Introduction
Aquaporin zero (AQP0) is a water channel expressed almost exclusively in fiber cells of the lens of the eye. There is a long-standing hypothesis that AQP0 functions as both a water channel and a cell-to-cell adhesion protein in the lens. This hypothesis was supported in 2004 by the observation of AQP0 mediated cell-to-cell junctions in the lens (1) and in 2013 by observation of in vitro AQP0-mediated cell-to-cell adhesion (2). Absence of AQP0 or certain mutations in it lead to cataract and sometimes complete maldevelopment of the lens, so the essential nature of the protein to lens function is well established experimentally. In addition, the water permeability of AQP0 in model systems shows regulation by both calcium and pH, and phosphorylation alters the regulation by calcium (3). In normal lenses, protein kinase A (PKA) and AQP0 are associated with the A-kinase anchor protein AKAP2. In organ-cultured lenses, breaking the link between AKAP2 association with PKA and AQP0 results in a cortical cataract (4), suggesting a connection among phosphorylation, water permeability regulation, and cataract in vivo. Zebrafish lenses express two isoforms of AQP0: AQP0a is a water channel; AQP0b appears to provide functions other than water permeability, possibly the cell-to-cell adhesion function. Knockdown of AQP0a cannot be rescued by either a permanently phosphorylated form of AQP0 or a form that can never be phosphorylated; neither of these have calcium-mediated regulation of water permeability. Regulation of the water permeability of AQP0 is clearly essential for proper lens function. But this is only part of the story.
A rising tide of data supports the view that the lens, which lacks blood vessels but is still too large to subsist on diffusion alone, generates its own intrinsic circulation of fluid (Fig. 1) (5–8). Compelling evidence argues that this circulation is powered by the Na/K ATPase in the equatorial epithelial cells, where sodium is extruded and a large transmembrane electrochemical potential for sodium entry is established. An inward current of sodium in lens extracellular spaces, combined with sodium leak channels in the lens fiber cells, generates an osmotic gradient to promote the flow of water through interstitial space and into the cells via osmotically driven flow through AQP0. The circulation carries nutrients and antioxidants into the lens, and it presumably also carries out waste products, although the evidence for this is circumstantial (9,10). The inward flow of water in the interior fiber cells of the lens leads to a buildup of fluid that generates a large intracellular hydrostatic pressure, of ∼1/3 of an atmosphere in the lens center, which drives fluid flow outward through gap junctions. This pressure may well be critical in increasing the index of refraction of the lens progressively toward the lens center and thus correcting for spherical aberration.
Figure 1.
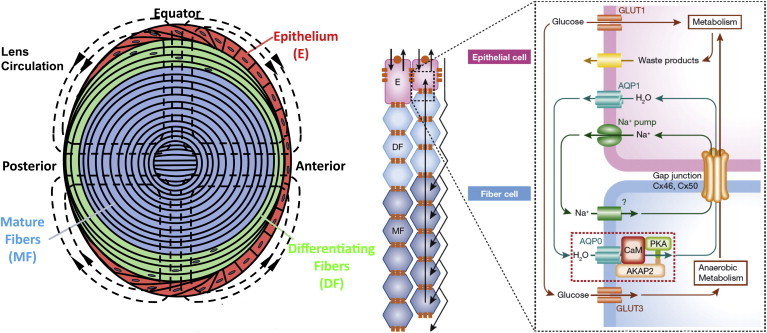
The intrinsic lens circulation. (Left-hand panel) Path of currents generated by the action of the NaK ATPase. (External dotted lines) Path of measured currents; (dotted lines inside the lens) proposed current pathways based on modeling. (Right-hand panel) Selection of proposed players in the circulation (reprinted with permission from EMBO Mol. Med. 2011, vol. 4, pp. 1–2). To see this figure in color, go online.
What is the Puzzle?
Because the fiber cell membrane water permeability is very large relative to the sodium permeability, altering water permeability of fiber cell membranes by a factor of 2–4 (similar to the regulatory changes in AQP0 water permeability) should have essentially no effect on fluid flow and produce only a very tiny change in the intracellular sodium concentration. (Note that we are interested only in the water permeability of the fiber cell membranes. There is plenty of aquaporin 1 in the epithelial cell surface layer, but this will have little effect on the internal lens circulation. In addition, aquaporin 5 has been found in fiber cells (2,11), but there is no experimental evidence on whether or not its water permeability is regulated or what effects its knockdown or knockout has on lens properties such as there is for AQP0.) This is because water flow entering the fiber cell membranes is essentially isotonic. To get a feeling for why the osmotic gradient is essentially zero, consider the limit of water permeability becoming infinite. If this is the case, even a vanishingly small osmotic gradient will produce very large fluid flow. This is called the isotonic limit (but it may help to think of it as the infinite water permeability limit).
Now realize that if the water permeability is large enough compared to the sodium permeability, then if water permeability increases, the sodium generated osmotic gradient will decrease, but water flow will remain essentially constant nearly at its isotonic limit. This is apparently the case in the lens. Sodium leak permeability is quite small, so water permeability only needs to be small to allow fluid flow driven by very small sodium concentration gradients, essentially at the isotonic limit. Why then does water permeability, especially its regulation, matter? And what other functions might aquaporin zero supply? The possible answers to these questions constitute the subject of this review.
The Evidence That Changing Water Permeability Should Not Matter
There are many fluid-transporting epithelia in the body, and where the osmolarity of the transported solution was measured, it was often indistinguishable from isotonic with the surrounding bathing solution. Mathias and Wang (12) used modeling to investigate the coupling of water flow and salt transport in planar epithelia to obtain insight on how osmosis could lead to near isotonic transport. Although they did not specifically consider the spherical lens, some of their conclusions should carry over to any geometry. They identified a fundamental parameter termed ε (first used by Segel (13) in a perturbation analysis of epithelial transport). The physical basis of ε was the ratio of membrane salt permeability to water permeability, and where data were available (14), ε was very small, of ∼10−3. A general conclusion was that for the transported solution to approach isotonic, ε had to be a very small parameter. In the mouse lens, the fiber cell membrane sodium permeability can be estimated from electrophysiology studies, and the water permeability from fiber-cell-membrane vesicle swelling assays. These estimates give ε = 4 × 10−4, so in the lens it is indeed small.
When ε is small, the analysis predicted water flow would be directly proportional to membrane salt transport. This prediction was supported in the lens by studies that altered fiber cell membrane sodium influx and observed the intracellular hydrostatic pressure gradient varied proportionally (7). Moreover, the idea that water flow is osmotically generated at the membrane was further supported in the lens by altering gap junction coupling conductance and observing the effect on the intracellular hydrostatic pressure gradient (7). The prediction was that water flow would not change because membrane salt transport had not changed, but the pressure gradient needed to drive the water flow would change in proportion to the resistance of the outflow pathway (5). The intracellular pressure gradient was indeed proportional to the intracellular hydraulic resistivity. Lastly, the analysis in Mathias and Wang (12) suggested that when ε is small and the width of extracellular clefts is small in comparison to their length, a situation that is maximally true for the lens relative to planar epithelia, then water flow would not be significantly affected by the membrane water permeability. The physical basis for this is that transmembrane sodium influx is rate-limiting, and because transmembrane water flow is near its isotonic limit, it would remain near that limit unless there was a dramatic reduction in membrane water permeability. Here, we provide data supporting this last prediction.
Mouse lenses that transgenically express AQP1 in fiber cell membranes have a membrane water permeability that is ∼3.5-fold larger than wild-type (15). Conversely, heterozygous AQP0+/− knockout lenses have a fiber-cell-membrane water permeability that is approximately half that of wild-type (16). To estimate the effect on water flow in wild-type and these two types of lenses, we measured intracellular hydrostatic pressure gradients (7) (Fig. 2, A and B). Altering the water permeability of fiber cell membranes had no effect on the pressure gradient profile, suggesting no effect on water flow. However, even if fluid circulation is constant, when gap junction coupling is decreased, the intracellular hydrostatic pressure gradient increases (7), so we also measured gap junction coupling in the three types of lenses.
Figure 2.
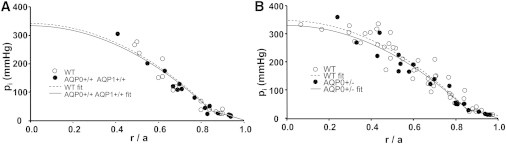
The intracellular pressure profile of the lens is not altered by changes in water permeability. Intracellular hydrostatic pressure is graphed as a function of normalized distance from the lens center (r/a), where r is the radial distance in centimeters and a is the lens radius in centimeters. (A) A comparison of wild-type (WT) lenses and lenses transgenically expressing AQP1 (AQP0+/+TgAQP1+/+) in their fiber cells (15). In WT lenses, a = 0.11 ± 0.003 cm and water permeability = 35 ± 5 μm/s. In AQP0+/+TgAQP1+/+ lenses a = 0.11 ± 0.004 cm and water permeability = 125 ± 19 μm/s. (B) A comparison of WT lenses and lenses lacking half of their AQP0 protein (AQP0+/−). In WT lenses, a = 0.12 ± 0.003 cm and water permeability = 36 ± 10 μm/s. In AQP0± lenses, a = 0.12 ± 0.004 cm and water permeability = 20 ± 5 μm/s.
The series resistance (17), which is the resistance of all the layers of gap junctions between the point of recording and the surface of the lenses, does not change when the water permeability is altered by changing the type and amount of water channel expressed. In fact, the pressure profile is maintained in the face of a number of challenges to lens transport and scales with the sodium flux and the size of the lens such that the pressure at the center of the lens is always ∼340 mmHg (17,18). Clearly the tenacity with which lenses of different species and different sizes cling to this particular value of hydrostatic pressure suggests that there is something fundamental about its importance to lens function. We suggest that this hydrostatic pressure gradient is one of the mechanisms for increasing the index of refraction toward the center of the lens and thus correcting for spherical aberration. Another may be variation in water permeability with distance into the lens. Both mechanisms could contribute to the level of hydration of intracellular proteins and thus regulate the index of refraction.
The Evidence That Water Permeability Matters
Experimental evidence makes a very solid case that water permeability provided by AQP0 (or added by AQP1) is essential for proper development of the lens, but the evidence that it is required to maintain the intrinsic circulation in the adult lens is less conclusive. Aquaporin 1(AQP1) expressed in mice lacking AQP0 can partially compensate for the absence of AQP0 (15). Because AQP1 water permeability is not regulated by calcium, and AQP1 cannot bind to other lens proteins such as AKAP2 (4) or filensin (19) and is probably not an adhesive protein, this partial restoration of lens clarity most probably results from AQP1 supplying the missing water permeability normally provided by AQP0.
Experiments in zebrafish also support the view that the water permeability provided by AQP0 is essential for lens clarity. Zebrafish have two AQP0s—Aqp0a, and Aqp0b. Aqp0a is a water channel, but Aqp0b is not. Knockdown/rescue experiments (summarized in Fig. 2) show that both aquaporins are essential for a clear lens and morpholino (MO) knockdown of either or both results in a cataract (20). MO knockdown of Aqp0a can be rescued by overexpression of MIPfun, an AQP0 from Fundulus heteroclitus, a telost fish distantly related to zebrafish, and MIPfun overexpression also rescues knockdown of Aqp0b. A mutant version of MIPfun, which completely lacks water permeability, MIPfun N68Q, cannot rescue the MO knockdown of Aqp0a. What is extremely interesting is that overexpression of MIPfun N68Q can rescue the MO knockdown of Aqp0b, demonstrating that MIPfun has whatever properties Aqp0b has over and beyond water permeability.
The zebrafish experiments provided two conclusions:
-
1.
The water permeability of Aqp0a is essential, and
-
2.
MIPfun and Aqp0b possess an additional property or properties also essential for lens clarity.
Of course the water permeability of other aquaporins, such as aquaporin 5 in fiber cells or aquaporin 1 in epithelial cells, may also be important, but there is no experimental evidence for or against an essential role of water permeability in the lens normally provided by these aquaporins.
Evidence That Regulation of Water Permeability Matters
The most direct evidence that regulation of water permeability is necessary for maintaining homeostasis in the adult lens comes from the work of Gold et al. (4). Gold and colleagues show that rat lenses maintained in organ culture remain clear for several days under control conditions but take on a cortical cloudiness when cultured in the presence of the drug Ht31, which breaks the link between AKAP2 and PKA. AKAP2 is a scaffolding protein that binds to the C-terminal tail of AQP0 and to PKA. Because AKAP2 connects PKA to AQP0, and phosphorylation of AQP0 alters the sensitivity of the water permeability of AQP0 to calcium (21), the further implication is that regulation of AQP0 water permeability is essential for clarity in the adult lens, but this conclusion assumes that the only effect of AQP0 phosphorylation is alteration of its water permeability.
Experiments in zebrafish also suggest that the ability to regulate water permeability is essential to lens clarity. Calcium concentration regulates the water permeability of MIPfun. As noted above, MIPfun can rescue the MO knockdown of Aqp0a, the zebrafish AQP0, which is a water channel. However, if serine 231 in the calmodulin binding region of MIPfun is phosphorylated, calcium concentration no longer alters MIPfun water permeability. The same is true if serine 231 is replaced by alanine, a residue that cannot be phosphorylated. Presumably both of these modifications alter the binding of calmodulin to the C-terminus. The payoff comes when rescue attempts are made with MIPfun S231D (the pseudophosphorylated mutant) or S231A (the mutant that cannot be phosphorylated). The water permeability of either of these mutants is not altered by changes in calcium concentration (22). Neither of the S231 site MIPfun mutants rescue the knockdown of Aqp0a but either can rescue the knockdown of Aqp0b (Fig. 3). Even though both MIPfun mutants have water permeability, the elimination of their ability to regulate their water permeability in response to changes in calcium concentration renders them unable to rescue knockdown of Aqp0a. Thus, two different systems suggest that not only is water permeability important, but so is its regulation by changes in phosphorylation. So water permeability seems to matter.
Figure 3.
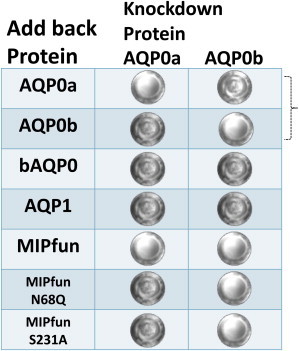
Mutations altering Aqp0 water permeability or its regulation result in cataract. The table shows the results of knockdown/rescue experiments in zebrafish. The two right-hand columns (AQP0a and AQP0b) show the protein knocked down by a morpholino. The clear lens icon shows that the rescue construct in the left-hand column can rescue the knockdown of the subject protein. The mottled cataract icon shows that the rescue construct (add back protein) in the left-hand column cannot rescue knockdown of the subject protein. (The icons are representative of normal and cataractous lenses and are not images from the particular experiments indicated.) Recall from the text that Aqp0a is a water channel, but Aqp0b is not. To see this figure in color, go online.
Alternatively, one could argue that, yes, changing the phosphorylation pattern does alter the ability of MIPfun and AQP0 to maintain lens clarity in fish and mouse, but this is due to effects of phosphorylation that have nothing to do with water permeability, perhaps binding to unknown proteins that change lens structure. The counter to this argument would be: it is the zebrafish water channel AQP0a, and not the AQP0b lacking water permeability, that is disrupted by these mutations. But even if regulation of water permeability is not essential, the conclusion remains that water permeability itself is essential, at least in the developing lens.
What Could be Going on?
We have confined ourselves to two avenues of investigation of the role of water permeability by examining the following:
-
1.
The predictions of a model of lens circulation that is enjoying accumulating evidence for its validity, and
-
2.
The experimental manipulation of water permeability by addition of AQP1 and deletion of wild-type or modified AQP0 in the lenses of experimental animals.
Of course there are other sources of water permeability in cells including the cells of the lens and there are other aquaporins in the lens, notably AQP5 and AQP1, but there are no experiments addressing the normal contribution of these proteins to lens clarity. The theoretical aspect of this review deals with water permeability, per se, from whatever source and finds that as long as there is still a tiny amount (much less than would be provided by all the AQP0 in the lens), the circulation will proceed normally. Thus, altering the amount of water permeability by whatever means should have no effect. But experimental manipulations contradict this notion.
Experiments in mouse and fish show clearly that interfering with regulation of water permeability mediated by AQP0 interferes with lens clarity (regardless of what any other aquaporins present might be doing). Normal regulatory changes in AQP0 water permeability are approximately two-to-fourfold. However, in the adult mouse lens, a twofold decrease or 3.5-fold increase in water permeability does not appreciably alter water flow (Fig. 2). So what is water permeability doing? Experimental interventions that eliminate regulation of water permeability are done at the embryonic stage and the resulting cataracts can be seen as soon as a lens is formed. This suggests regulation of AQP0 water permeability is critical for the developing lens, but we do not know how. Regulatory changes in membrane water permeability do not measurably affect water flow, so how are they important? This is the puzzle.
Changes in membrane water permeability are predicted to alter the transmembrane osmolarity (12). If membrane water permeability decreases, transmembrane osmolarity increases and water flow remains about the same. Could regulation of intracellular osmolarity be the target of regulation of AQP0 water permeability? Transmembrane osmolarity cannot be measured in the lens, but based on the same model calculations that predicted the results in Fig. 1, it is given by εco, where ε is the ratio of membrane salt permeability to water permeability and co is bulk osmolarity, ∼300 mM in mammals. In the outer fiber cells, there are data on water permeability (16) and sodium fluxes (23), and these data suggest ε ≈ 4 × 10−4, suggesting a transmembrane osmotic difference of 0.12 mM. This seems too small to have any significant physiological effects other than to generate fluid flow.
However, we do not know exactly what is happening in central fiber cells, where water permeability has not been measured. We know that the C-terminus of AQP0 is cleaved toward the center of the lens and that C-terminal cleavage tends to promote the junctional form of AQP0 over the single plasma membrane form (1), and there is no structural rational for the junctional form to contribute to plasma membrane water permeability. Whether or not the junctional form provides cell-to-cell water permeability is unknown. Thus, water permeability could decrease as AQP0 is converted from membrane form to junctional form. How much could the osmolarity change?
Varadaraj et al. (24) reported the water permeability of normal outer fiber cells was 35 μm/s whereas the water permeability of vesicles formed from fiber cell membrane lipids had a water permeability of just 1 μm/s, so the limit is an ∼35-fold decrease in water permeability. This would lead to an increase in osmolarity of ∼4 mM. This could potentially have some small effect on the lens refractive index gradient, which is generated by a center-to-surface gradient in the concentration of intracellular protein. There is a center-to-surface gradient in intracellular calcium concentration (17) that could potentially upregulate nonjunctional AQP0 water permeability. This might come into play for fine-tuning, especially at the cortical-nuclear transition where Gold et al. (4) found loss of regulation of AQP0 by PKA induced a cortical cataract.
Does water permeability matter in the adult lens? Based on the data in Varadaraj et al. (15), the answer is “probably”. Heterozygous KO of AQP0 results in a cataract that begins as diffraction of light in the central fibers of the young lens and eventually progresses to a full nuclear cataract. Transgenic expression of AQP1 in the fiber cells of the AQP0+/− lenses delays the onset so that at any point in time the transgenic lenses have a less severe opacity. This suggests water permeability does matter in the adult lens, particularly in the central fiber cells. However, the puzzle remains: what is water permeability doing? It is not affecting fluid circulation, at least as assayed by the pressure gradient, so perhaps its effect on intracellular osmolarity is the answer.
Acknowledgments
This work was supported by National Institutes of Health grants No. EY06391 (to R.T.M.) and No. EY5661 (to J.E.H.).
References
- 1.Gonen T., Cheng Y., Walz T. Aquaporin-0 membrane junctions form upon proteolytic cleavage. J. Mol. Biol. 2004;342:1337–1345. doi: 10.1016/j.jmb.2004.07.076. [DOI] [PubMed] [Google Scholar]
- 2.Kumari S.S., Varadaraj K. Aquaporin 5 knockout mouse lens develops hyperglycemic cataract. Biochem. Biophys. Res. Commun. 2013;441:333–338. doi: 10.1016/j.bbrc.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichow S.L., Clemens D.M., Gonen T. Allosteric mechanism of water-channel gating by Ca2+-calmodulin. Nat. Struct. Mol. Biol. 2013;20:1085–1092. doi: 10.1038/nsmb.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold M.G., Reichow S.L., Scott J.D. AKAP2 anchors PKA with aquaporin-0 to support ocular lens transparency. EMBO Mol. Med. 2012;4:15–26. doi: 10.1002/emmm.201100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathias R.T. Steady-state voltages, ion fluxes, and volume regulation in syncytial tissues. Biophys. J. 1985;48:435–448. doi: 10.1016/S0006-3495(85)83799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaghefi E., Pontre B.P., Donaldson P.J. Visualizing ocular lens fluid dynamics using MRI: manipulation of steady state water content and water fluxes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R335–R342. doi: 10.1152/ajpregu.00173.2011. [DOI] [PubMed] [Google Scholar]
- 7.Gao J., Sun X., Mathias R.T. Lens intracellular hydrostatic pressure is generated by the circulation of sodium and modulated by gap junction coupling. J. Gen. Physiol. 2011;137:507–520. doi: 10.1085/jgp.201010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Candia O.A., Alvarez L.J. Fluid transport phenomena in ocular epithelia. Prog. Retin. Eye Res. 2008;27:197–212. doi: 10.1016/j.preteyeres.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B., Li L., Lim J.C. Dynamic regulation of GSH synthesis and uptake pathways in the rat lens epithelium. Exp. Eye Res. 2010;90:300–307. doi: 10.1016/j.exer.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson P.J., Musil L.S., Mathias R.T. Point: A critical appraisal of the lens circulation model—an experimental paradigm for understanding the maintenance of lens transparency? Invest. Ophthalmol. Vis. Sci. 2010;51:2303–2306. doi: 10.1167/iovs.10-5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumari S.S., Varadaraj M., Varadaraj K. Spatial expression of aquaporin 5 in mammalian cornea and lens, and regulation of its localization by phosphokinase A. Mol. Vis. 2012;18:957–967. [PMC free article] [PubMed] [Google Scholar]
- 12.Mathias R.T., Wang H. Local osmosis and isotonic transport. J. Membr. Biol. 2005;208:39–53. doi: 10.1007/s00232-005-0817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segel L.A. Standing-gradient flows driven by active solute transport. J. Theor. Biol. 1970;29:233–250. doi: 10.1016/0022-5193(70)90020-2. [DOI] [PubMed] [Google Scholar]
- 14.Whittembury G., Echevarría M. Pathways for water absorption in isosmotic transporting epithelia. Mt. Sinai J. Med. 1994;61:311–319. [PubMed] [Google Scholar]
- 15.Varadaraj K., Kumari S.S., Mathias R.T. Transgenic expression of AQP1 in the fiber cells of AQP0 knockout mouse: effects on lens transparency. Exp. Eye Res. 2010;91:393–404. doi: 10.1016/j.exer.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varadaraj K., Kumari S., Mathias R.T. Regulation of aquaporin water permeability in the lens. Invest. Ophthalmol. Vis. Sci. 2005;46:1393–1402. doi: 10.1167/iovs.04-1217. [DOI] [PubMed] [Google Scholar]
- 17.Gao J., Wang H., Mathias R.T. The effects of age on lens transport. Invest. Ophthalmol. Vis. Sci. 2013;54:7174–7187. doi: 10.1167/iovs.13-12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J., Sun X., Mathias R.T. The effect of size and species on lens intracellular hydrostatic pressure. Invest. Ophthalmol. Vis. Sci. 2013;54:183–192. doi: 10.1167/iovs.12-10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsey Rose K.M., Gourdie R.G., Schey K.L. The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest. Ophthalmol. Vis. Sci. 2006;47:1562–1570. doi: 10.1167/iovs.05-1313. [DOI] [PubMed] [Google Scholar]
- 20.Froger A., Clemens D., Hall J.E. Two distinct aquaporin 0s required for development and transparency of the zebrafish lens. Invest. Ophthalmol. Vis. Sci. 2010;51:6582–6592. doi: 10.1167/iovs.10-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalman K., Németh-Cahalan K.L., Hall J.E. Phosphorylation determines the calmodulin-mediated Ca2+ response and water permeability of AQP0. J. Biol. Chem. 2008;283:21278–21283. doi: 10.1074/jbc.M801740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemens D.M., Németh-Cahalan K.L., Hall J.E. In vivo analysis of aquaporin 0 function in zebrafish: permeability regulation is required for lens transparency. Invest. Ophthalmol. Vis. Sci. 2013;54:5136–5143. doi: 10.1167/iovs.13-12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H., Gao J., Mathias R.T. The effects of GPX-1 knockout on membrane transport and intracellular homeostasis in the lens. J. Membr. Biol. 2009;227:25–37. doi: 10.1007/s00232-008-9141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varadaraj K., Kushmerick C., Mathias R.T. The role of MIP in lens fiber cell membrane transport. J. Membr. Biol. 1999;170:191–203. doi: 10.1007/s002329900549. [DOI] [PubMed] [Google Scholar]


