Abstract
Background
There are limited data on the failure of second-line antiretroviral therapy (ART) and the use of third-line ART in people living with HIV in resource-limited settings. Since 2011, the Médecins Sans Frontières (MSF) HIV/tuberculosis programme in Mumbai, India, has been providing third-line ART to patients in care.
Objective
To describe the experiences and programmatic challenges during management of suspected second-line ART failure and third-line ART therapy for patients living with HIV, including the use of HIV viral load (VL) testing.
Design
This was a retrospective, observational cohort study of patients with suspected second-line ART treatment failure, who were followed for at least 12 months between January 2011 and March 2014.
Results
A total of 47 patients with suspected second-line failure met the inclusion criteria during the study period. Twenty-nine of them (62%) responded to enhanced adherence support, had a subsequent undetectable VL after a median duration of 3 months and remained on second-line ART. The other 18 patients had to be initiated on a third-line ART regimen, which consisted of darunavir–ritonavir, raltegravir, and one or more appropriate nucleoside or nucleotide reverse transcriptase inhibitors, based on the results of HIV genotype testing. Of the 13 patients for whom follow-up VL results were available, 11 achieved virological suppression after a median duration of 3 months on third-line ART (interquartile range: 2.5–3.0). No serious treatment-related adverse events were recorded.
Conclusions
With intensive counselling and adherence support in those suspected of failing second-line ART, unnecessary switching to more expensive third-line ART can be averted in the majority of cases. However, there is an increasing need for access to third-line ART medications such as darunavir and raltegravir, for which national ART programmes should be prepared. The cost of such medications and inadequate access to VL monitoring and HIV genotype testing are currently major barriers to optimal management of patients failing second-line ART.
Keywords: viral load, HIV, adherence, counselling, antiretroviral therapy, genotyping, India
India is home to approximately 2.1 million people living with HIV (1). Of the 0.6 million people living with HIV and on antiretroviral therapy (ART) in 2012, approximately 5,503 (0.9%) were receiving second-line ART and none were on third-line ART under the National AIDS Control Programme.
Because a certain percentage of patients living with HIV fail their ART regimens every year, and there is the possibility of transmission of drug-resistant HIV (2, 3), a gradual rise in the number of patients failing second-line ART regimens can be expected, as well as a growing need for access to third-line ART in the future. Furthermore, the cost of a third-line regimen represents a major challenge, because it is nearly 15 times higher than that of a first-line ART regimen and over six times that of a typical second-line regimen (4).
Routine HIV viral load (VL) testing is an indispensable requirement in any ART programme. The World Health Organization (WHO) recommends VL monitoring as the preferred approach to provide an early and more accurate indication of treatment failure, thereby reducing the accumulation of drug-resistance mutations and improving clinical outcomes (5). However, VL testing is not routinely offered in many countries owing to its cost and lack of availability.
To date, there are limited data describing experiences of routine VL monitoring in the contexts of patients living with HIV who have suspected second-line ART failure and the use of third-line ART in resource-limited settings. To address this knowledge gap, this study describes the experiences and programmatic challenges during treatment and VL testing of patients living with HIV who are suspected of second-line ART failure and those needing third-line ART in Mumbai within a non-governmental organization (NGO)-run programme that provides ART and VL testing free of charge.
Methods
Study design
This was a retrospective, observational cohort study of patients living with HIV who had suspected failure of second-line ART using data collected under routine programmatic conditions.
Setting and study population
Since 2006, Médecins Sans Frontières (MSF) has been operating an HIV/tuberculosis (TB) programme in Mumbai, India (6), for those unable to access essential HIV care and treatment services elsewhere. During the study period, the size of the entire clinic cohort was approximately 290 patients. One of the main objectives of the MSF programme has been to provide free second-line or third-line ART as appropriate to patients living with HIV who are not able to access these through national ART centres. These patients living with HIV are referred from government ART centres, public–private ART centres, and a network of community NGOs. The programme initially started providing third-line ART in 2011. Patients were eligible for inclusion in this study if they were suspected of failing a second-line ART regimen that included a protease inhibitor (PI) and 2–3 nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs), and they were followed for at least 12 months between January 2011 and March 2014.
Definitions and treatment protocol
Second-line ART was defined as the regimen used for treatment of patients living with HIV who failed a first-line regimen, and typically it would consist of a PI (e.g. atazanavir or ritonavir) and two or three NRTIs (e.g. lamivudine and tenofovir±zidovudine). Third-line ART regimen in the MSF programme included the second-generation PI darunavir boosted with ritonavir (DRV/r) and the integrase inhibitor raltegravir (RAL), together with one or more NRTIs likely to be effective on the basis of HIV genotyping results. As part of routine clinical care, HIV VL and CD4 cell count testing was carried out for each patient every 6 months in the MSF programme. An undetectable VL was defined as a result with fewer than 50 copies/ml. Treatment failure was defined as a VL result >5,000 copies/ml in two consecutive results in a 3-month time frame, as previously recommended by WHO (7).
Patients in the programme were suspected of failing second-line ART if they had received and failed a first-line regimen, were subsequently treated with a second-line regimen for at least three consecutive months, and then had a single VL test result measuring >5,000 copies/ml. All patients suspected of failing second-line ART underwent a thorough assessment, including structured client-centred adherence counselling and repeated VL measurement after 3 months of good adherence. The assessment involved a multidisciplinary team consisting of an HIV physician, psychologist, social worker, and nurse. Each member of the team had special predefined tasks and responsibilities (Box 1). The time to virological suppression, if achieved, was measured and reported.
Box 1. Predefined tasks and responsibilities of members of the multidisciplinary team assessing those suspected of failing second-line ART.
HIV Physician: Physicians perform a detailed history and examination at the first visit, and they arrange for a number of laboratory tests, including complete blood count, alanine transaminase, serum creatinine, serum bilirubin, blood sugar, serum lipids, Western blot (for HIV-1 and 2), HIV-1 viral load (VL), CD4 count, chest X-ray, abdominal ultrasound, and fundoscopy, as well as a pregnancy test in females. Second-line ART is restarted only after ruling out opportunistic infections. Any treatment-related adverse events are symptomatically managed. Initially, the patient is assessed every two weeks for the first three visits and then monthly. At every visit, adherence is reinforced, and after 2–3 months of good adherence, VL testing is repeated. VL and CD4 count testing are repeated every 6 months. If VL suppression is not achieved, genotyping is performed, and a treatment regimen is designed based on the genotyping results.
Psychologist: Every patient is evaluated at baseline, including assessment with the PHQ9 Patient Depression questionnaire. Baseline HIV knowledge is tested using 30 basic HIV questions: a score of <10 is defined as poor knowledge, 11–20 as average knowledge, and 21 or more as good knowledge. Barriers for adherence are identified. The patients see the psychologist every two weeks for the first three visits, and then every month.
Social Worker: Every patient receives a social assessment at their first visit, during which social barriers for adherence are identified and solutions discussed with the patient.
Nurses: Dosage and timing of all pills are explained to all patients. A pill count is done at every visit, and the percentage of pills taken is entered into a file. Pillboxes are used for patients who have difficulties with timing of their ARVs.
The adherence levels of all patients were assessed with patient self-reports. These levels and any adherence concerns were recorded by the team following individual interviews. In patients observed to have adherence issues, the team provided education to the patient about HIV, VL and CD4 testing, and treatment failure. Psychological and emotional support were offered to the patients during treatment, and they were also referred to other NGOs and services as needed.
A two-log decrease in the repeat VL measurement 3 months after interventions to support adherence in those suspected of failing a second-line regimen was defined as virological suppression, and these patients were continued on second-line ART.
Those patients who failed to achieve virological suppression were investigated for antiretroviral (ARV) resistance using genotyping with the ViroSeq HIV-1 genotyping system method (Celera Diagnostics, Alameda, CA, USA); the instrument used was the ABI 310 Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA). The Stanford genotypic resistance interpretation algorithm was used to analyse resistance of HIV in these patients. Patients in need of a third-line regimen were prescribed the regimen based on genotyping results.
Data collection and analysis
Demographic and clinical information of all patients living with HIV was recorded in patient files. The clinical data that were routinely collected for each patient, including treatment and laboratory data, were entered into an electronic database. A full-time data manager routinely supervised data entry for accuracy and completeness. Data from all patients suspected of second-line failure between January 2011 and March 2014 were included in the analyses. Descriptive statistics were used to analyse the data of these patients using SPSS (Release 20, 2011; SPSS, Inc., Chicago, IL, USA).
Ethics
The study satisfied the criteria for reports using routinely collected programmatic data set by the MSF independent Ethics Review Board in Geneva, Switzerland. As this was a study of routinely collected monitoring data, informed consent from the patients was not obtained. The named ethics committee specifically approved the study and waived the need for consent.
Results
Patient characteristics
A total of 47 patients living with HIV were suspected to have failed second-line ART during the study period. The median age of patients was 40 years (interquartile range (IQR): 35–46), and almost three-fourths of them (35/47) were male. The median baseline CD4 count for these patients at the time of enrolment was 110 cells/µl (IQR: 47.0–208.8). The monthly family income was less than 7,000 Indian rupees in 27/44 (61.4%) cases, which is less than the cost of second-line ART in a pharmacy in Mumbai (8). Thirty-one (66%) patients were in WHO clinical stage IV at the time of enrolment (9). Three patients were co-infected with HIV and multidrug-resistant TB (MDR-TB). The median duration of exposure to ART of these patients prior to enrolment in this study was 7 years (IQR: 5.0–9.0). Twenty-two (47%) patients had suffered from ARV medication–related grade III or IV toxicity (10).
Treatment outcomes
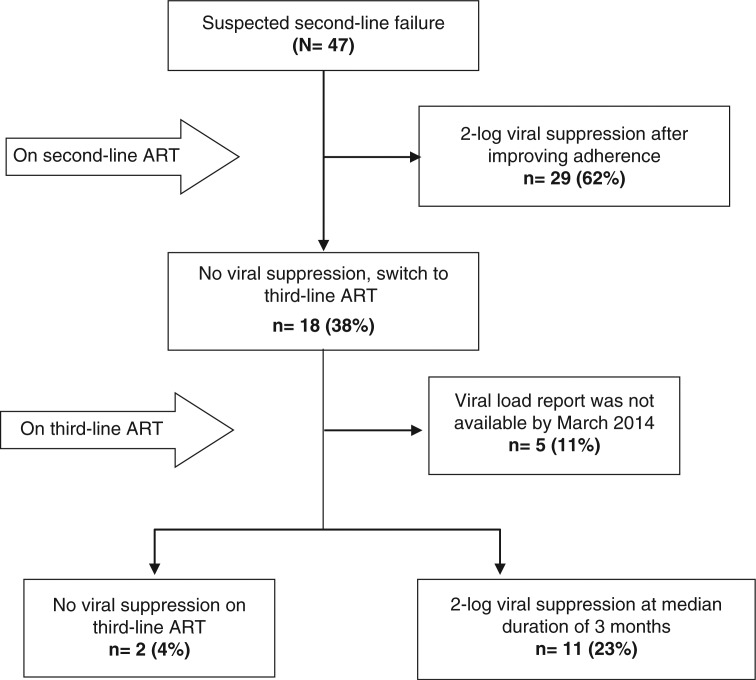
Of the 47 patients suspected of second-line ART failure, 29 patients (62%) responded to enhanced adherence support and had a two-log decrease in their level of HIV on subsequent VL testing (Fig. 1). The other 18 patients (38%) did not respond to enhanced adherence support and were eventually switched to third-line ART. The median duration of prior ART exposure of these 18 patients (Table 1) was 9.0 years (IQR: 7.0–10.3). Out of 18 patients who switched to third-line ART, 13 had VL follow-up before the end of March 2014, of whom 11 achieved viral suppression after a median duration of 3 months (IQR: 2.5–3.0). Two patients failed to achieve viral suppression, one of whom died in April 2013 due to Pneumocystis jirovecii pneumonia (PCP).
Fig. 1.
Patients living with HIV and suspected of second-line antiretroviral therapy failure.
Table 1.
Clinical characteristics of 18 patients living with HIV and started on third-line ART, Mumbai, India, January 2011–January 2014
| Age | Sex | WHO stage | Prior duration of ART (years) | Comorbidity | Drugs in third-line ART regimen | Duration on third-line ART (months) | Adverse events | |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | M | III | 8 | DL, DM | DRV/R, RAL, ATV | 30 | Transient rise in ALT |
| 2 | 40 | M | IV | 8 | DL | DRV/R, RAL, EFV, TDF, 3TC | 33 | No adverse event |
| 3 | 38 | M | IV | 8.5 | – | DRV/R, RAL, ABC, 3TC | 33 | No adverse event |
| 4 | 32 | F | III | 7 | DL | DRV/R, RAL, EFV | 32 | No adverse event |
| 5 | 60 | F | IV | 10 | DL, HTN | DRV/R, RAL, ABC | 32 | No adverse event |
| 6 | 38 | M | IV | 7 | – | DRV/R, RAL, TDF, 3TC | 18 | No adverse event |
| 7 | 40 | M | II | 10 | DL | DRV/R, RAL, TDF, 3TC | 30 | No adverse event |
| 8 | 45 | M | IV | 10 | HTN | DRV/R, RAL, TDF, 3TC | 15 | No adverse event |
| 9 | 38 | F | IV | 9 | DL | DRV/R, RAL, TDF, 3TC | 17 | No adverse event |
| 10 | 49 | M | IV | 13 | DL | DRV/R, RAL, ABC, 3TC | 16 | No adverse event |
| 11 | 46 | M | IV | 4 | HBV | DRV/R, RAL, ABC, 3TC | 3 | No adverse event |
| 12 | 45 | M | IV | 16 | DL | DRV/R, RAL, ABC, 3TC, EFV | 10 | No adverse event |
| 13 | 16 | F | IV | 6 | – | DRV/R, RAL, ABC, 3TC | 3 | No adverse event |
| 14 | 40 | M | IV | 15 | DM | DRV/R, RAL, TDF, 3TC | 5 | No adverse event |
| 15 | 48 | M | IV | 9 | DM | DRV/R, RAL, ABC, 3TC | 6 | No adverse event |
| 16 | 47 | M | IV | 11 | – | DRV/R, RAL, TDF, 3TC | 1 | No adverse event |
| 17 | 42 | F | IV | 6 | – | DRV/R, RAL, TDF, 3TC | 4 | No adverse event |
| 18 | 52 | M | IV | 9 | – | DRV/R, RAL, TDF, 3TC | 3 | No adverse event |
ART: antiretroviral therapy; DM: diabetes mellitus; DL: dyslipidemia; HTN: hypertension; HBV: hepatitis B; ATV: atazanavir; ABC: abacavir; DRV/R: darunavir boosted with ritonavir; EFV: efavirenz; RAL: raltegravir; TDF: tenofovir; 3TC: lamivudine; ALT: alanine aminotransferase.
Discussion
As a result of enhanced adherence support and routine HIV VL monitoring, the majority of patients living with HIV who had suspected second-line ART failure in the MSF programme in Mumbai did not have to be switched to third-line ART. In fact, most of the patients suspected to be failing second-line ART in this study had adherence issues rather than HIV strains that were resistant to the ARV drugs, a finding which is in line with the results of previous studies, including a similar South African cohort and several other programmatic cohorts in resource-limited settings (11–13).
One of the main reasons behind the lack of adherence reported by patients was the high cost of second-line ART (prior to enrolment in the MSF programme). These patients had initially opted to receive their HIV treatment in private facilities rather than public ones, in an effort to reduce HIV-related stigma and discrimination; however, they were eventually no longer able to afford the medications and were forced to stop the treatment. Patients co-infected with HIV and TB had a high pill burden, which has the potential to negatively affect adherence. Additional reasons for poor adherence in our cohort included anxiety, in part due to fear of disclosure of their HIV status, and alcohol abuse.
Routine VL monitoring is an important tool to identify poor adherence and ART failure at an early stage, and with proper management this will prevent the accumulation of further resistance mutations and preserve treatment options (14). Routine HIV VL monitoring is not currently available in the public sector in India; instead, CD4 count monitoring continues to be used as the main test to identify treatment failure. VL testing is available within the public sector on only a case-by-case basis, after being approved by an ART centre expert committee. The authors believe that routine HIV VL monitoring should no longer be considered as a luxury, but a necessity in HIV ART programmes, and that it should be rapidly scaled up in the public sector. Efforts should be made to ensure that VL testing is affordable and easily accessible in all resource-limited settings, including the removal of any barriers, such as reliance on a committee to sanction each and every VL test, as this can result in significant delay. Until such time that VL testing is performed routinely in those on ART, actual treatment failure is likely to be grossly underdiagnosed (15).
HIV resistance surveillance or genotyping in patients (16, 17) assists in designing appropriate treatment regimens for patients needing third-line ART. Thus, genotyping should be considered before regimen preparation for all those requiring a switch to a third-line ART regimen. The lack of access to affordable antiretroviral drugs that can be used in robust combinations is another barrier to management of those failing second-line ART. National ART programmes need to be prepared to offer third-line ART (18), and they should already start preparing by making supplies of darunavir and raltegravir accessible. Furthermore, management of patients needing third-line ART can be complicated and may require palliative care (19). A medical dilemma exists for those failing third-line ART regarding whether they should continue costly treatment that consists of many pills and is only weakly effective or switch to another ARV regimen that has a lower pill burden and is less expensive.
The small number of patients in the study is one of the limitations, and therefore generalisation of the results may not be possible. However, this study is one of the few reports from resource-limited settings describing operational feasibility and programmatic challenges in relation to management of patients living with HIV who have suspected second-line ART failure and patients requiring third-line ART. There may be additional factors influencing treatment adherence in such patients; however, it would be difficult to comment on these factors on the basis of routine programme data.
Conclusion
Early detection of ART failure through routine VL monitoring and HIV resistance surveillance should be prioritised in settings where ART programmes have been successfully established. Adequate adherence support and extensive counselling should be provided to all patients suspected of second-line ART failure since the underlying reason for suspected failure is often not genetic mutation of HIV but improper adherence by patients. HIV viral load testing should be offered after 3 months of enhanced adherence support in all patients with suspected failure based on a single VL measurement.
With intensive psychosocial and medical intervention in those suspected to be failing second-line ART, an unnecessary switch to more expensive and often unavailable third-line ART can be averted in the majority of cases. However, there is still an urgent need for improved access to third-line ART regimens in India, for which the national ART programme should be prepared. The cost of such medications and the additional costs of VL monitoring and genotyping are currently major barriers to the optimal management of patients failing second-line ART.
Acknowledgements
The authors are grateful for the contribution of healthcare workers from the MSF clinic and the Infectious Diseases Department, Mahatma Gandhi Medical College, as well as the patients living with HIV and their families.
Authors’ contributions
SK and PI conceived and designed the experiments. SK and AA performed the experiments. PI, MD, and AA analysed the data. HM, AD, PI, and PS contributed reagents, materials, and analysis tools. SK, PI, MD, and AA wrote the paper. AD, HM, PS, PI, MD, and AA reviewed the final manuscript.
Conflict of interest and funding
The authors declare no conflicts of interest, and they have not received any funds for the study.
References
- 1.Department of AIDS Control, National AIDS Control Organization. Ministry of Health & Family Welfare, Government of India; 2013. Annual report 2012–2013. [Google Scholar]
- 2.World Health Organization, WHO Res Net, including –. Jordan MR, Barcarolo J, Parkin N, de Oliveira T, Bertagnolio S. WHO HIV drug resistance report 2012, WHO report; Geneva, Switzerland: WHO. 2012. [Google Scholar]
- 3.Zhang F, Dou Z, Ma Y, Zhao Y, Liu Z, Bulterys M, et al. Five year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med. 2009;151:241–51. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 4.Médecins Sans Frontières (MSF) Untangling the web of antiretroviral price reductions. 16th ed. Geneva, Switzerland: Médecins Sans Frontières; 2013. Access campaign. [Google Scholar]
- 5.WHO. Consolidated guidelines on the use of antiretroviral drugs for treatment and preventing HIV infection; Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 6.Isaakidis P, Varghese B, Mansoor H, Cox HS, Ladomirska J, Saranchuk P, et al. Adverse events among HIV/MDR-TB co-infected patients receiving antiretroviral and second line anti-TB treatment in Mumbai, India. PloS ONE. 2012;7:e40781. doi: 10.1371/journal.pone.0040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Antiretroviral Therapy for HIV infection in adults and adolescents: recommendations for a public health approach-2010 revision; Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 8.Lorina Publications (India) Inc. Vol. 1. India: Lorina Publications; 2010. Drug today – ready reckoner of current medical formulations. [Google Scholar]
- 9.World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. 2007. Available from: http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf [cited 4 June 2014]
- 10.AIDS Clinical Trials Group. Division of AIDS table for grading the severity of adult and pediatric adverse events Version 1.0, 2004 Dec. Clarification 2009 Aug; Available from: http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf [cited 30 April 2014]
- 11.Ajose O, Mookerjee S, Mills EJ, Boulle A, Ford N. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. AIDS. 2012;26:929–38. doi: 10.1097/QAD.0b013e328351f5b2. [DOI] [PubMed] [Google Scholar]
- 12.Pujades-RodrIguez M, Balkan S, Arnould L, Brinkhof MAW, Calmy A- for the AIDS Working Group of MSF Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. JAMA. 2010;304:303–12. doi: 10.1001/jama.2010.980. [DOI] [PubMed] [Google Scholar]
- 13.Garone DB, Conradie K, Patten G, Cornell M, Goemaere E, Kunene J, et al. High rate of virological re-suppression among patients failing second-line antiretroviral therapy following enhanced adherence support: a model of care in Khayelitsha, South Africa. South Afric J HIV Med. 2013;14:166–69. [Google Scholar]
- 14.Aghokeng AF, Kouanfack C, Eymard-Duvernay S, Butel C, Edoul GE, Laurent C, et al. Virological outcome and patterns of HIV-1 drug resistance in patients with 36 months’ antiretroviral therapy experience in Cameroon. J Int AIDS Soc. 2013;16:18004. doi: 10.7448/IAS.16.1.18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renaud-Théry AF, Duncombe C, Kerr S, Thierry S, Perriens J. Adult antiretroviral therapy in resource limited settings: a systematic review of first-line failure and attrition rates. Available from: http://www.who.int/hiv/topics/treatment/First_Line_ART_failure_RLS_metanalysis.pdf [cited 30 April 2014]
- 16.Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization’s global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13(Suppl 2):1–13. [PubMed] [Google Scholar]
- 17.Jordan MR, Bennett DE, Wainberg MA, Havlir D, Hammer S, Yang C, et al. Update on World Health Organization HIV drug resistance prevention and assessment strategy: 2004–2011. Clin Infect Dis. 2012;54(Suppl 4):S245–9. doi: 10.1093/cid/cis206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pujari SN, Makane A, Lodha A, Bele V, Joshi K. Short-term effectiveness and safety of third-line antiretroviral regimens among patients in Western India. J Acquir Immune Defic Syndr. 2014;65:e82–4. doi: 10.1097/QAI.0b013e3182a6104a. [DOI] [PubMed] [Google Scholar]
- 19.Sigaloff KC, Hamers RL, Wallis CL, Kityo C, Siwale M, Ive P, et al. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr. 2011;58:23–31. doi: 10.1097/QAI.0b013e318227fc34. [DOI] [PubMed] [Google Scholar]