Abstract
Osteomyelitis (OM) is a common complication of diabetic foot ulcers and/or diabetic foot infections. This review article discusses the clinical presentation, diagnosis, and treatment of OM in the diabetic foot. Clinical features that point to the possibility of OM include the presence of exposed bone in the depth of a diabetic foot ulcer. Medical imaging studies include plain radiographs, magnetic resonance imaging, and bone scintigraphy. A high index of suspicion is also required to make the diagnosis of OM in the diabetic foot combined with clinical and radiological studies.
Keywords: osteomyelitis, diabetic foot, ulcer, infection, antibiotics, amputation
‘Osteomyelitis’ (OM) is derived from three Greek words: osteon, myelos, and itis. It refers to the inflammation or infection of the bone and bone marrow. It is frequently missed and underdiagnosed in patients with diabetic foot problems. A high index of clinical suspicion is required to make a diagnosis since undiagnosed and untreated OM often leads to the dreaded complication of limb amputation. The risk for amputation in acute diabetic infections is four times higher with OM than with soft tissue infection alone (1). Also, the presence of OM requires a longer duration of antibiotic therapy and a longer duration of hospital stay, thereby raising the hospitalization costs specifically for diabetic patients with OM.
The medical and surgical treatment of OM will also be outlined in this review article. The key to the successful treatment of OM in the diabetic foot is a combination of antibiotic therapy and surgical procedures. The latter may include surgical debridement with excision of the osteomyelitic bone and/or minor amputations. In addition, stabilization of the foot is also an important factor in the surgical management of OM in the diabetic foot. Adjuncts to this treatment may include the use of antibiotic-loaded cement spacers or beads after surgical debridement. The role of conservative treatment of OM in the diabetic foot with antibiotic therapy only is controversial and this has been discussed in this paper. However, in these cases surgery may need to be performed for a relapse in the pathological entity.
The ultimate goal of treatment is to achieve limb salvage wherever possible in the diabetic patient. However, in some cases major amputation such as below the knee amputation may be warranted. Early diagnosis and appropriate treatment is necessary to reduce the morbidity and mortality of this disease.
Pathogenesis
Unlike hematogenous OM (seeding via bloodstream) and direct inoculation (via open fractures), in the diabetic foot, there is a contiguous spread of pathogens from infection, complicating a diabetic foot ulcer to the bones underlying these ulcers (2). Histologically, it is characterized by the presence of leukocytes or inflammatory cells, such as lymphocytes and plasma cells, and by the presence of bone necrosis. OM is a common complication in the diabetic foot. Underlying OM is seen in 15% of patients with diabetic foot ulcers (3), and in 20% of patients with diabetic foot infections (4). The risk of developing OM increases with ulcers larger than 2 cm2 or a diabetic foot ulcer with exposed bone or joint (5). The most common sites of OM are in the forefoot (90%) followed by the midfoot (5%) and hindfoot (5%) (6–10). The most common bones involved are the weight-bearing bones of the foot, particularly to what it is referred as the ‘tripod of the foot’ that includes the first metatarsal head, fifth metatarsal head, and the calcaneum (Fig. 1). Other sites include the bones underlying lateral decubitus ulcers such as the lateral malleolus, base of the fifth metatarsal and the calcaneum (11).
Fig. 1.
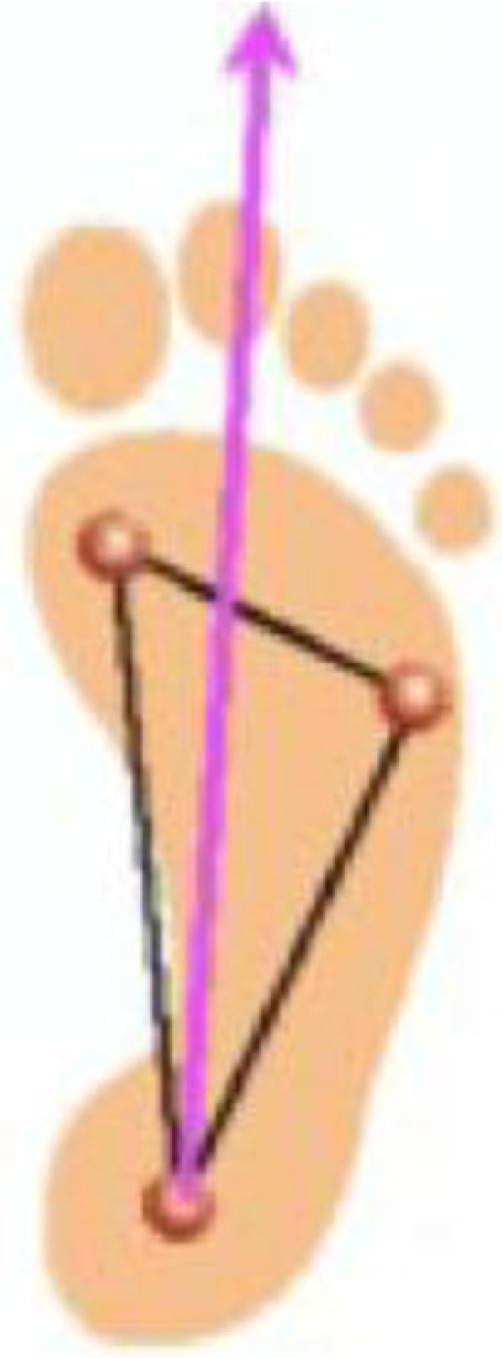
Diagram of weight-bearing tripod of the foot.
Clinical examination
A diabetic foot ulcer must be assessed meticulously in terms of site, size, periphery, depth, content, and adjacent skin environment. The foot and lower extremity must also be assessed for the presence of any vasculopathy, neuropathy, and extent of underlying infection. Peripheral vascular disease may be present in 45–65% of patients with diabetic foot complications (6–8). Lower limb pulses must be palpated and features of vascular disease need to be assessed for skin color, temperature, and capillary refill time. Basic non-invasive vascular studies should also be conducted, including the ankle–brachial and toe–brachial pressure indices. Duplex ultrasound can help assess for significant arterial stenoses that may warrant vascular intervention to enhance distal blood flow (11). Peripheral neuropathy is seen in 88% of patients with diabetic foot problems (7). This is frequently assessed by using a Semmes Weinstein 5.07 gauge monofilament to test 10 points on the foot for touch sensation. A score of 7 or less indicates neuropathy and identifies a ‘foot at risk’ (11). Infection is clinically assessed for by the presence of cardinal features of inflammation (redness, warmth, swelling, pain) as well as ulcer discharge or wet gangrene. The depth and extent of infection must be assessed since it is progressing rapidly in the diabetic foot (11).
Laboratory and clinical findings
Laboratory findings that should be performed include markers of infection [white blood cell (WBC) count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR)] and markers of healing (Hemoglobin A1C, blood urea nitrogen/creatinine, albumin and hemoglobin). Mutluoglu et al. (1) compared OM with soft tissue infection and found no difference in terms of WBC, CRP, and renal function. However, ESR was found to be significantly higher in the OM group (90 vs. 70 mm/h) and the mean hemoglobin to be lower in the OM group (10.8 vs. 12.0). A meta-analysis by Butalia et al. (12) showed that ESR >70 mm/h indicates an 11 fold greater risk of OM. Michail et al. (13) found WBC, CRP, ESR and Pro-Calcitonin to be significantly higher in OM compared to soft tissue infection. However, it should be noted that evidence is limited and no clear consensus exists on the ability for laboratory tests to diagnose OM (2).
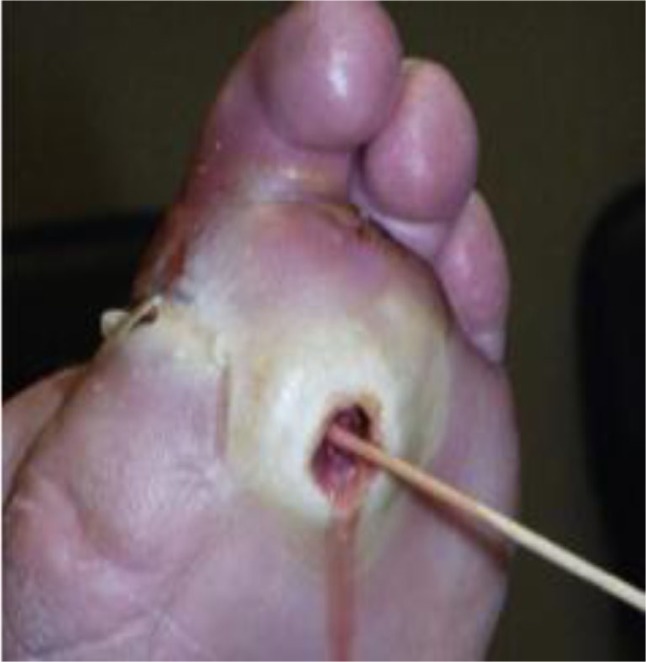
Clinical testing may include the probe-to-bone test (PTB) that could be performed using a sterile, blunt metal probe (Fig. 2). This test is easy to perform and evaluates the ability to contact a bone in the depth of the ulcer. Some authors advocate it is a reliable test to diagnose OM (14, 15). Morales Lozano et al. (15) found a sensitivity of 98% and a specificity of 79%. Aragon-Sanchez et al. (14) found a sensitivity and specificity of 95 and 93%, respectively.
Fig. 2.
Probe-to-bone test for osteomyelitis in the diabetic foot.
A summary of studies on PTB tests is shown in Table 1. There are variable results seen with sensitivity ranging from 38 to 98% and specificity from 78 to 92%. These studies have different populations and methodology. Two studies assessed patients admitted to hospital with diabetic foot infections. In one, the prevalence of OM was 66% (16), and in the other it was 72% (14). The three studies in the outpatient setting revealed OM prevalence in 23.5% (17), 79.5% (15), and 12% (18). Variation was also seen in mean ulcer duration in outpatient studies: 44 weeks (15) and 25 weeks (18). Finally, these studies did not all use the same reference markers for confirmation of OM and some studies did not use a standard reference marker (16, 17) for confirmation. The PTB is a widely debated clinical sign (2) and more evaluation is needed to determine the setting where the test is most reliable. However, it is useful to perform this test in the clinical setting where an expedited evaluation for OM can be made before detailed imaging can be performed.
Table 1.
Results from probe-to-bone test in diagnosis of osteomyelitis
Medical imaging for OM in the diabetic foot
Plain radiography
The sensitivity of plain films in the diagnosis of OM has shown variable results. It is related to the chronicity of the infection and at least 30–50% bone loss is required to show visible changes on plain radiographs and such changes take at least 2–3 weeks to manifest (2). The specificity of radiographs is also lowered due to difficulty in distinguishing OM from Charcot neuroathropathy joint disease (2). The most common OM changes that may be seen on radiographs include osteopenia, periosteal thickening, cortical erosions, and new bone formation (19). Overall, the sensitivity and specificity are 54 and 68% respectively according to one meta-analysis (20). Nevertheless, plain radiographs should be performed initially as a baseline to assess the development and presentation of OM in a bone (Fig. 3).
Fig. 3.
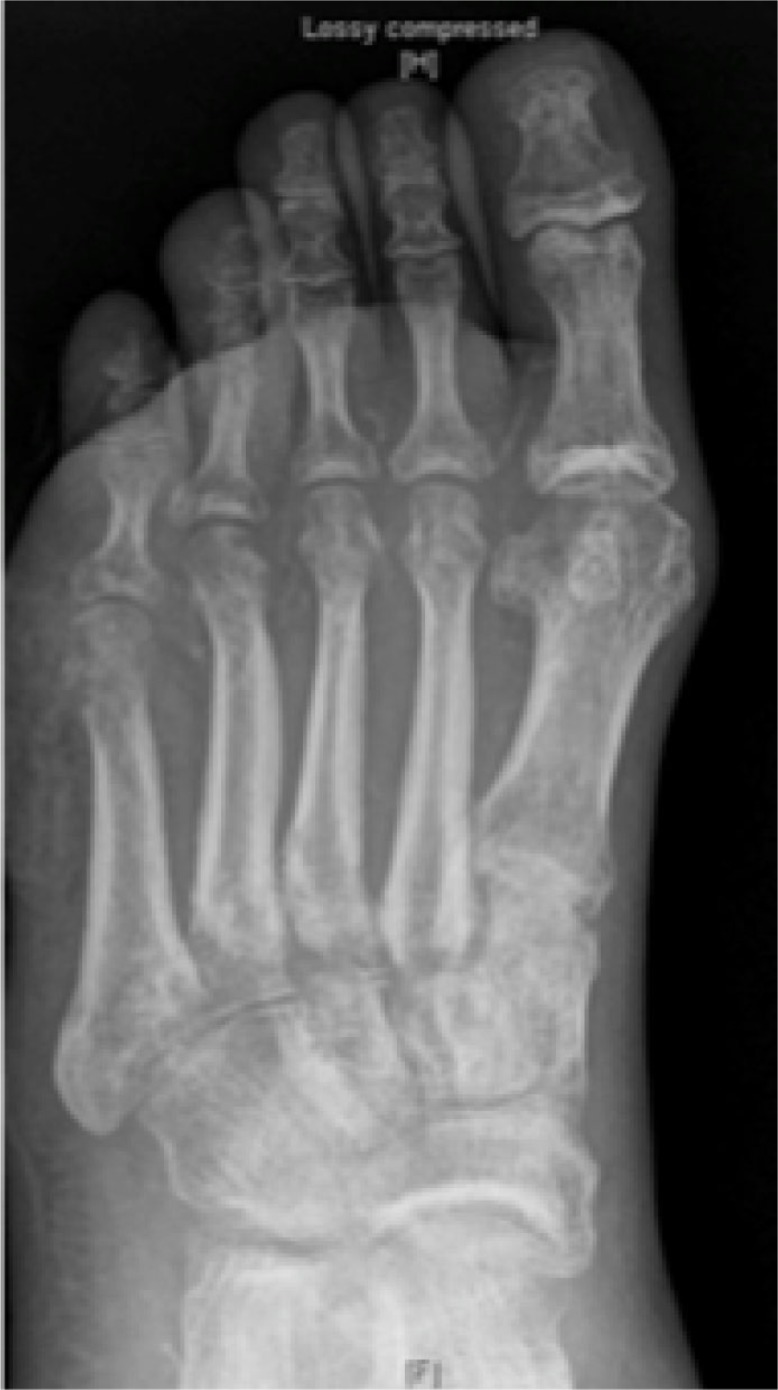
Plain radiograph. Evidence of cortical erosion of the fifth metatarsal head in a patient with osteomyelitis.
Magnetic resonance imaging
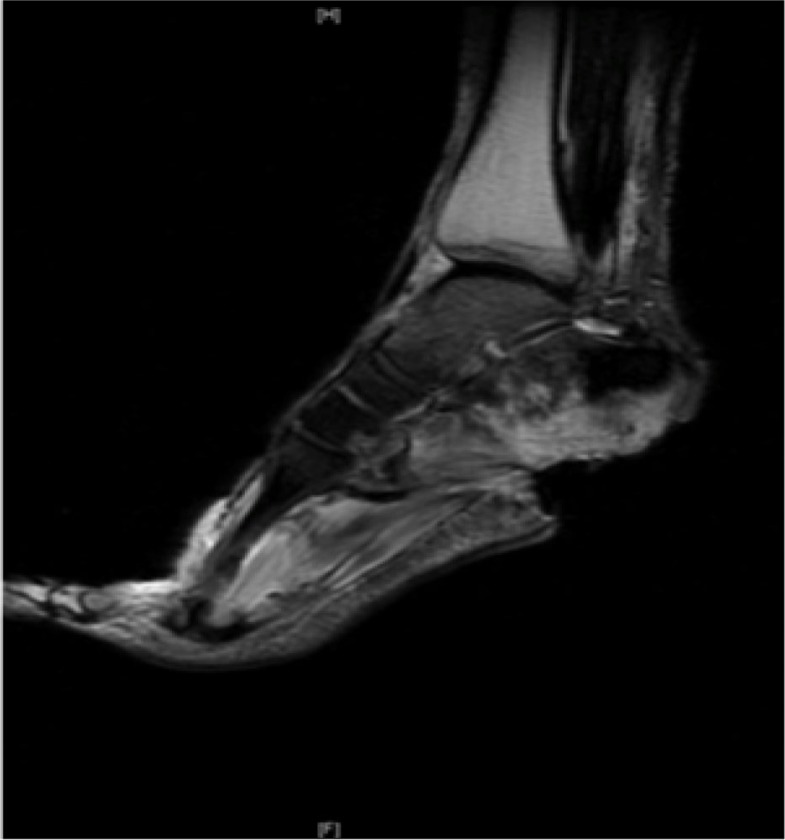
Magnetic resonance imaging (MRI) is presently considered the investigation of choice for diagnosing diabetic foot OM (21). In OM, the loss of signal in T1-weighted images and higher intensity on T2-weighted images can reveal the pathology as early as 3 days after infection (22). However, this bone edema can sometimes be difficult to differentiate from non-infectious causes of edema (23). The accuracy of MRI is challenged when Charcot neuroarthropathy joint disease or recent surgical change is present. Meta-analyses and reviews show that MRI is probably the most useful imaging modality for assessing OM with a sensitivity of about 90% and a specificity of about 80% (12, 20, 24). MRI provides a good anatomical correlation but it is limited in terms of functional correlation (Fig. 4).
Fig. 4.
MRI. T2-weighted image – irregular ‘whitening’ of calcaneum suggests edema and osteomyelitis in a patient with a calcaneal ulcer.
Bone scintigraphy
The three-phase bone scan using Technetium-99m-Medronic Acid Bisphosphonate provides a two-dimensional image of areas in bone with active bone turnover. For diabetic foot OM, bone scans have a sensitivity of 80–90% but a specificity of less than 50% (25–27). The poor specificity relates to inability of the bone scan to distinguish OM from other inflammatory or traumatic conditions involving the bone, such as Charcot neuroarthropathy joint disease, bone metastasis, gout, fracture, or even recent surgery (28, 29). It must also be noted that it is difficult to delineate the exact anatomical location or extent of infection with a bone scan (Fig. 5).
Fig. 5.
Bone scan. Bone scan showing increased uptake localized to the base of the fifth metatarsal, indicating osteomyelitis.
Single photon emission computerized tomography
Single photon emission computerized tomography (SPECT) combines bone scan with computerized tomography to improve the anatomical–functional correlation since it provides three-dimensional images of the foot. However, the technology is still not widely available and its diagnostic potential for diabetic foot OM is still being researched (22).
White blood cell scan
Leukocytes can also be removed from the patient, tagged with a radioactive tracer and re-infused into the patient where they accumulate at an infected focus. WBC scans have a sensitivity of at least 80% and a specificity of at least 70% for diabetic foot OM (25–27). WBC scanning shows soft tissue infection and is not bone specific and therefore correlation with the results of a bone scan is useful with a sensitivity and specificity of 80–90% (30). This combination, however, would be time consuming, expensive and perhaps best conducted when a MRI is contraindicated.
Positron emission tomography
Only a few studies have been done using PET scan to evaluate its diagnostic potential for OM in the diabetic foot. PET scan images radioactive fluorine attached to 2-fluoro-2deoxy-D-glucose, which accumulates at sites of increased intracellular glucose metabolism such as infection, inflammation, or malignancy. Its sensitivity ranges from 80 to 100% while its specificity has been reported as 93% (26, 31). Combining PET with computerized tomography improves the anatomical detail available (26).
Bone biopsy
The gold standard for the diagnosis of OM is histopathological and microbiological assessment of bone (32–34). The bone may be sampled percutaneously through a site of non-infected skin, or intra-operatively during debridement/amputation. Knowing the pathogens involved is highly desirable especially when planning conservative treatment. Histological features of OM are specific and rarely seen in normal bone (35). Possible histological criteria to diagnose OM include the presence of sequestrum, involucrum, necrotic bone, necrotic-inflammatory exudates, fat necrosis, marrow edema, marrow fibrosis, bone erosions, and cellular changes of acute or chronic inflammation (36). However, some evidence suggests possibility of subjectivity among pathologists and perhaps the criteria for histological diagnosis have to be clarified (36).
Concurrent soft tissue infection may result in bacterial contamination of bone sample and false positives are possible and are still worthwhile as antibiotics can be guided by sensitivities (32). Bone cultures are preferable over soft tissue cultures (37). Successful antibiotic treatment is more likely to occur when the choice of antibiotics is guided by cultures from bone biopsy (38). If surgery is not performed, bone cultures may be obtained via percutaneous biopsy through an area of uninvolved skin (39). The precision of this approach can be questioned and Senneville et al. (27) found that after transcutaneous biopsies for culture, 1 in 4 ‘normal’ results were false negatives. Needle aspiration when compared to transcutaneous bone biopsy was shown to be unreliable with only 33% concordance with transcutaneous biopsy culture results (40). Cultures from ulcer swabs may be useful for predicting deep tissue cultures (41) but are only identical to bone cultures between 12 and 30% of the time (12, 37, 42). Therefore, bone is best sampled under direct vision, or a reasonably size percutaneous biopsy should be undertaken to guide the choice of antibiotics.
Systemic antibiotic treatment
Staphylococcus aureus is the most common pathogen in diabetic foot OM. However, it may be part of a polymicrobial infection and frequent gram-negative bacterial prevalence makes empirical antibiotic choices difficult (32). Antibiotics may be less effective in treating areas of necrotic bone and where biofilm formation impairs penetration of antibiotics to the infective focus and thus the standard surgical practice adopted is that chronic OM must be treated by surgical removal of infected and necrotic bone (21). However, successful treatment of diabetic foot OM has been shown with antibiotic therapy alone (6, 10, 31, 38). Recommended antibiotic regimes do not have strong evidence. Guidelines from the Infectious Diseases Society of America (IDSA) (32) suggested treatment based on likely residual infection. They recommended antibiotics for 1–3 weeks for any residual soft tissue infection, 4–6 weeks for residual but viable osteomyelitic bone, or at least 3 months for non-operative cases.
Surgical treatment
The operative aim for management of diabetic foot OM is to resect the affected bone and avoid leaving residual disease (43). IDSA guidelines in 2012 recommended effective surgical debridement followed by antibiotic treatment, with duration dependent on clearance of the infection (32). Surgical removal of an osteomyelitic digit is likely to be more acceptable to a patient as it still provides a satisfactory weight-bearing foot. For OM involving the distal phalanx or middle phalanx of a toe, disarticulation may be performed. However, for OM involving the proximal phalanx or the metatarsal head, a ray amputation is advised (Fig. 6) (11).
Fig. 6.

Showing bones removed during a second ray amputation (second toe and partial resection of the second metatarsal).
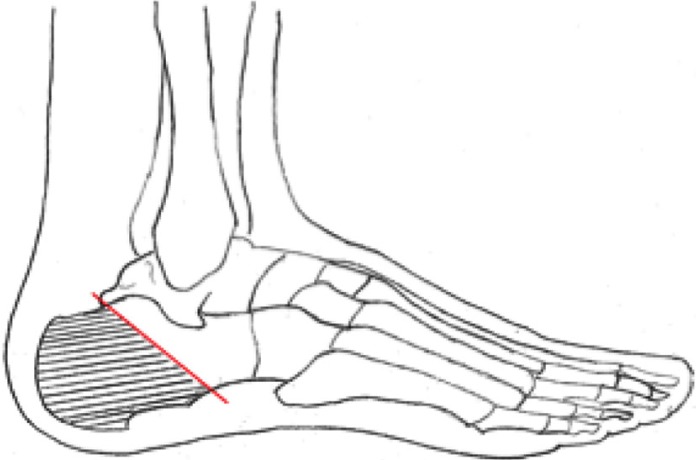
For OM of the calcaneum, partial or sub-total calcanectomy (Fig. 7) may be required with a wound direct closure or via a flap. This operation is less likely to be acceptable to a patient than digital amputation as the operation usually does not result in a satisfactory weight-bearing foot (11).
Fig. 7.
Diagram showing the bone resection for partial calcanectomy.
Inadequate surgical resection is one of the causes for re-operation. About a quarter of patients treated for OM have been shown to require operations. This was more likely if initial surgery was conservative or if necrosis or ischemia were involved (8). The risk of below the knee amputation was significantly higher with hindfoot OM (50%), compared to forefoot OM (0.33%) and midfoot OM (18.5%) (9). The additional benefit of surgery is the access to bone biopsy for cultures and histopathological analysis. One drawback of surgery is the change of foot dynamics and anatomy. Partial amputations and even conservative surgery can result in biomechanical changes in the feet which may provoke re-ulceration at a new site (44).
Foot deformity stabilization and antibiotic-loaded cement spacers/beads
Stabilization of a foot deformity is critical for ulcer/wound management in the diabetic foot and surgical correction is gaining favor over accommodative bracing (45). Deformity is common in Charcot neuroathropathy feet and when there is concurrent OM, management is difficult. Pinzur et al. (45) showed that radical debridement for OM, correction of deformity, and external fixation is achievable with a healing and ambulation rate of 83% after a single procedure and an overall limb salvage of 96%.
Polymethylmethacrylate (PMMA) cement has been loaded with antibiotics since the 1970s for use in orthopedic surgery. In this technique, the antibiotic diffuses out to local tissues where is reaches far higher local concentrations than can be obtained through systemic treatment (46, 47). Antibiotics delivered systemically cannot attain optimal levels in avascular sequestrae, and in the presence of vasculopathy commonly seen in diabetic patients (48, 49). The use of antibiotic impregnated beads following debridement has shown successful outcomes in the treatment of diabetic foot OM (50). Biodegradable options for local antibiotic therapy are of interest that may soon develop popularity (46, 47). Regardless of delivery, after bony debridement these methods allow dead space to be reduced while permitting residual infection to be controlled with elution of drugs over several weeks, which is desirable in the management of deep infection (46, 47). More research, such as comparative trials would be useful to prove that local antibiotics are more effective than systemic antibiotic therapy in terms of clinical outcomes (2).
Conservative treatment (antibiotics-only) vs. operative management of OM in the diabetic foot
The efficacy of antibiotic-only treatment for diabetic foot OM has been debated. In the last decade, a few key studies have been done to support the treatment of suspected diabetic foot OM with antibiotics alone (6, 10, 31, 38). Bone biopsies were not routinely taken for confirmation in these studies and antibiotics were often empirically selected. Duration of antibiotics also varied with minimum treatment of 6 weeks to 3 months. Successful treatment was also defined differently, some requiring complete ulcer healing and others requiring a prolonged period without recurrence of infection. Therefore, these studies are not fully comparable but report that between 63 and 83% of patients may be treated with systemic antibiotics alone (6, 10, 31, 38). As these studies were predominantly based on outpatients, the extent of infection may be less severe and conservative treatment is more likely to succeed.
On the other hand, these studies show that between 13 and 28% of patients may worsen during treatment and require early surgery (6, 10, 31). Recurrence of ulceration/infection is common during or after successful treatment as high as 30% (6, 10, 31), although about two thirds of these recurrences may also be successfully controlled conservatively rather than surgically (6, 31). One randomized study (51) of 52 patients has compared antibiotic and surgical treatment. Either a tailored antibiotic plan was used for 90 days of ulcer healing or conservative surgery was done followed by 10 days of antibiotics. There was no statistical difference in time to ulcer/wound healing between the surgical group (6 weeks) and the antibiotic group (7 weeks) (51). This small trial did not histologically confirm OM in the antibiotic group. The authors also excluded those patients with severe infections, vasculopathy, renal failure or involvement of the midfoot/hindfoot. Thus, their conclusions are only applicable to the healthier diabetic patients with forefoot involvement.
Some cases where antibiotics may be suitable for treatment of OM in the diabetic may include patient's own decision, patient unfit for surgery, infection involving a small area of the forefoot or if the extent of surgery required would cause significant destabilization of the foot (and the aim is limb salvage) (52). Surgery is recommended where the infection is multi-resistant or where there is a non-reversible ischemia, as systemic antibiotics would be of questionable efficacy. A non-salvageable limb will require a proximal amputation (52).
Conclusion
OM is a common complication of a diabetic foot ulcer or diabetic foot infection. A high index of clinical suspicion is required for diagnosis. Useful radiological studies include a combination of plain radiographs, bone scans, and MRI. Bone specimen should be sent for culture and sensitivity and histological studies. The key to surgical treatment of chronic OM in the diabetic foot is excision of the bone involved. Inadequate removal may lead to re-operation. In the recent years, however, some studies and one randomized control trial have shown that systemic antibiotics can be used long term to treat OM avoiding the need for amputation. Although a subset of these patients will eventually require surgery, it may be feasible to consider systemic antibiotics in the appropriate group of patients based on bone cultures. However, surgery can provide adequate debridement and also allow for the use of antibiotic-loaded cement spacers/beads. Patients with diabetic foot OM should be appropriately counseled regarding their options and medically optimized to ensure best outcomes with the treatment offered.
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study.
References
- 1.Mutluoglu M, Sivrioglu AK, Eroglu M, Uzun G, Turhan V, Ay H, et al. The implications of the presence of osteomyelitis on outcomes of infected diabetic foot wounds. Scand J Infect Dis. 2013;45:497–503. doi: 10.3109/00365548.2013.765589. [DOI] [PubMed] [Google Scholar]
- 2.Game FL. Osteomyelitis in the diabetic foot: diagnosis and management. Med Clin North Am. 2013;97:947–56. doi: 10.1016/j.mcna.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–7. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 4.Lavery LA, Peters EJ, Armstrong DG, Wendel CS, Murdoch DP, Lipsky BA. Risk factors for developing osteomyelitis in patients with diabetic foot wounds. Diabetes Res Clin Pract. 2009;83:347–52. doi: 10.1016/j.diabres.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Newman LG, Waller J, Palestro CJ, Schwartz M, Klein MJ, Hermann G, et al. Unsuspected osteomyelitis in diabetic foot ulcers. Diagnosis and monitoring by leukocyte scanning with indium in 111 oxyquinoline. JAMA. 1991;266:1246–51. doi: 10.1001/jama.266.9.1246. [DOI] [PubMed] [Google Scholar]
- 6.Acharya S, Soliman M, Egun A, Rajbhandari SM. Conservative management of diabetic foot osteomyelitis. Diabetes Res Clin Pract. 2013;101:18–20. doi: 10.1016/j.diabres.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Aragon-Sanchez FJ, Cabrera-Galvan JJ, Quintana-Marrero Y, Hernandez-Herrero MJ, Lazaro-Martinez JL, Garcia-Morales E, et al. Outcomes of surgical treatment of diabetic foot osteomyelitis: a series of 185 patients with histopathological confirmation of bone involvement. Diabetologia. 2008;51:1962–70. doi: 10.1007/s00125-008-1131-8. [DOI] [PubMed] [Google Scholar]
- 8.Aragon-Sanchez J, Lazaro-Martinez JL, Hernandez-Herrero C, Campillo-Vilorio N, Quintana-Marrero Y, Garcia-Morales E, et al. Does osteomyelitis in the feet of patients with diabetes really recur after surgical treatment? Natural history of a surgical series. Diabet Med. 2012;29:813–18. doi: 10.1111/j.1464-5491.2011.03528.x. [DOI] [PubMed] [Google Scholar]
- 9.Faglia E, Clerici G, Caminiti M, Curci V, Somalvico F. Influence of osteomyelitis location in the foot of diabetic patients with transtibial amputation. Foot Ankle Int. 2013;34:222–7. doi: 10.1177/1071100712467436. [DOI] [PubMed] [Google Scholar]
- 10.Valabhji J, Oliver N, Samarasinghe D, Mali T, Gibbs RG, Gedroyc WM. Conservative management of diabetic forefoot ulceration complicated by underlying osteomyelitis: the benefits of magnetic resonance imaging. Diabet Med. 2009;26:1127–34. doi: 10.1111/j.1464-5491.2009.02828.x. [DOI] [PubMed] [Google Scholar]
- 11.Nather A. The diabetic foot; Singapore: World Scientific; 2013. [Google Scholar]
- 12.Butalia S, Palda VA, Sargeant RJ, Detsky AS, Mourad O. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806–13. doi: 10.1001/jama.299.7.806. [DOI] [PubMed] [Google Scholar]
- 13.Michail M, Jude E, Liaskos C, Karamagiolis S, Makrilakis K, Dimitroulis D, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94–9. doi: 10.1177/1534734613486152. [DOI] [PubMed] [Google Scholar]
- 14.Aragon-Sanchez J, Lipsky BA, Lazaro-Martinez JL. Diagnosing diabetic foot osteomyelitis: is the combination of probe-to-bone test and plain radiography sufficient for high-risk inpatients? Diabet Med. 2011;28:191–4. doi: 10.1111/j.1464-5491.2010.03150.x. [DOI] [PubMed] [Google Scholar]
- 15.Morales Lozano R, Gonzalez Fernandez ML, Martinez Hernandez D, Beneit Montesinos JV, Guisado Jimenez S, Gonzalez Jurado MA. Validating the probe-to-bone test and other tests for diagnosing chronic osteomyelitis in the diabetic foot. Diabetes Care. 2010;33:2140–5. doi: 10.2337/dc09-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA. 1995;273:721–3. [PubMed] [Google Scholar]
- 17.Shone A, Burnside J, Chipchase S, Game F, Jeffcoate W. Probing the validity of the probe-to-bone test in the diagnosis of osteomyelitis of the foot in diabetes. Diabetes Care. 2006;29:945. doi: 10.2337/diacare.29.04.06.dc05-2450. [DOI] [PubMed] [Google Scholar]
- 18.Lavery LA, Armstrong DG, Peters EJ, Lipsky BA. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270–4. doi: 10.2337/dc06-1572. [DOI] [PubMed] [Google Scholar]
- 19.Pineda C, Espinosa R, Pena A. Radiographic imaging in osteomyelitis: the role of plain radiography, computed tomography, ultrasonography, magnetic resonance imaging, and scintigraphy. Semin Plast Surg. 2009;23:80–9. doi: 10.1055/s-0029-1214160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519–27. doi: 10.1086/590011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters EJ, Lipsky BA. Diagnosis and management of infection in the diabetic foot. Med Clin North Am. 2013;97:911–46. doi: 10.1016/j.mcna.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Pineda C, Pena A, Espinosa R, Hernandez-Diaz C. Imaging of osteomyelitis: the key is in the combination. Int J Clin Rheumatol. 2011;6:25–33. [Google Scholar]
- 23.Roug IK, Pierre-Jerome C. MRI spectrum of bone changes in the diabetic foot. Eur J Radiol. 2012;81:1625–9. doi: 10.1016/j.ejrad.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 24.Kapoor A, Page S, Lavalley M, Gale DR, Felson DT. Magnetic resonance imaging for diagnosing foot osteomyelitis: a meta-analysis. Arch Intern Med. 2007;167:125–32. doi: 10.1001/archinte.167.2.125. [DOI] [PubMed] [Google Scholar]
- 25.Capriotti G, Chianelli M, Signore A. Nuclear medicine imaging of diabetic foot infection: results of meta-analysis. Nucl Med Commun. 2006;27:757–64. doi: 10.1097/01.mnm.0000230065.85705.b3. [DOI] [PubMed] [Google Scholar]
- 26.Kagna O, Srour S, Melamed E, Militianu D, Keidar Z. FDG PET/CT imaging in the diagnosis of osteomyelitis in the diabetic foot. Eur J Nucl Med Mol Imaging. 2012;39:1545–50. doi: 10.1007/s00259-012-2183-z. [DOI] [PubMed] [Google Scholar]
- 27.Senneville E, Gaworowska D, Topolinski H, Devemy F, Nguyen S, Singer B, et al. Outcome of patients with diabetes with negative percutaneous bone biopsy performed for suspicion of osteomyelitis of the foot. Diabet Med. 2012;29:56–61. doi: 10.1111/j.1464-5491.2011.03414.x. [DOI] [PubMed] [Google Scholar]
- 28.Harmer JL, Pickard J, Stinchcombe SJ. The role of diagnostic imaging in the evaluation of suspected osteomyelitis in the foot: a critical review. Foot (Edinb) 2011;21:149–53. doi: 10.1016/j.foot.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 29.van der Bruggen W, Bleeker-Rovers CP, Boerman OC, Gotthardt M, Oyen WJ. PET and SPECT in osteomyelitis and prosthetic bone and joint infections: a systematic review. Semin Nucl Med. 2010;40:3–15. doi: 10.1053/j.semnuclmed.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Tomas MB, Patel M, Marwin SE, Palestro CJ. The diabetic foot. Br J Radiol. 2000;73:443–50. doi: 10.1259/bjr.73.868.10844873. [DOI] [PubMed] [Google Scholar]
- 31.Game FL, Jeffcoate WJ. Primarily non-surgical management of osteomyelitis of the foot in diabetes. Diabetologia. 2008;51:962–7. doi: 10.1007/s00125-008-0976-1. [DOI] [PubMed] [Google Scholar]
- 32.Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clinic Inf Dis. 2012;54:132–73. doi: 10.1093/cid/cis346. [DOI] [PubMed] [Google Scholar]
- 33.Lipsky BA, Peters EJ, Senneville E, Berendt AR, Embil JM, Lavery LA, et al. Expert opinion on the management of infections in the diabetic foot. Diabetes Metab Res Rev. 2012;28:163–78. doi: 10.1002/dmrr.2248. [DOI] [PubMed] [Google Scholar]
- 34.Berendt AR, Peters EJ, Bakker K, Embil JM, Eneroth M, Hinchliffe RJ, et al. Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev. 2008;24:145–61. doi: 10.1002/dmrr.836. [DOI] [PubMed] [Google Scholar]
- 35.Aragon-Sanchez J, Lazaro-Martinez JL, Cabrera-Galvan JJ. Additional information on the role of histopathology in diagnosing diabetic foot osteomyelitis. Diabetic Med. 2014;31:113–16. doi: 10.1111/dme.12283. [DOI] [PubMed] [Google Scholar]
- 36.Meyr AJ, Singh S, Zhang X, Khilko N, Mukherjee A, Sheridan MJ, et al. Statistical reliability of bone biopsy for the diagnosis of diabetic foot osteomyelitis. J Foot Ankle Surg. 2011;50:663–7. doi: 10.1053/j.jfas.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Elamurugan TP, Jagdish S, Kate V, Chandra Parija S. Role of bone biopsy specimen culture in the management of diabetic foot osteomyelitis. Int J Surg. 2011;9:214–16. doi: 10.1016/j.ijsu.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Senneville E, Lombart A, Beltrand E, Valette M, Legout L, Cazaubiel M, et al. Outcome of diabetic foot osteomyelitis treated nonsurgically: a retrospective cohort study. Diabetes Care. 2008;31:637–42. doi: 10.2337/dc07-1744. [DOI] [PubMed] [Google Scholar]
- 39.Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Clin Inf Dis. 2004;39:885–910. doi: 10.1086/424846. [DOI] [PubMed] [Google Scholar]
- 40.Senneville E, Morant H, Descamps D, Dekeyser S, Beltrand E, Singer B, et al. Needle puncture and transcutaneous bone biopsy cultures are inconsistent in patients with diabetes and suspected osteomyelitis of the foot. Clin Inf Dis. 2009;48:888–93. doi: 10.1086/597263. [DOI] [PubMed] [Google Scholar]
- 41.Slater RA, Lazarovitch T, Boldur I, Ramot Y, Buchs A, Weiss M, et al. Swab cultures accurately identify bacterial pathogens in diabetic foot wounds not involving bone. Diabetic Med. 2004;21:705–9. doi: 10.1111/j.1464-5491.2004.01221.x. [DOI] [PubMed] [Google Scholar]
- 42.Senneville E, Melliez H, Beltrand E, Legout L, Valette M, Cazaubiel M, et al. Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: concordance with ulcer swab cultures. Clin Inf Dis. 2006;42:57–62. doi: 10.1086/498112. [DOI] [PubMed] [Google Scholar]
- 43.Simpson AH, Deakin M, Latham JM. Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J Bone Joint Surg Br. 2001;83:403–7. doi: 10.1302/0301-620x.83b3.10727. [DOI] [PubMed] [Google Scholar]
- 44.Molines-Barroso RJ, Lazaro-Martinez JL, Aragon-Sanchez J, Garcia-Morales E, Beneit-Montesinos JV, Alvaro-Afonso FJ. Analysis of transfer lesions in patients who underwent surgery for diabetic foot ulcers located on the plantar aspect of the metatarsal heads. Diabetic Med. 2013;30:973–6. doi: 10.1111/dme.12202. [DOI] [PubMed] [Google Scholar]
- 45.Pinzur MS, Gil J, Belmares J. Treatment of osteomyelitis in Charcot foot with single-stage resection of infection, correction of deformity, and maintenance with ring fixation. Foot Ankle Int. 2012;33:1069–74. doi: 10.3113/FAI.2012.1069. [DOI] [PubMed] [Google Scholar]
- 46.Roeder B, Van Gils CC, Maling S. Antibiotic beads in the treatment of diabetic pedal osteomyelitis. J Foot Ankle Surg. 2000;39:124–30. doi: 10.1016/s1067-2516(00)80037-x. [DOI] [PubMed] [Google Scholar]
- 47.Gogia JS, Meehan JP, Di Cesare PE, Jamali AA. Local antibiotic therapy in osteomyelitis. Semin Plast Surg. 2009;23:100–7. doi: 10.1055/s-0029-1214162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LoGerfo FW, Coffman JD. Current concepts. Vascular and microvascular disease of the foot in diabetes. Implications for foot care. N Engl J Med. 1984;311:1615–19. doi: 10.1056/NEJM198412203112506. [DOI] [PubMed] [Google Scholar]
- 49.Seabrook GR, Edmiston CE, Schmitt DD, Krepel C, Bandyk DF, Towne JB. Comparison of serum and tissue antibiotic levels in diabetes-related foot infections. Surgery. 1991;110:671–6. [PubMed] [Google Scholar]
- 50.Calhoun JH, Klemm K, Anger DM, Mader JT. Use of antibiotic-PMMA beads in the ischemic foot. Orthopedics. 1994;17:453–7. doi: 10.3928/0147-7447-19940501-12. [DOI] [PubMed] [Google Scholar]
- 51.Lazaro-Martinez JL, Aragon-Sanchez J, Garcia-Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: a randomized comparative trial. Diabetes Care. 2014;37:789–95. doi: 10.2337/dc13-1526. [DOI] [PubMed] [Google Scholar]
- 52.Lipsky BA. Treating diabetic foot osteomyelitis primarily with surgery or antibiotics: have we answered the question? Diabetes Care. 2014;37:593–5. doi: 10.2337/dc13-2510. [DOI] [PubMed] [Google Scholar]