Abstract
Background
Major Depressive Disorder is common, often recurrent and/or chronic. Theoretically, assessing quality of life (QoL) in addition to the current practice of assessing depressive symptoms has the potential to offer a more comprehensive evaluation of the effects of treatment interventions and course of illness.
Methods
Before and after acute-phase cognitive therapy (CT), 492 patients from Continuation Phase Cognitive Therapy Relapse Prevention trial (Jarrett et al., 2013, Jarrett and Thase, 2010) completed the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q), Inventory of Depressive Symptomatology Self-report (IDS-SR) & Beck Depression Inventory (BDI); clinicians completed Hamilton Rating Scale for Depression-17-items. Repeated measures analysis of variance evaluated the improvement in QoL before/after CT and measured the effect sizes. Change analyses to assess clinical significance (Hageman and Arrindell, 1999) were conducted.
Results
At the end of acute-phase CT, a repeated measure analysis of variance produced a statistically significant increase in Q-LES-Q scores with effect sizes of 0.48 - 1.3; 76.9 - 91.4% patients reported clinically significant improvement. Yet, only 11 - 38.2% QoL scores normalized. An analysis of covariance showed that change in depression severity (covariates=IDS-SR, BDI) completely accounted for the improvement in Q-LES-Q scores.
Limitations
There were only two time points of observation; clinically significant change analyses lacked matched normal controls; and generalizability is constrained by sampling characteristics. Conclusions: Quality of life improves significantly in patients with recurrent MDD after CT; however, this improvement is completely accounted for by change in depression severity. Normalization of QoL in all patients may require targeted, additional, and/or longer treatment.
Keywords: Quality of life, Major depressive disorder, Cognitive therapy
Introduction
Major Depressive Disorder (MDD) is often a chronic and/or recurrent illness (Holma et al., 2008, Judd, 2001, Keller et al., 1992, Keller MB, 1984, Patten et al., 2010) that affects 5-7% of adults in United States annually (Hasin et al., 2005, Kessler et al., 2003). Psychosocial impairments almost always accompany depression (Judd et al., 2008, Miller et al., 1998) and worsen with increased depression severity (Judd et al., 2000). Moreover, psychosocial dysfunction may persist after treatment and increases the risk of future relapse or recurrence (Kennedy et al., 2007, Solomon et al., 2004, Vittengl et al., 2007, Vittengl et al., 2009). Hence, it is not adequate to rely solely on relief of depressive symptoms as primary outcome of treatment (Greer et al., 2010).
The World Health Organization's (WHO) definition of health as “a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity” (http://www.who.int/about/definition/en/print.html) offers a more comprehensive definition of health which could be embraced by and also improve current practice in mental health. Quality of life (QoL), a measure of well-being, has gained recent attention in treatment of depression (Bech, 2005, Frisch et al., 2005, Grant et al., 1995, IsHak et al., 2011, Kilnkman, 2009, Papakostas et al., 2004, Frisch, 2009).
Quality of life can be assessed using a variety of instruments such as Quality of Life in Depression Scale (McKenna and Hunt, 1992, Tuynman-Qua et al., 1997), Quality of Well-Being Scale (Kaplan et al., 1998), Quality of Life Enjoyment and Satisfaction Questionnaire (Endicott et al., 1993), Quality of Life Inventory (Frisch et al., 2005) and WHO Quality of Life Assessment Instruments (Skevington et al., 2004, Skevington and Wright, 2001). Here we used the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q; (Endicott et al., 1993), a frequently used QoL measure, that evaluates patients' enjoyment and satisfaction with different aspects of their lives through its eight summary scales of physical health, subjective feelings, work, household duties, school/course work, leisure time activities, social relationships and general activities (Endicott et al., 1993). Consistent with the definition of health by WHO, the multidimensional nature of Q-LES-Q (Bishop et al., 1999) can comprehensively capture a patient's subjective evaluation of well-being and satisfaction with life. The General activities summary scale of Q-LES-Q is often used as a short form instrument (Q-LES-Q SF) (Stevanovic, 2011).
Lower scores on Q-LES-Q are associated with increased depressive symptom severity (Endicott et al., 1993), lifetime history of MDD even in absence of any current psychiatric illnesses (Schechter et al., 2007), being unemployed, having high school education or more and being divorced or separated (Daly EJ, 2010). In a like manner, Q-LES-Q scores increase with both pharmacological (Demyttenaere et al., 2008, Keitner et al., 2009, Kocsis, 1997, Lydiard RB, 1997, Miller et al., 1998, Shelton RC, 2006, Trivedi et al., 2004a, Versiani et al., 2005) and psychosocial (Drymalski and Washburn, 2011, Swan et al., 2009) treatment interventions.
While statistically significant change in QoL has been demonstrated above, it is also important to evaluate how clinically important such changes are. Toward this end to evaluate the clinical significance of this increase in Q-LES-Q score, Swan et al. (Swan et al., 2009) used the two-fold criteria proposed by Jacobson and Truax (Jacobson and Truax, 1991) and Cohen's d effect size. As a measure of clinical significance, Jacobson and Truax (Jacobson and Truax, 1991) proposed a two-fold criterion of post-treatment score being more than cut off score (CS) and reliable change index (RCI) > 1.96 to determine the extent to which a treatment intervention moves a patient out of dysfunctional range or within functional range and beyond the range of measurement error (Jacobson et al., 1984). Hageman and Arrindell (Hageman and Arrindell, 1999) proposed further refinements to RCI and CS by distinguishing individual versus group level analyses and correcting for ‘regression to mean’ of observed scores and labeled individual level analyses as RCindv and CSindv and proposed group level analyses for proportionCHANGED and proportionBEYOND CUTOFF.
Increases in Q-LES-Q scores with treatment interventions are related to improvement in depressive symptoms but may not be completely accounted for by it. Endicott et al. (Endicott et al., 1993) estimated correlation coefficients of change in Q-LES-Q with change in Hamilton Rating Scale for Depression17-items (HRSD-17) which ranged from -0.34 to -0.54 suggesting Q-LES-Q is sensitive to change in depressive symptom but may not be totally redundant. Using hierarchical multiple regression analysis, Swan et al. (Swan et al., 2009) reported that between 37% and 53% variance in Q-LES-Q SF is not accounted for by the change in depression severity measured by Beck Depression Inventory (BDI) II. Defining “normal” quality of life as within 10% of community norm of Q-LES-Q SF score of 58 {per (Rapaport et al., 2005)}, Demyttenaere et al. (Demyttenaere et al., 2008) found that 40% individuals who attained remission of depressive symptoms {defined as Montgomery Asberg Depression Rating Score (Montgomery and Asberg, 1979) less than or equal to 12} did not have a “normal” quality of life.
Cognitive therapy (CT) is a commonly used and extensively researched treatment for MDD. Compared to discontinued pharmacotherapy, CT significantly reduces the risk of relapse/recurrence of MDD (Vittengl et al., 2007). Only a limited number of studies have evaluated effect of CT on QoL (Jarrett et al., 2013, Swan et al., 2009, Vittengl et al., 2007, Watson and Nathan, 2008), although the findings of these studies suggest that QoL in depressed patients improves with effective treatment. For a detailed quality of life assessment, Jarrett and Thase used long form of Q-LES-Q in Continuation Phase Cognitive Therapy Relapse Prevention (C-CT-RP) and included acute phase CT provided to adults presenting with recurrent MDD (Jarrett et al., 2013, Jarrett and Thase, 2010). As far as we know this is the first study to use the long form of Q-LES-Q to assess the outcomes of people with recurrent major depressive disorder.
In the current report, we attempt to replicate and extend previous findings by asking the following: 1) After treatment, is quality of life better than before in adult outpatients exposed to individual cognitive therapy (CT) for recurrent MDD? 2) To what extent is pre-post CT improvement in quality of life clinically significant? and 3) To what extent does pre-post CT change in depression severity account for the improvement in quality of life?
Previous studies used only the general activities summary scale from the Q-LES-Q to evaluate the effect of CT on QoL. Here we provide a comprehensive and multidimensional evaluation of QoL (Jarrett et al., 2013, Jarrett and Thase, 2010) by relying on a large sample (N= 492) who completed the long form of Q-LES-Q complete with summary scales (i.e., physical health, subjective feelings, work, household duties, school/course work, leisure time activities, social relationships and general activities). We also rely upon the use of multiple measures of depression severity making replication of previously published reports possible (Endicott et al., 1993, Swan et al., 2009) in a general attempt to better understand of the influence of change in depression severity on change in QoL in recurrent MDD patients.
Methods
Details of the C-CT-RP trial, focused on relapse/recurrence prevention, have been described elsewhere by Jarrett & Thase (Jarrett et al., 2013, Jarrett and Thase, 2010) (clinicaltrials.gov identifiers NCT00118404, NCT00183664, and NCT00218764). Out of the 523 patients who met inclusion and exclusion criteria and consented for treatment in C-CT-RP, 492 filled long form of Q-LES-Q prior to starting acute-phase CT and hence constituted the modified intention to treat (mITT) sample for the current report. During acute-phase CT, patients received 16 to 20 individual sessions spread over 12 weeks with up to 2 additional weeks to accommodate scheduling needs. Sixteen therapists provided acute-phase CT and demonstrated competence by achieving and maintaining Cognitive Therapy Scale (CTS) scores ≥ 40.
Patients
The C-CT-RP trial was approved by Institutional Review Boards at The University of Texas Southwestern Medical Center and University of Pittsburgh, Western Psychiatric Institute and Clinic. With their verbal consent, potential participants were screened over the phone and/or in-person by the clinic staff and scheduled for initial diagnostic evaluation and a second, confirmatory interview to determine eligibility. Patients included in C-CT-RP provided written informed consent, scored 14 or more on HRSD-17 at both initial diagnostic evaluation and confirmatory interview and were diagnosed with recurrent Major Depressive disorder using Structured Clinical Interview for DSM-IV with either a) remission between episodes; b) one prior episode with complete inter-episode recovery; or c) antecedent dysthymic disorder. Patients were excluded if they: a) had concurrent severe or poorly controlled medical disorder or required medications that may cause depression; b) had concurrent bipolar disorder, any psychotic or organic mental disorder, active alcohol or drug dependence, primary (i.e. associated with most impairment) obsessive compulsive disorder or eating disorders; c) were unable to complete questionnaires in English; d) presented an active suicide risk; e) had a previous non-response to at least 8 weeks of CT or at least 6 weeks of 40mg of Fluoxetine; g) were pregnant or planned to become pregnant during the first 11 months after intake.
Assessments
Demographic information on patients was collected at diagnostic evaluation with a self-report form.
Inventory of Depressive Symptomatology Self-report (IDS-SR), HRSD-17 and BDI were used as measures of depressive symptom severity in C-CT-RP trial. Clinicians administered HRSD-17 and patients completed 30 item IDS-SR and 21 item BDI at initial evaluation and at the end of acute-phase of CT. Higher scores on these measures indicate greater depressive symptom severity. Patients filled out 93 item long form of Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) prior to and at the end of acute-phase CT.
Beck Depression Inventory (BDI)
BDI has 21 items with 4 choices for each item which are scored from 0-3. Total score, generated by adding all 21 items, categorizes depression severity as minimal (0-9), mild (10-18), moderate (19-29) and severe (30-63) (Beck et al., 1961). The measure of internal consistency of BDI for psychiatric populations in previously published literature is 0.86 (Beck et al., 1988) and Cronbach's α is 0.83 in MDD patients (Rush et al., 1996). The correlation coefficient (Pearson product moment correlation) between BDI and HRSD is 0.74 (Beck et al., 1988). In C-CT-RP, the Cronbach's α was 0.88 (range = 0.83 to 0.92); median convergent validity with HRSD was r = 0.72 (range = 0.44 - 0.80) & with IDS-SR was r = 0.86 (range = 0.79 to 0.90) (Dunn et al., 2012).
Hamilton Rating Scale for Depression 17-items (HRSD-17)
Individual items have 3-5 choices which are scored from 0-2 or 0-4. Sum of scores of individual items can indicate depression severity of none (<6), mild (6-13), moderate (14-18), severe (19-23) and very severe (>24) (Hamilton, 1960). With highly trained raters, HRSD has a high inter-rater reliability {r = 0.94; (Trajković et al., 2011)}. Previously reported Cronbach's α of HRSD-17 in MDD patients ranged from 0.53 (Rush et al., 1996) to 0.83 (Rush et al., 2003). In C-CT-RP, HRSD-17 inter rater reliability was r = 0.91, Cronbach's α was 0.68 and median concurrent validity with IDS-SR was r = 0.76 (Dunn et al., 2012).
Inventory of Depressive Symptomatology Self-report (IDS-SR)
IDS-SR has 30 items with 4 choices for each item scored from 0-3. Total score is sum of 28 of 30 items (range 0-84), categorizing depression severity as none (<13), mild (14-25), moderate (26-38), severe (39-48) and very severe (>49). In 2 different samples, the internal consistency of IDS-SR was Cronbach's α = 0.92 (Rush et al., 2003, Trivedi et al., 2004b) which is close to the Cronbach's α = 0.86 in C-CT-RP (Dunn et al., 2012).
For the current analyses, we decided to use IDS-SR as the primary measure of depression severity because when compared to HRSD-17 it evaluates atypical symptoms of depression and is thought to cover the depressive symptom constructs more completely (Gullion and Rush, 1998). We used HRSD-17 and BDI in addition to IDS-SR to replicate the results of Endicott et al. (Endicott et al., 1993) and Swan et al. (Swan et al., 2009) to evaluate the change in QoL with change in depression severity.
Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q)
93 items of this scale are grouped in 8 summary scales and 2 individual questions. Physical Health, Subjective Feelings, Leisure Time Activities, Social Relationships, General Activities and the 2 individual questions are scored for all patients. Work, Household Duties and School/Course Work are scored only for patients for whom they are applicable. Each question is scored on a 5-point scale and higher values signify better quality of life. Across the 8 summary scales, Endicott et al. (Endicott et al., 1993) report test-retest reliability ranging from 0.63 to 0.89 and α coefficients of internal consistency ranging from 0.90 to 0.96. Using factor analyses, Bishop et al. (Bishop et al., 1999) reported good construct validity of Q-LES-Q. The eight summary scales and the individual item regarding overall satisfaction were included in the current study. The individual item regarding medication was not pertinent to acute-phase CT and hence was excluded. In C-CT-RP, across Q-LES-Q summary scales and pre- and post-CT visit, α coefficients of internal consistency ranged from 0.84 to 0.94 with a median of 0.89.
Statistical analyses
The modified intention to treat (mITT) sample included 492 patients. To use all available data and adequately reflect variance of imputed data (Schafer, 1999), we used multiple imputation procedure for missing Q-LES-Q and IDS-SR values at the end of CT by using Markov chain Monte Carlo (MCMC) method and m=10 imputations. We used SAS 9.2 to perform statistical analyses. We used the methods described by Paul Allison Ph.D. at http://www.ssc.upenn.edu/∼allison/ to combine the individual level analyses of the multiple imputed datasets to arrive at the combined results in our study. We used Bonferroni correction for multiple analyses and set the level of significance at .05
We used repeated measures analysis of variance (ANOVA) with one within subject factor for each outcome to assess if change in QoL at the end of CT was statistically significant for the eight summary scales and overall individual item of Q-LES-Q. We calculated the Cohen's d effect sizes to evaluate the magnitude of changes in the summary scales and individual item.
We performed group level Clinically Significant Change (CSC) analyses using proportionCHANGED and proportionBEYOND CUTOFF as described by Hageman and Arrindell (Hageman and Arrindell, 1999). We classified post acute-phase CT patients in following CSC categories: a) Unchanged/deteriorated: includes patients who had either no significant change with or worsened after treatment with CT, b) Improved but still impaired: includes patients with a statistically significant change but not large enough to cross the cut-off of clinically significant threshold and c) Unimpaired: includes patient with statistically significant change large enough to cross the above mentioned cut-off. For these analyses, we calculated index for individual reliable change (RCindv) and the index for individual reliably passing the cutoff for clinical significance (CSindv). Using the methods outlined by Jacobson and Truax (Jacobson and Truax, 1991), calculation of CSindv (Hageman and Arrindell, 1999) requires cut-off of 2 SD from the mean of either control/functional population or dysfunctional population in functional direction, or midpoint of means of control/functional and dysfunctional populations. As C-CT-RP did not include a control (non-MDD) population, we considered using previously published normal control sample of Q-LES-Q study (Schechter et al., 2007). However, we found significant differences in mean age (9.93 years), sex (χ2 = 8.03, p < 0.005), ethnicity (χ2 = 42.105, p< 0.0001), education (t= 103.481, p<0.0001) and marital status (χ2 = 69.624, p<0.0001) between the C-CT-RP sample and the control sample from Schechter 2007. Hence due to the unavailability of control sample, we used the cut-off of 2 SD from pre-treatment mean of C-CT-RP in functional direction to calculate CSindv.
We included pre- and post-CT IDS-SR as covariates in the analysis of covariance model (ANCOVA) to evaluate if the change in depression severity accounts for the change in QoL. A non-significant F statistic for change in Q-LES-Q in the ANCOVA model when accounting for changes in IDS-SR will suggest that change in depression severity completely accounts for the change in Q-LES-Q. To replicate the reported results of Swan et al. (Swan et al., 2009), we performed the above analysis with BDI in the ANCOVA model. We also replicated the analyses of Endicott et al. (Endicott et al., 1993) for redundancy of depression severity and QoL by calculating the correlation coefficients between Q-LES-Q summary scales and HRSD-17 at the end of CT, as well as change in Q-LES-Q summary scales and HRSD-17 with CT.
Results
Prior to starting acute-phase CT, 396 patients filled out work summary scale, 479 patients filled out household duties summary scale, and 87 patients filled out the school summary scale of Q-LES-Q. Out of the 492 patients, 356 patients completed Q-LES-Q and 361 completed IDS-SR at the end of acute-phase CT. Rate of missing values for Q-LES-Q was 27.7% and for IDS-SR was 26.6%. Markov chain Monte Carlo method was used to impute these missing values.
Does quality of life improve after acute-phase CT, compared to before?
Yes. After CT patients reported that their quality of life was significantly better than before CT, as indicated by pre- and post- acute-phase CT Q-LES-Q scores within a repeated measures ANOVA. These analyses revealed statistically significant (p value <0.003 to <0.001) increases in all 8 summary scales and overall individual item of Q-LES-Q. The greatest improvement was seen in the general activities summary scale and least improvement was observed in school summary scale. The summary statistics of change in Q-LES-Q summary scales and overall individual item along with F statistics for ANOVA and Cohen's d values are listed in table 2. The effect size of change was large in all summary scales except school where it was medium {using Cohen's conventional criteria where values of Cohen's d of 0.2, 0.5 and 0.8 suggest small, medium and large effect sizes respectively (Cohen J, 1988).
Table 2. Pre- and Post-Cognitive Therapy Change in Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) Summary Scales.
| Q-LES-Q Summary Scales | Pre CT Mean (SD) | Post CT Mean (SD) | ANOVA F-Statistic(df) | p Value* | Cohen's d effect size |
|---|---|---|---|---|---|
| Physical | 39.74 (16.88) | 62.78 (21.09) | F(1,347)= 416.6 | <0.001 | 0.98 |
| Feelings | 41.39 (15.42) | 65.65 (18.83) | F(1,348)= 556.0 | <0.001 | 1.12 |
| Work | 47.05 (24.96) | 71.09 (21.29) | F(1,258)= 326.5 | <0.001 | 0.86 |
| Household | 45.22 (19.9) | 66.88 (21.1) | F(1,333)= 377.5 | <0.001 | 0.94 |
| School | 29.92 (30.11) | 67.79 (30.85) | F(1,30)= 63.0 | <0.003 | 0.48 |
| Leisure | 45.88 (19.69) | 66.33 (20.76) | F(1,347)= 239.9 | <0.001 | 0.80 |
| Social | 45.68 (16.97) | 66.91 (19.43) | F(1,343)= 403.6 | <0.001 | 0.90 |
| General | 40.91 (15.14) | 65.38 (18.69) | F(1,344)= 743.1 | <0.001 | 1.3 |
| Overall | 28.62 (21.56) | 62.19 (25.16) | F(1,326)= 376.4 | <0.001 | 1.08 |
adjusted after Bonferroni correction; CT is Cognitive Therapy; SD is standard deviation; and ANOVA is analysis of variance.
Is the statistically significant improvement in quality of life clinically significant?
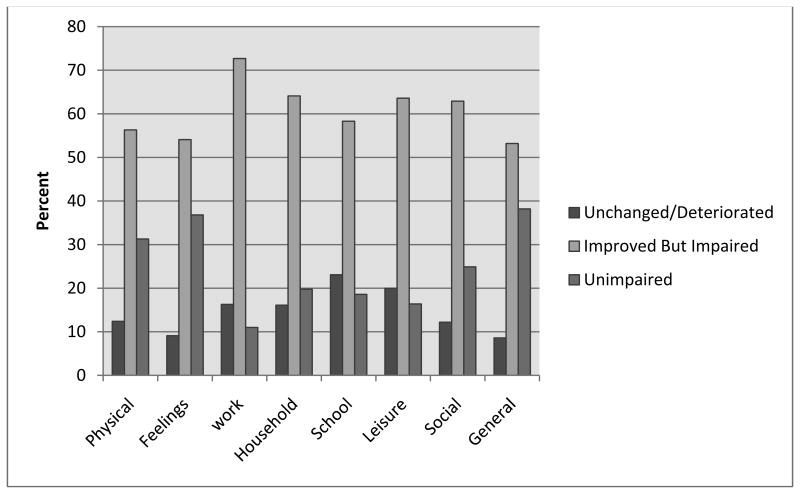
Yes. The majority of patients reported improved quality of life post CT. Only a small fraction of patients (8.6% to 23.1%) reported that their quality of life after acute phase CT was unchanged or had deteriorated. For all Q-LES-Q summary scales, a majority of patients were in improved but still impaired CSC category; ranging from the lowest of 52.7% for general activities and the largest of 72.7% for work. Details are in figure 1.
Figure 1. Percentage of patients in CSC categories after CT for Q-LES-Q summary scales.
CT is cognitive therapy; Q-LES-Q is Quality of Life Enjoyment and satisfaction questionnaire; CSC categories are clinically significant change categories of unchanged/deteriorated, improved but still impaired and unimpaired.
Does the pre-post CT change in depression severity account for improvement in QoL?
Yes. Improvement in depression severity at post-CT compared to pre-CT accounted for improvement in Q-LES-Q over CT for all summary scales and the overall individual item. We used repeated measures ANCOVA analyses of pre- and post-CT Q-LES-Q scores with pre- and post- acute-phase IDS-SR as a covariate and used the F-test to assess if the improvement in depressive severity accounts for improvement in Q-LES-Q. For all summary scales, we found a non-significant F-statistic suggesting that change in depressive symptom severity completely accounted for improvement in QoL. Replicating the analyses of Swan et al. (Swan et al., 2009) , we repeated the repeated measures ANCOVA analyses with pre- and post- acute-phase BDI as the covariate and arrived at a similar conclusion. Details are given in table 3.
Table 3. Pre- to Post-Cognitive Therapy Change in Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) Summary Scales adjusted for change in depression severity.
| Q-LES-Q Summary Scales | F-Statistics from ANCOVA Analysis with Depressive Severity Measures by | |||
|---|---|---|---|---|
| IDS-SR F-Statistic(df) | p Value* | BDI F-Statistic(df) | p Value* | |
| Physical | F(1,341)= 3.06 | <0.73 | F(1,184)= 2.56 | >0.99 |
| Feelings | F(1,342)= 0.98 | >0.99 | F(1,35)= 0.49 | >0.99 |
| Work | F(1,254)= 0.85 | >0.99 | F(1,51)= 4.93 | <0.28 |
| Household | F(1,327)= 0.03 | >0.99 | F(1,222)= 3.76 | <0.48 |
| School | F(1,29)= 0.10 | >0.99 | F(1,6)= 0.35 | >0.99 |
| Leisure | F(1,341)= 4.93 | <0.25 | F(1,146)= 0.00 | >0.99 |
| Social | F(1,337)= 0.00 | >0.99 | F(1,49)= 3.31 | <0.67 |
| General | F(1,338)= 1.04 | >0.99 | F(1,68)= 3.35 | <0.64 |
| Overall | F(1,320)= 0.33 | >0.99 | F(1,69)= 3.46 | <0.60 |
adjusted after Bonferroni correction; ANCOVA is analysis of covariance; IDS-SR is Inventory of Depressive Symptomatology Self-report; and BDI is Beck Depression Inventory.
Following Endicott et al. (Endicott et al., 1993) , we calculated the correlation coefficients of pre-post CT changes in Q-LES-Q summary scales and overall individual item with changes in HRSD-17 or IDS-SR which ranged from -0.24 to -0.63 for HRSD-17 and -0.34 to -0.68 for IDS-SR. We also calculated the correlation coefficients between Q-LES-Q summary scales and overall individual item and HRSD-17 or IDS-SR at the end of acute-phase CT. These correlation coefficients are listed in table 4.
Table 4. Correlation coefficients of pre- to post-CT change and post-CT Q-LES-Q summary scales with IDS-SR and HRSD-17.
| QLESQ Scales | Pre- to Post-CT Change in Q-LES-Q | Post-CT Q-LES-Q | ||
|---|---|---|---|---|
| IDS-SR | HRSD-17 | IDS-SR | HRSD-17 | |
| Physical | -0.61 | -0.57 | -0.72 | -0.70 |
| Feelings | -0.68 | -0.63 | -0.78 | -0.77 |
| Work | -0.46 | -0.36 | -0.55 | -0.54 |
| Household | -0.46 | -0.50 | -0.59 | -0.58 |
| School | -0.34 | -0.24 | -0.53 | -0.64 |
| Leisure | -0.48 | -0.46 | -0.64 | -0.60 |
| Social | -0.53 | -0.46 | -0.62 | -0.64 |
| General | -0.67 | -0.63 | -0.80 | -0.80 |
| Overall | -0.62 | -0.60 | -0.78 | -0.79 |
CT is cognitive therapy; Q-LES-Q is Quality of Life Enjoyment and satisfaction questionnaire; IDS-SR is Inventory of Depressive Symptomatology Self-report; and HRSD-17 is Hamilton Rating Scale for Depression 17-items.
Discussion
These results after CT replicate previous findings that quality of life improves with treatment of depression. After acute-phase CT, patients reported increased satisfaction and enjoyment with different aspects of their lives like physical health, feelings, household, occupational, educational, leisure time or social activities. The magnitude of these improvements are similar to the effect sizes reported in a previous study of CT for depression (Swan et al., 2009). A large majority of patients experienced clinically significant improvement in different aspects of quality of life although full normalization (compared to available norms) was limited. Moreover, as 75.1% to 89% of patients showed impairment in the area of work and social activities after acute-phase CT, our findings emphasize the relatively persistent burden imposed by recurrent depression and highlight the need for greater therapeutic focus on normalizing role and social functioning during and after the acute phase of treatment.
Contrary to previously published reports (Endicott et al., 1993, Swan et al., 2009) that variance in Q-LES-Q is only partly explained by changes in depressive symptom severity, we found that improvement in depressive symptoms completely accounted for the improvement in quality of life in all 8 summary scales, including general activities, and overall question. We found correlations between changes in HRSD-17 and Q-LES-Q similar to those reported by Endicott et al. (Endicott et al., 1993) but our finding of change in BDI completely accounting for changes in Q-LES-Q general activities summary scale differed from the previously published report (Swan et al., 2009) .
The nuances in results across studies may be due to differences in study designs and/or analyses. For example, sample characteristic here differ from that of Swan et al. (Swan et al., 2009), which had 47.6 % patients diagnosed with major affective disorder, recurrent ; with rest of the patients being diagnosed as major affective disorder, single episode (28.3%), dysthymia (22.2%) or melancholia (1.9%). Swan and associates also used short form of Q-LES-Q, a combination of group and individual cognitive therapy and completer sample to evaluate shared variance. In contrast our report included only recurrent MDD patients and used long form of Q-LES-Q, only individual cognitive therapy and multiple imputations for a modified intention to treat sample. We used a more refined approach (Hageman and Arrindell, 1999)to analyze clinically significant changes in Q-LES-Q with treatment. We also differed (Demyttenaere et al., 2008, Swan et al., 2009) by checking for demographic differences between the study sample and historical control sample while calculating a cut-off threshold for clinical significance.
The results highlight the distinctions between statistically and even clinically significant improvement in quality of life compared to full normalization. A valid question is to what extent do longer courses of or targeted treatment facilitate moving QoL improvement to true normalization, especially in the areas of role and social functioning?
The current study has limitations. With only 2 time points of assessment, our study is limited in understanding the relationship between changes in Q-LES-Q and depressive symptom severity and future studies should use more frequent measurement so that cross-lagged correlations between these instruments can be examined (Dunn et al., 2012) . The differences in demographics between our sample and historical control (Schechter et al., 2007) resulted in use of 2 SD from pre-treatment mean in functional range instead of the mid-point between means of patient and control samples. This might have caused a higher threshold for clinical significance in our sample leading to lower estimates of percentage of individuals in unimpaired range of Q-LES-Q scores. As treatment occurred in academic medical center clinic setting by highly proficient therapists and focused on well characterized patients with recurrent depression, the generalizability of these findings may be limited in community samples (Blanco et al., 2008). Another limitation of our report is that quality of life was not a primary treatment outcome for C-CT-RP and hence we did not check for the power to detect differences in Q-LES-Q a priori.
In conclusion, these findings demonstrate statistically and clinically significant improvement, but incomplete normalization, in different aspects of QoL in patients with recurrent MDD after acute-phase cognitive therapy. While statistically these improvements are completely accounted for by the changes in depressive symptom severity, the fine grained analysis of the components of quality of life (shown in the subscales), can guide targets for change during treatment and additional assurance of the broad and positive effect of cognitive therapy for recurrent depression. The implications of these findings will be on evaluating quality of life as an outcome of treatment interventions for major depression and choice of appropriate measures of QoL.
Table 1. Summary statistics of C-CT-RP sample and previously published normal sample.
| C-CT-RP sample (n=492) | Normal Sample*(n=529) | |
|---|---|---|
| Mean Age yrs (SD) | 42.63 (11.99) | 32.7 (n.a.) |
| Male % (n) | 31.91 (157) | 40.5 (214) |
| Female % (n) | 68.09 (335) | 59.5 (315) |
| Caucasian ethnicity % (n) | 81.71 (402) | 63.6 (336) |
| Non-Caucasian ethnicity % (n) | 18.29 (90) | 36.4 (193) |
| ≥16 years education % (n) | 47.36 (233) | 78.05 (413) |
| <16 years education % (n) | 52.64 (259) | 21.95 (116) |
| Paired marital status % (n) | 56.71 (279) | 30.85 (163) |
| Unpaired marital status % (n) | 43.29 (213) | 69.15 (366) |
n.a. is not available; C-CT-RP is Continuation Phase Cognitive Therapy Relapse Prevention trial; and
normal sample according to Schecter, D., Endicott, J. & Nee, J. 2007. Quality of life of ‘normal’ controls: Association with lifetime history of mental illness. Psychiatry research, 152, 45-54.
Acknowledgments
We are grateful to our patients who made this trial possible. We appreciate the dedication and longstanding commitment of our research teams and our many colleagues at the University of Texas Southwestern Medical Center at Dallas, the University of Pittsburgh (where Dr. Thase was located during patient accrual), and the University of Pennsylvania. We also appreciate the diligence of the members of the Data Safety and Monitoring Board. We have acknowledged these contributors by name in a previous paper (Jarrett and Thase, 2010).
Role of funding source: This report was supported by Grants Number K24MH001571, R01MH058397, R01MH069619 (to Robin B. Jarrett, Ph.D.) and R01MH058356 and R01MH069618 (to Michael E. Thase, M.D.) from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health (NIMH) or the National Institutes of Health (NIH).
Footnotes
Conflicts of Interest: Drs. Jha and Minhajuddin report no financial relationships with commercial interests.
Dr. Thase has served as a consultant to and was a member of various advisory boards for Eli Lilly and Company and received honoraria for talks sponsored by this company. In addition to Eli Lilly and Company, during the past 3 years Dr. Thase has consulted with, served on advisory boards for, or received honoraria for talks from: Aldolor, Alkermes, AstraZeneca, Bristol-Myers Squibb Company, Dey, Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, Lundbeck, MedAvante, Inc., Merck, Neuronetics, Inc., Otsuka, PamLab, Pfizer Pharmaceuticals, PGx (now Forest), PharmaNeuroboost, Rexahn, Schering-Plough (now Merck), Shire US Inc., Supernus Pharmaceuticals, Transcept Pharmaceuticals, and Wyeth Pharmaceuticals (now Pfizer). During the past 2 years, he has received grant support from Eli Lilly and Company, Forest, GlaxoSmithKline, Otsuka, and Rexahn, in addition to funding from the National Institute of Mental Health and the Agency for Healthcare Research and Quality. He has equity holdings for MedAvante, Inc. and has received royalties from American Psychiatric Publishing, Inc. (APPI), Guilford Publications, Herald House, and W.W. Norton & Company, Inc. One book currently promoted by the APPI specifically pertains to cognitive therapy. Dr. Thase also discloses that his spouse is an employee of Embryon, Inc (formerly Advogent and Cardinal Health), which does business with several pharmaceutical companies that market medications used to treat depression.
Dr. Jarrett's medical center receives the fees from the cognitive therapy she provides to patients. Dr. Jarrett is a paid consultant to the NIMH.
Contributors: All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drs. Jarrett and Thase.
Acquisition of data: Drs. Jarrett and Thase.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Drs. Jha, Minhajuddin and Jarrett.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Dr. Minhajuddin
Obtained funding: Drs. Jarrett and Thase.
Study supervision: Drs. Jarrett and Thase.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bech P. Social Functioning: Should it Become an Endpoint in Trials of Antidepressants? CNS Drugs. 2005;19:313–324. doi: 10.2165/00023210-200519040-00004. [DOI] [PubMed] [Google Scholar]
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Bishop SL, Walling DP, Dott SG, Folkes CC, Bucy J. Refining quality of life: validating a multidimensional factor measure in the severe mentally ill. Quality of Life Research. 1999;8:151–160. doi: 10.1023/a:1026489331009. [DOI] [PubMed] [Google Scholar]
- Blanco C, Olfson M, Goodwin RD, Ogburn E, Liebowitz MR, Nunes EV, Hasin DS. Generalizability of clinical trial results for major depression to community samples: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69:1276–80. doi: 10.4088/jcp.v69n0810. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Daly EJ, T M, Wisniewski SR, Nierenberg AA, Gaynes BN, Warden D, Morris DW, Luther JF, Farabaugh A, Cook I, Rush AJ. Health-related quality of life in depression: a STAR*D report. ANNALS OF CLINICAL PSYCHIATRY. 2010;22:43–55. [PubMed] [Google Scholar]
- Demyttenaere K, Andersen HF, Reines EH. Impact of escitalopram treatment on Quality of Life Enjoyment and Satisfaction Questionnaire scores in major depressive disorder and generalized anxiety disorder. Int Clin Psychopharmacol. 2008;23:276–86. doi: 10.1097/YIC.0b013e328303ac5f. [DOI] [PubMed] [Google Scholar]
- Drymalski WM, Washburn JJ. Sudden gains in the treatment of depression in a partial hospitalization program. Journal of Consulting and Clinical Psychology. 2011;79:364–368. doi: 10.1037/a0022973. [DOI] [PubMed] [Google Scholar]
- Dunn TW, Vittengl JR, Clark LA, Carmody T, Thase ME, Jarrett RB. Change in psychosocial functioning and depressive symptoms during acute-phase cognitive therapy for depression. Psychological Medicine. 2012;42:317–326. doi: 10.1017/S0033291711001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–6. [PubMed] [Google Scholar]
- Frisch MB. Handbook: A Practical Guide for Laypersons, Clients, and Coaches. Minneapolis, MN: Pearson Assessments; 2009. The Quality of Life Inventory (QOLI') [Google Scholar]
- Frisch MB, Clark MP, Rouse SV, Rudd MD, Paweleck JK, Greenstone A, Kopplin DA. Predictive and treatment validity of life satisfaction and the quality of life inventory. Assessment. 2005;12:66–78. doi: 10.1177/1073191104268006. [DOI] [PubMed] [Google Scholar]
- Grant GM, Salcedo V, Hynan LS, Frisch MB, Puster K. Effectiveness of quality of life therapy for depression. Psychol Rep. 1995;76:1203–8. doi: 10.2466/pr0.1995.76.3c.1203. [DOI] [PubMed] [Google Scholar]
- Greer TL, Kurian BT, Trivedi MH. Defining and Measuring Functional Recovery from Depression. CNS Drugs. 2010;24:267–284. doi: 10.2165/11530230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Gullion CM, Rush AJ. Toward a generalizable model of symptoms in major depressive disorder. Biological psychiatry. 1998;44:959–972. doi: 10.1016/s0006-3223(98)00235-2. [DOI] [PubMed] [Google Scholar]
- Hageman WJ, Arrindell WA. Establishing clinically significant change: increment of precision and the distinction between individual and group level of analysis. Behaviour Research and Therapy. 1999;37:1169–1193. doi: 10.1016/s0005-7967(99)00032-7. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of Major Depressive Disorder: Results From the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Holma KM, Holma IA, Melartin TK, Rytsala HJ, Isometsa ET. Long-term outcome of major depressive disorder in psychiatric patients is variable. J Clin Psychiatry. 2008;69:196–205. doi: 10.4088/jcp.v69n0205. [DOI] [PubMed] [Google Scholar]
- Ishak WW, Greenberg JM, Balayan K, Kapitanski N, Jeffrey J, Fathy H, Fakhry H, Rapaport MH. Quality of Life: The Ultimate Outcome Measure of Interventions in Major Depressive Disorder. Harvard Review of Psychiatry. 2011;19:229–239. doi: 10.3109/10673229.2011.614099. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Follette WC, Revenstorf D, Hahlweg K, Baucom DH, Margolin G. Variability in outcome and clinical significance of behavioral marital therapy: A reanalysis of outcome data. Journal of Consulting and Clinical Psychology. 1984;52:497–504. doi: 10.1037//0022-006x.52.4.497. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, Thase ME. Preventing depressive relapse and recurrence in higher-risk cognitive therapy responders: a randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. JAMA Psychiatry. 2013;70:1152–60. doi: 10.1001/jamapsychiatry.2013.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Thase ME. Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: Design of a double-blinded, fluoxetine- and pill placebo-controlled, randomized trial with 2-year follow-up. Contemporary Clinical Trials. 2010;31:355–377. doi: 10.1016/j.cct.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL. Major depressive disorder: longitudinal symptomatic structure, relapse and recovery. Acta Psychiatrica Scandinavica. 2001;104:81–83. doi: 10.1034/j.1600-0447.2001.t01-1-00003.x. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Zeller PJ, Paulus M, Leon AC, Maser JD, Endicott J, Coryell W, Kunovac JL, Mueller TI, Rice JP, Keller MB. Psychosocial Disability During the Long-term Course of Unipolar Major Depressive Disorder. Arch Gen Psychiatry. 2000;57:375–380. doi: 10.1001/archpsyc.57.4.375. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Solomon DA, Maser JD, Coryell W, Endicott J, Akiskal HS. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. Journal of affective disorders. 2008;108:49–58. doi: 10.1016/j.jad.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Kaplan RM, Ganiats TG, Sieber WJ, Anderson JP. The Quality of Well-Being Scale: critical similarities and differences with SF-36. Int J Qual Health Care. 1998;10:509–20. doi: 10.1093/intqhc/10.6.509. [DOI] [PubMed] [Google Scholar]
- Keitner GI, Garlow SJ, Ryan CE, Ninan PT, Solomon DA, Nemeroff CB, Keller MB. A randomized, placebo-controlled trial of risperidone augmentation for patients with difficult-to-treat unipolar, non-psychotic major depression. Journal of Psychiatric Research. 2009;43:205–214. doi: 10.1016/j.jpsychires.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, K G, Lavori PW, Coryell W, Endicott J, Taylor J. Long-term outcome of episodes of major depression. Clinical and public health significance. Journal of American Medical Association. 1984;252:788–792. [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RMA, Shea T. Time to Recovery, Chronicity, and Levels of Psychopathology in Major Depression: A 5-Year Prospective Follow-up of 431 Subjects. Arch Gen Psychiatry. 1992;49:809–816. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- Kennedy N, Foy K, Sherazi R, Mcdonough M, Mckeon P. Long-term social functioning after depression treated by psychiatrists: a review. Bipolar Disorders. 2007;9:25–37. doi: 10.1111/j.1399-5618.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The Epidemiology of Major Depressive Disorder. JAMA: The Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kilnkman KS. Assessing functional outcomes in clinical practice. The American Journal of Managed Care. 2009;15:S 335–342. [PubMed] [Google Scholar]
- Kocsis JH. Double-blind comparison of sertraline, imipramine, and placebo in the treatment of dysthymia: psychosocial outcomes. American Journal of Psychiatry. 1997;154:390–395. doi: 10.1176/ajp.154.3.390. [DOI] [PubMed] [Google Scholar]
- Lydiard RB, S S, Hertzman M, Harrison WM. Journal of Clinical Psychiatry. A double-blind, placebo-controlled study comparing the effects of sertraline versus amitriptyline in the treatment of major depression. 1997;58:484–491. doi: 10.4088/jcp.v58n1104. [DOI] [PubMed] [Google Scholar]
- Mckenna SP, Hunt SM. A new measure of quality of life in depression: testing the reliability and construct validity of the QLDS. Health Policy. 1992;22:321–30. doi: 10.1016/0168-8510(92)90005-v. [DOI] [PubMed] [Google Scholar]
- Miller IW, Keitner GI, Schatzberg AF, Klein DN, Thase ME, Rush AJ, Markowitz JC, Schlager DS, Kornstein SG, Davis SM, Harrison WM, Keller MB. The treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramine. J Clin Psychiatry. 1998;59:608–19. doi: 10.4088/jcp.v59n1108. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British Journal of Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Mahal Y, Mischoulon D, Nierenberg AA, Fava M. Quality of life assessments in major depressive disorder: a review of the literature. General hospital psychiatry. 2004;26:13–17. doi: 10.1016/j.genhosppsych.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Patten SB, Wang JL, Jeanne W, Lavorato VA, Khaled DH, Bulloch SM, M AG. Predictors of the Longitudinal Course of Major Depression in a Canadian Population Sample. Prédicteurs du cours longitudinal de la depression majeure dans un échantillon de la population canadienne. 2010;55:669–676. doi: 10.1177/070674371005501006. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Clary C, Fayyad R, Endicott J. Quality-of-Life Impairment in Depressive and Anxiety Disorders. American Journal of Psychiatry. 2005;162:1171–1178. doi: 10.1176/appi.ajp.162.6.1171. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Schafer JL. Multiple imputation: a primer. Statistical Methods in Medical Research. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- Schechter D, Endicott J, Nee J. Quality of life of ‘normal’ controls: Association with lifetime history of mental illness. Psychiatry research. 2007;152:45–54. doi: 10.1016/j.psychres.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, H K, Rapaport MH, Kiev A, Smith WT, Hirschfeld RM, Lydiard RB, Zajecka JM, Dunner DL. A randomized, double-blind, active-control study of sertraline versus venlafaxine XR in major depressive disorder. Journal of Clinical Psychiatry. 2006;67:1674–1681. doi: 10.4088/jcp.v67n1102. [DOI] [PubMed] [Google Scholar]
- Skevington SM, Lotfy M, O'connell KA. The World Health Organization's WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004;13:299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- Skevington SM, Wright A. Changes in the quality of life of patients receiving antidepressant medication in primary care: validation of the WHOQOL-100. Br J Psychiatry. 2001;178:261–7. doi: 10.1192/bjp.178.3.261. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Leon AC, Endicott J, Mueller TI, Coryell W, Shea MT, Keller MB. Psychosocial impairment and recurrence of major depression. Comprehensive Psychiatry. 2004;45:423–430. doi: 10.1016/j.comppsych.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Stevanovic D. Quality of Life Enjoyment and Satisfaction Questionnaire-short form for quality of life assessments in clinical practice: a psychometric study. J Psychiatr Ment Health Nurs. 2011;18:744–50. doi: 10.1111/j.1365-2850.2011.01735.x. [DOI] [PubMed] [Google Scholar]
- Swan A, Watson HJ, Nathan PR. Quality of life in depression: An important outcome measure in an outpatient cognitive–behavioural therapy group programme? Clinical Psychology & Psychotherapy. 2009;16:485–496. doi: 10.1002/cpp.588. [DOI] [PubMed] [Google Scholar]
- Trajković G, Starčević V, Latas M, Leštarević M, Ille T, Bukumirić Z, Marinković J. Reliability of the Hamilton Rating Scale for Depression: A meta-analysis over a period of 49 years. Psychiatry research. 2011;189:1–9. doi: 10.1016/j.psychres.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Pigotti TA, Perera P, Dillingham KE, Carfagno ML, Pitts CD. Effectiveness of low doses of paroxetine controlled release in the treatment of major depressive disorder. J Clin Psychiatry. 2004a;65:1356–64. doi: 10.4088/jcp.v65n1010. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004b;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Tuynman-Qua H, De Jonghe F, Mckenna SP. Quality of life in depression scale (QLDS). Development, reliability, validity, responsiveness and application. Eur Psychiatry. 1997;12:199–202. doi: 10.1016/S0924-9338(97)89105-5. [DOI] [PubMed] [Google Scholar]
- Versiani M, Moreno R, Ramakers-van Moorsel CJA, Schutte AJ, Antidepressants Study Grp, CEO Comparison of the Effects of Mirtazapine and Fluoxetine in Severely Depressed Patients. CNS Drugs. 2005;19:137–146. doi: 10.2165/00023210-200519020-00004. [DOI] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: A comparative meta-analysis of cognitive-behavioral therapy's effects. Journal of Consulting and Clinical Psychology. 2007;75:475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB. Deterioration in psychosocial functioning predicts relapse/recurrence after cognitive therapy for depression. Journal of affective disorders. 2009;112:135–143. doi: 10.1016/j.jad.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson HJ, Nathan PR. Role of gender in depressive disorder outcome for individual and group cognitive–behavioral treatment. Journal of Clinical Psychology. 2008;64:1323–1337. doi: 10.1002/jclp.20524. [DOI] [PubMed] [Google Scholar]