence tomography as tools to study adult retina regeneration in zebrafish. PLoS ONE. 2013;8:e80483. doi: 10.1371/journal.pone.0080483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–40. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–13. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–24. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montgomery JE, Parsons MJ, Hyde DR. A novel model of retinal ablation demonstrates that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors. J Comp Neurol. 2010;518:800–14. doi: 10.1002/cne.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu DM, Schneiderman T, Burgett J, Gokhale P, Barthel L, Raymond PA. Cones regenerate from retinal stem cells sequestered in the inner nuclear layer of adult goldfish retina. Invest Ophthalmol Vis Sci. 2001;42:2115–24. [PubMed] [Google Scholar]
- 8.Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish Muller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013;140:4510–21. doi: 10.1242/dev.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thummel R, Enright JM, Kassen SC, Montgomery JE, Bailey TJ, Hyde DR. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res. 2010;90:572–82. doi: 10.1016/j.exer.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966;5:450–73. [PubMed] [Google Scholar]
- 12.Wenzel A, Grimm C, Marti A, Kueng-Hitz N, Hafezi F, Niemeyer G, Remé CE. c-fos controls the “private pathway” of light-induced apoptosis of retinal photoreceptors. J Neurosci. 2000;20:81–8. doi: 10.1523/JNEUROSCI.20-01-00081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karin M, Chang L. AP-1–glucocorticoid receptor crosstalk taken to a higher level. J Endocrinol. 2001;169:447–51. doi: 10.1677/joe.0.1690447. [DOI] [PubMed] [Google Scholar]
- 14.Thomas JL, Nelson CM, Luo X, Hyde DR, Thummel R. Characterization of multiple light damage paradigms reveals regional differences in photoreceptor loss. Exp Eye Res. 2012;97:105–16. doi: 10.1016/j.exer.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas JL, Thummel R. A novel light damage paradigm for use in retinal regeneration studies in adult zebrafish. Journal of visualized experiments. JoVE. 2013;80:e51017. doi: 10.3791/51017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vihtelic TS, Soverly JE, Kassen SC, Hyde DR. Retinal regional differences in photoreceptor cell death and regeneration in light-lesioned albino zebrafish. Exp Eye Res. 2006;82:558–75. doi: 10.1016/j.exer.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–13. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Kassen SC, Ramanan V, Montgomery JE. T Burket C, Liu CG, Vihtelic TS, Hyde DR. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67:1009–31. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010;12:1101–7. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapp LM, Williams TP. The role of ocular pigmentation in protecting against retinal light damage. Vision Res. 1980;20:1127–31. doi: 10.1016/0042-6989(80)90050-4. [DOI] [PubMed] [Google Scholar]
- 21.Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Muller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22:334–47. doi: 10.1016/j.devcel.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson CM, Ackerman KM, O'Hayer P, Bailey TJ, Gorsuch RA, Hyde DR. Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Muller glia proliferation during zebrafish retinal regeneration. J Neurosci. 2013;33:6524–39. doi: 10.1523/JNEUROSCI.3838-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]