Summary
We have compared the melanogenic activities of cultured melanocytes carrying two common TYR alleles as homozygous 192S-402R wildtype, heterozygous and homozygous variant. This includes assays of TYR protein, DOPAoxidase activity, glycosylation and temperature sensitivity of protein and DOPAoxidase levels. Homozygous wildtype strains on average had higher levels of TYR protein and enzyme activity than other genotypes. Homozygous 402Q/Q melanocytes produced significantly less TYR protein, displayed altered trafficking and glycosylation, with reduced DOPAoxidase. However, near wildtype TYR activity levels could be recovered at lower growth temperature. In a sample population from Southeast Queensland these two polymorphisms were present on four TYR haplotypes, designated as WT 192S-402R, 192Y-402R, 192S-402Q with a double variant 192Y-402Q of low frequency at 1.9%. Based on cell culture findings and haplotype associations, we have used an additive model to assess the penetrance of the ten possible TYR genotypes derived from the combination of these haplotypes.
Keywords: Tyrosinase, Pigmentation, Melanocyte, Albinism, Melanin
Introduction
Multiple genetic loci and polymorphisms both within and between populations have been associated with normal variation of human pigmentary phenotypes (Sturm, 2009), with natural selection acting upon different subsets of pigmentation genes in geographically isolated populations (Sturm and Duffy, 2012). Tyrosinase (TYR) was one of the first human pigmentation genes identified, with reports showing it to be mutated in OCA1 albinism, accounting for 46% of cases of albinism in European populations (Rooryck et al., 2009). The TYR locus is located on chromosome 11q14.3 and encodes a 529 amino acid protein coded by five exons, with an inactive tyrosinase pseudogene TYRL also located on the same chromosome at 11p11.12 (Giebel et al., 1991; Ponnazhagan et al., 1994). TYR is a melanosomal membrane-bound glycoprotein with a native molecular weight of 55kD, which increases to 65-75kD following post-translational modification at six potential N-glycosylation sites as it traffics to the mature melanosome (Wang and Hebert, 2006). It is the key enzyme for pigmentation that catalyzes the oxidation of its substrates tyrosine and DOPA to form the intermediate DOPAquinone.
Polymorphisms within the TYR locus were initially reported during molecular cloning of the coding region of the TYR cDNA sequence, and later upon the analysis of OCA1 patient mutations (albinism data base, albinismdb.med.umn.edu). Two common changes, rs1042602*C/A S192Y in exon 1 (Giebel and Spritz, 1990) and rs1126809*G/A R402Q in exon 4 (Giebel et al., 1991; Tripathi et al., 1991), occur at high frequency but were not immediately correlated with pigmentation phenotypes in normal Caucasians. The TYR 192Y allele was shown as an ancestry informative marker contributing to skin pigmentation differences between African and European populations (Shriver et al., 2003); later, in a population of South Asian descent the 192Y allele was found to be associated with lighter skin colour (Stokowski et al., 2007). The relationship of these TYR alleles to normal variation in skin, hair and eye colour in Europeans has been studied by several groups (Branicki et al., 2011; Duffy et al., 2010; Gudbjartsson et al., 2008; Hu et al., 2011; Nan et al., 2009; Sulem et al., 2007), with reported associations for lighter eye colour, brown hair colour, freckling and melanoma risk, but such effects are not always detectable (Candille et al., 2012).
Functional studies of TYR enzyme activity have reported the 192Y allele to be only 60% active, possibly due to steric hindrance effects in the TYR protein copper-A catalytic site (Chaki et al., 2011). The 402Q allele also has reduced enzyme activity, with exogenous expression showing that it encodes a thermolabile variant protein with only 25% activity (Spritz et al., 1997; Tripathi et al., 1992). However, despite these TYR alleles encoding variant enzymes that are clearly deficient relative to the WT TYR protein, the role of these polymorphisms in relation to albinism and normal pigmentation variation remains controversial (Oetting et al., 2009; Rooryck et al., 2009; Simeonov et al., 2013).
We have combined biochemical and cellular analysis of these common TYR polymorphisms with genetic tests for phenotypic association, so as to further characterise and define the effects these variants may play on human pigmentation characteristics. To do this we first compared the in vitro and in vitro melanogenic activities of primary human melanocytes genotyped for these TYR alleles carried as homozygous WT, heterozygous and homozygous variants. We then assessed TYR S192Y and R402Q haplotypes and phased genotypes in a large collection of adolescent twins and melanoma patients with phenotypic data for skin, hair and eye colour, skin reflectance and mole count.
Results
Pigmentation genotype screen of clonal melanocytic strains
We have previously reported on a collection of primary human melanocyte strains that were genotyped for polymorphisms within the MC1R, SLC45A2, SLC24A5 and OCA2 loci (Cook et al., 2009; Leonard et al., 2003). We have continued an analysis of 266 of these strains propagated as melanoblasts (Cook et al., 2003), assaying for 31 SNPs within a number of pigmentation genes (Duffy et al., 2010) to ascertain clonal cell strains of defined genotypes. In this collection we found the frequency for the minor alleles of TYR rs1042602*C/A S192Y and rs1126809*G/A R402Q to be 0.34 (A) and 0.26 (A) respectively, frequencies in line with those seen in the general population of Southeast Queensland (Duffy et al., 2010). To analyse the melanogenic activity based on TYR alleles these strains carry, we first designated 192S/S and 402R/R combined genotypes as WT for the TYR protein, consistent with the ancestral origin of these alleles (Norton et al., 2007). We also restricted the selected strains to a similar European genetic background, being homozygous SLC45A2 MATP-374F/F, SLC24A5 NCKX5-111T/T, OCA2 rs12913832*C/C and MC1R+/+ genotypes, although this was not always the case with MC1R (Table S1). We selected 5 strains WT for the TYR protein to compare with 5 strains each of those carrying variant TYR alleles 192S/Y and 402R/Q in the heterozygous state, or 192Y/Y and 402Q/Q in the homozygous state.
Endogenous expression levels of TYR in genotyped melanocytic strains and human skin tissue samples
Total soluble cell extracts were assayed by immunoblot to determine the endogenous level of TYR protein in a range of our primary QF melanocytic strains and human skin tissue samples genotyped for the two common TYR polymorphisms (Tables S1 and S2). In comparison to WT cell extracts, Western blot analysis revealed significant reduction in TYR expression for heterozygous 192S/Y (Fig. 1A; P<0.003) and 402R/Q (Fig. 1B; P<0.021) extracts, with a greater than 2-fold reduction when averaged for the cells strains of each genotype that we used. Although not statistically significant, a consistent decrease in TYR protein levels was also noted for 192Y/Y cells (Fig. 1C). Assay of homozygous 402Q/Q melanocytes demonstrated a consistent and significant reduction, on average greater than 3-fold, when compared to WT melanocytes (Fig. 1D; P=0.001). Differences in the average expression of TYR protein levels of these strains are unlikely to be due changes in transcription of these alleles, as the average levels of TYR mRNA were comparable when WT and 402Q/Q melanocytes were quantitated by RT-PCR (Fig. S1A and B). Levels of TYRP1 protein varied between TYR genotyped strains, with a decrease observed in 192S/Y and 402R/Q melanocytes (Fig. 1A, B) but little to no change was seen in 192Y/Y and 402Q/Q melanocytes (Fig. 1C, D). Extracts of human skin tissue samples also genotyped at TYR were used to determine TYR protein levels present in human skin (Fig. 1E). Consistent with the observation seen in cultured cell strains, variation of TYR protein levels was observed between genotypes, exhibiting high and consistent TYR expression in two WT skin samples, but a 2-fold or greater reduction in expression in the 402R/Q, 402Q/Q, 192S/Y and 192Y/Y skin samples that were available for testing.
Figure 1. Endogenous protein expression and activity of TYR in primary melanocytes of defined genotype.
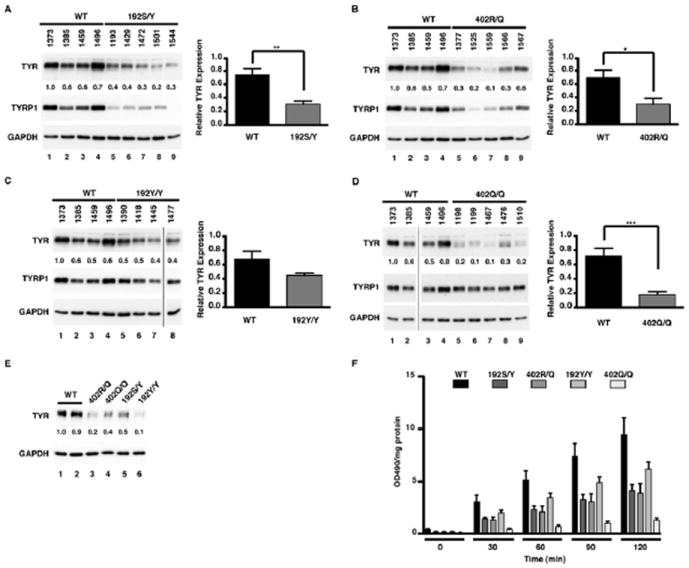
(A) WT (lanes 1-4) and 192S/Y (lanes 5-9). Numbers represent normalised ratio of protein expression (lane 1 set to 1) for TYR. A graph of this ratio between genotypes WT (n=4) and 192S/Y (n=5) determined a difference with a significance of **P<0.003. (B) WT (lanes 1-4) and 402R/Q (lanes 5-9). Numbers represent normalised ratio of protein expression (lane 1 set to 1) for TYR. A graph of this ratio between genotypes WT (n=4) and 402R/Q (n=5) determined a difference with a significance of *P<0.021. (C) WT (lanes 1-4) and 192Y/Y (lanes 5-8). Numbers represent normalised ratio of protein expression (lane 1 set to 1) for TYR. A graph of this ratio between genotypes WT (n=4) and 192Y/Y (n=4) determined a difference but with no significance. (D) WT (lanes 1-4) and 402Q/Q (lanes 5-9). Numbers represent normalised ratio of protein expression (lane 1 set to 1) for TYR. A graph of this ratio between genotypes WT (n=4) and 402Q/Q (n=5) determined a difference with a significance of ***P<0.0014. (E) Endogenous protein expression of TYR in foreskin tissue samples (lanes 1-6). Numbers represent normalised ratio of protein expression (lane 1 set to 1) for TYR. GAPDH was used to determine protein loading. (F) The rate of L-DOPA oxidation in extracted cell lysates of primary melanocytes, WT (n=4), 192S/Y (n=5), 402R/Q (n=5), 192Y/Y (n=4), 402Q/Q (n=5) was determined over a 2hr time course at 30min intervals. Values indicate mean + SEM (n=4-5) of 4-5 different melanocyte strains of each genotype normalised to total soluble protein.
TYR enzymatic activity is compromised in 402Q/Q melanocytes
The enzymatic activity of TYR as determined by the rate of L-DOPA oxidation was also assessed in these same cultured human melanocytes genotyped for TYR polymorphisms (Fig. 1F). Previous work (Tripathi et al., 1992) has shown transfected HeLa cells artificially expressing the 402Q allele have reduced levels of L-DOPA oxidation when compared to HeLa cells artificially expressing WT TYR. Consistent with this report are our findings that primary human melanocytes of TYR 402Q/Q genotype upon averaging are severely compromised in the rate of L-DOPA oxidation when compared with that observed in WT melanocytes (P<0.05 linear regression analysis followed by one way ANOVA and Dunnet’s post hoc comparisons). Furthermore, TYR 192S/Y, 402R/Q and 192Y/Y melanocytes also exhibited a significant (P<0.05) reduction in L-DOPA oxidation rates when compared with WT cells. In comparing this series of cell strains the average level of TYR activity in 192Y/Y homozygotes was slightly greater than the TYR 192S/Y and 402R/Q heterozygotes. Although this difference was not statistically significant it is consistent with the higher TYR and TYRP1 protein levels observed in these strains (Fig. 1C compared to 1A and 1B).
TYR protein levels and catalytic activity in 402Q/Q melanocytes is temperature sensitive
When tested using in vitro exogenous expression studies, the TYR 402Q allele confers a temperature sensitive effect on enzyme activity at 37°C, causing a reduction of L-DOPA oxidase activity (Tripathi et al., 1992) with potential phenotypic effects (King et al., 1991). Western blot analysis was initially used to assess the endogenous temperature sensitivity of the TYR protein in 402Q/Q melanocyte strains grown at 37°C and 31°C (Fig. 2A) to test both the thermostability and catalytic activity of the protein. Using three 402Q/Q stains, on average a greater than 2-fold increase in protein levels was seen when cells were grown at 31°C compared to growth at 37°C for a 24hr period. When three WT TYR strains were also subjected to altered temperature growth conditions for a 24hr period, only a small average increase in TYR protein expression at 31°C was noted, although one WT strain QF1459 did show a 1.9 fold change.
Figure 2. Temperature sensitive recovery of TYR activity in melanocyte strains.
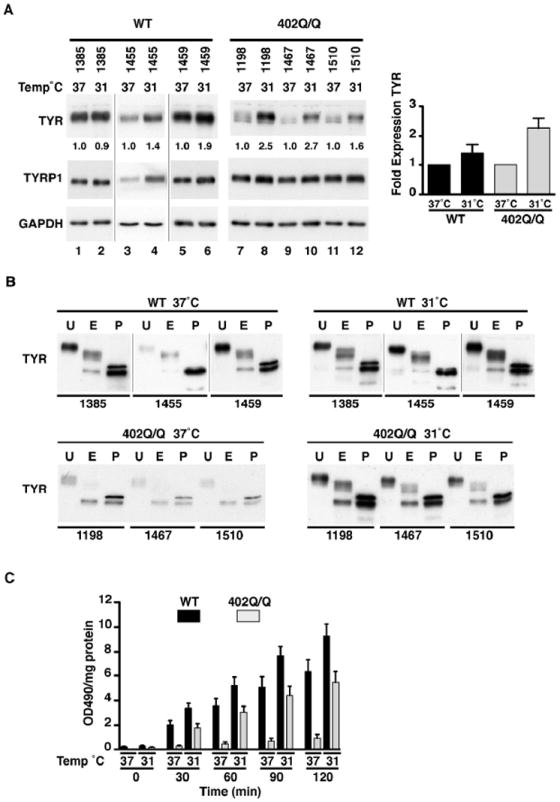
(A) Endogenous protein expression of TYR and TYRP1 in genotyped primary melanocytes, WT (n=3) and 402Q/Q (n=3) incubated at 37°C and 31°C for a 24hr time period. Numbers represent normalised ratio of protein expression for TYR (37°C sample for each melanocyte strain set to 1) (lane 1,3,6,7,9,11 set to 1). A graph of this ratio represents a fold difference of TYR temperature sensitive protein expression between 37°C and 31°C in WT (n=3) and 402Q/Q (n=3) genotypes. Values indicate mean + SEM (n=3) of 3 different melanocyte strains of each genotype. GAPDH was used to determine protein loading. (B) Protein cell lysates of genotyped primary melanocytes, WT (n=3) and 402Q/Q (n=3) were untreated (U) or digested with the glycosidase EndoH (E) or PNGaseF (P). Samples were immunoblotted and probed with anti-TYR antibody to determine the extent of digestion. (C) The rate of L-DOPA oxidation in extracted cell lysates of primary melanocytes, WT (n=3) and 402Q/Q (n=3) at 37°C and 31°C was determined over a 2hr time course at 30min intervals. Values indicate mean + SEM (n=3) of 3 different melanocyte strains of each genotype normalised to total soluble protein.
Temperature sensitive effects on TYR glycosylation in 402Q/Q melanocytes
Previous work (Berson et al., 2000; Halaban et al., 1997; Halaban et al., 2000) has shown the presence of an immature glycoform of TYR 402Q, lacking essential monoglucosylated glycans, that is retained in the endoplasmic reticulum due to protein misfolding, with a mature glycoform able to be partially rescued when cells expressing the 402Q allele are grown at 31°C. Glycosylation analysis of extracts prepared from each of the three 402Q/Q melanocyte strains indicated a high sensitivity of TYR to endoH digestion when cells were grown at 37°C (Fig. 2B lower panels). Analysis of the three WT strains however exhibited a form of TYR more resistant to endoH digestion at 37°C (Fig. 2B upper panels). In contrast, a more complex and mature TYR glycoform is observed for the 402Q/Q strains grown at 31°C, indicating that a higher degree of resistance to endoH digestion is induced by growth at the lower temperature. Digestion with PNGaseF cleaves both mature and immature glycoproteins and represents the de-glycosylated form of TYR, observed as the fastest migrating bands on the gels shown in Figure 2B. As expected there is little difference in the pattern of the PNGaseF treated extracts, but the lower levels of TYR protein in the 402Q/Q strains grown at 37°C compared with 31°C is consistent with the thermo-instability of this form of the TYR protein (Fig. 2A). The glycosylation status of TYRP1 showed no change (Fig. S2). Resistance to endoH digestion was also examined in 402R/Q and 192Y/Y melanocyte strains grown at 37°C (Fig. S3), however no change in sensitivity to endoH digestion of TYR or TYRP1 was observed compared to WT cells.
TYR enzymatic activity is rescued in 402Q/Q by growth of melanocytes at 31°C
We next investigated whether a rescue of TYR enzymatic activity could be achieved by growing 402Q/Q strains at 31°C. The rate of L-DOPA oxidation was evaluated and averaged for three WT and three 402Q/Q strains grown at 37°C or 31°C for a 24hr period (Fig. 2C). The rate of L-DOPA oxidation was significantly increased at 31°C for both WT (P<0.05) and 402Q/Q (P<0.001) melanocytes when compared to growth of cells at 37°C. Furthermore, as the L-DOPA oxidation levels for 402Q/Q cells grown at 31°C are comparable, though slightly weaker, to those in WT cells grown at 37°C, partial rescue of TYR activity in 402Q/Q cells can be attained by growth at lower temperature.
Subcellular localisation and trafficking of TYR in WT and 402Q/Q melanocytes
The subcellular localisation of TYR and TYRP1 together with calnexin, a known chaperone present in the endoplasmic reticulum and necessary for accurate and functional protein folding (Ellgaard et al., 1999; Zhang et al., 1997), was studied by immunofluorescence to further evaluate the temperature sensitive nature of the TYR protein expressed in 402Q/Q melanocytes. Both WT and 402Q/Q melanocyte strains grown at 37°C and 31°C were probed with antibodies to TYR (red) and calnexin (green) (Fig. 3). Examination of the staining pattern seen in WT cells clearly indicates the existence of TYR in the dendrites of melanocytes at 37°C, which also co-localises (merged as yellow) with calnexin in the perinuclear endoplasmic reticulum (Fig. 3A top panels). In contrast, 402Q/Q melanocytes grown at 37°C exhibited an abundance of TYR staining that largely co-localised with calnexin in the endoplasmic reticulum, but an absence of TYR staining in the dendrites of these cells (Fig. 3A bottom panels). This is consistent with retention of misfolded TYR in the endoplasmic reticulum in 402Q/Q melanocytes as may be expected from the endoH digestion pattern seen in extracts prepared from these cells under the same temperature growth conditions (Fig. 1B). WT melanocytes grown at 31°C had a similar subcellular localisation pattern to cells grown at 37°C, with high levels of TYR staining in the dendrites (Fig. 3B upper panel). Notably, 402Q/Q melanocytes grown at 31°C expressed a subcellular localisation pattern distinctly different to that observed when these cells are grown at 37°C, with TYR staining predominantly in the dendrites (Fig. 3B lower panel). Co-localisation with calnexin was reduced, indicating an increase in correctly folded TYR and less retention in the endoplasmic reticulum, in line with that seen in WT cells and with the return of near normal levels of DOPAoxidase activity (Fig. 2C). No difference was observed in the TYRP1 (red) subcellular localisation pattern when comparing WT and 402Q/Q melanocyte strains grown at 37°C (Fig. 3C).
Figure 3. Localization of TYR in 402Q/Q melanocytic cells is temperature dependent.
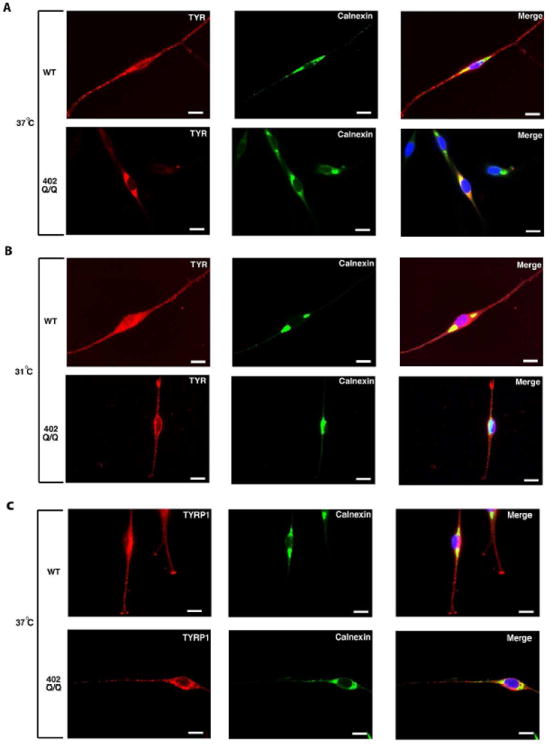
The images show immunofluorescent staining of WT (QF 1459) and homozygous variant 402Q/Q (QF 1510) melanocyte strains grown on coverslips at 37°C (A and C) or shifted to 31°C for 24h (B). In panels A and B, cells grown at indicated temperature were fixed and stained with mouse TYR-Alexa-594 (red) and rabbit calnexin-Alexa-488 (green). In panel C, cells grown at 37°C were fixed and stained with mouse TYRP1-Alexa-594 (red) and rabbit calnexin-Alexa-488 (green). Left to right with the antibody indicated in the upper right: TYR or TYRP1 (red), calnexin (green) and an overlay of the coloured images also including DAPI (blue). Yellow indicates colocalization between TYR/TYRP1 and calnexin. Scale bars in white represent 50μm.
Pigmentation characteristics of 402Q/Q melanocytes at 31°C
Given the increase in 402Q/Q cell strain TYR protein expression (Fig. 2A) and enzyme activity observed at 31°C (Fig. 2C), we expected melanin levels to rise in those cells grown at 31°C. However, total melanin content did not change significantly either in WT or 402Q/Q samples (Fig. S4A). Electron microscopy was undertaken to further characterise the state of melanosomes (Cook et al., 2009) in both WT and 402Q/Q melanocytes grown at 37°C and 31°C for a 24hr period (Fig. S4B). Again there was no clear evidence to support an increase in melanisation at 31°C either in the WT or 402Q/Q samples, and despite an increase in TYR protein, there was no observable change in melanosome maturation.
TYR allele and haplotype associations with pigmentation phenotype
To complement the biochemical functional assays performed in cultured melanocyte cells, human pigmentation phenotypic data from two population collections that we have previously reported upon were examined for associations with the TYR S192Y and R402Q protein polymorphisms. These sets included 5423 samples from the Brisbane Twin Nevus Study (BTNS) of adolescent twins, siblings and parents, and 1738 CMM case samples (Duffy et al., 2010; Sturm et al., 2008). These were combined for an assessment of pigmentary characteristics although not all measurements were available for each genotyped individual. Using the MENDEL program first on the BTNS collection, four haplotype phases were predicted for these alleles designated as 1-1 (S-R) for WT 192S-402R, 2-1 (Y-R) for 192Y-402R, 1-2 (S-Q) for 192S-402Q, and 2-2 (Y-Q) for the double variant 192Y-402Q (Table 1). The frequencies for each haplotype were 0.35 for 1-1, 0.32 for 2-1, 0.3 for 1-2, and 0.019 for 2-2, which are similar to those reported in a recent study of European vitiligo patients (Jin et al., 2012). A linear progression of highest to lowest penetrance was seen as 1-1> 2-1> 1-2> 2-2 for olive/dark skin (14> 9> 7 > 5%) and brown eye (26> 23> 20> 17%) colour with the reciprocal situation for fair skin (46< 50< 55< 64%) and blue eye colour (39< 44< 51< 61%). The hair colour penetrance of these haplotypes was consistent, 1-1> 2-1=1-2 >2-2 for dark brown hair colour (38> 36=36> 25%), with the reciprocal situation for fair/blond hair (15< 18=18< 22%). Given that our biochemical studies using homozygous genotyped melanocytes showed the greatest TYR protein and enzyme activity was found in WT (1-1/1-1) followed by 192Y/Y (2-1/2-1) then 402Q/Q (1-2/1-2) (Figure 1) we propose the allele strength designation of 3 to 1 for each of these TYR haplotypes respectively (Table 1), and 0 for the double variant for which we have no biochemical data. However, two individuals who were homozygous for the 2-2 haplotype were found in the study collections. Each was fair skinned with fair/blond hair and blue eye colour, suggesting that a general hypopigmentation phenotype is associated with this haplotype.
Table 1. Skin, hair and eye colour distribution for each TYR haplotype.
| TYR phasea | TYR proteinb | Allele Strengthc | Proposed Frequency (95% CI) | % Penetrance Skind | % Penetrance Haire | % Penetrance Eyef | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fair | Medium | Olive/ dark | Red | Fair/ blond | Light brown | Dark brown | Black | Blue | Green | Brown | ||||
| 1-1 | S-R | 3 | 0.35 (0.34-0.37) | 46 | 41 | 14 | 7 | 15 | 33 | 38 | 7 | 39 | 35 | 26 |
| 2-1 | Y-R | 2 | 0.32 (0.31-0.34) | 50 | 42 | 9 | 7 | 18 | 36 | 36 | 4 | 44 | 33 | 23 |
| 1-2 | S-Q | 1 | 0.30 (0.29-0.31) | 55 | 38 | 7 | 7 | 18 | 35 | 36 | 4 | 51 | 30 | 20 |
| 2-2 | Y-Q | 0 | 0.019 (0.016-0.023) | 64 | 31 | 5 | 8 | 22 | 41 | 25 | 5 | 61 | 021 | 17 |
Designated for rs1042602*C/A = 1/2 - rs1126809*G/A = 1/2
Protein phases designated at S192Y-R402Q as 192S = 1, 192Y = 2; 402R = 1, 402Q = 2
Based on a four point scale S-R = 3, Y-R =2, S-Q =1, Y-Q = 0
Sample set of 5,394 for skin colour,
Sample set of 5,393 for hair colour,
Sample set of 4,942 for eye colour, in BTNS and/or melanoma cases in which haplotype phase was predicted from the MENDEL program
In the subset of BTNS samples for which skin reflectance and nevi counts were recorded a similar gradation correlating with the allele strength as designated was seen (Table 2). Both inner and outer arm reflectance representing constitutive and facultative skin colour, showed a highest to lowest progression for 1-1> 2-1> 1-2> 2-2 in reflectance of 61.8 to 63.5% and 52.5 to 53.6% respectively, with average nevi counts progressing linearly with the designated allele strength at 89.8> 90.6> 97.6> 110.8.
Table 2. Mean Skin Reflectance and Nevi count for each TYR haplotype.
| TYR phasea | TYR proteinb | Allele Strengthc | Proposed Frequency (95% CI) | Inner arm % reflectance (SD)d | Outer arm % reflectance (SD)d | Nevi (95%CI)e,f |
|---|---|---|---|---|---|---|
| 1-1 | S-R | 3 | 0.36 (0.34-0.37) | 61.8 (0.14) | 52.5 (0.21) | 89.8 (86.1-93.7) |
| 2-1 | Y-R | 2 | 0.33 (0.31-0.34) | 62.4 (0.14) | 53.4 (0.22) | 90.6 (86.6-94.8) |
| 1-2 | S-Q | 1 | 0.29 (0.29-0.31) | 62.9 (0.15) | 53.6 (0.22) | 97.6 (93.1-102.3) |
| 2-2 | Y-Q | 0 | 0.017 (0.016-0.023) | 63.5 (0.34) | 53.6 (0.52) | 110.8 (99.1-123.9) |
Designated for rs1042602*C/A = 1/2 - rs1126809*G/A = 1/2
Protein phases designated at S192Y-R402Q as 192S = 1, 192Y = 2; 402R = 1, 402Q = 2
Based on a four point scale S-R = 3, Y-R =2, S-Q =1, Y-Q = 0
Sample set of 2287 for skin relectance, and
Sample set of 1,979 for nevi counts, in BTNS and/or melanoma cases in which haplotype phase was predicted from the MENDEL program
Geometric mean with 95% confidence interval.
Pigmentation phenotype association shows additive penetrance of TYR genotypes
The combination of each of the four TYR haplotypes and expected population frequencies are summarized as a matrix of 10 genotypes A (1-1/1-1) to J (2-2/2-2) in Table S3. An additive model for each allele was used to designate the strength of these genotypes correlating with TYR activity; as such this series ranged from a maximum of 6 (3+3) for genotype A to a minimum of zero (0+0) for J with a total of 7 categories (A> B > C/E > D/F > G/H > I > J) of combined allelic strength (6 > 5 > 4/4 > 3/3 > 2/2> 1> 0). We did not attempt to separate D/ F (1-1/2-2 vs 2-1/1-2), as the phase was not obvious from genotype data alone. As the expected frequency of D was low (1.33%) compared to F (19.2%) and the predicted genotype strength of 3 was the same (3+0 = 2+1), they were combined together in tests of penetrance for each TYR genotype.
Remarkably, an approximately linear progression series of highest to lowest penetrance of A to J correlated with the designated TYR genotype strengths for skin, hair and eye colour (Table 3), skin reflectance and nevi counts (Table 4), though this was not always strictly linear in each case. The strongest genotype A (strength of 6) had the greatest penetrance for olive/dark skin (21.9%), black hair colour (9.6%) and brown eye colour (36.2%), lowest inner and outer arm reflectance measurements (61.06/51.61), and mean nevi count (80.3), with the reciprocal situation of lowest frequency for fair skin (33.3%), fair/blond hair (12.8%), and blue eye colour (32.6%). As stated above there were only 2 individuals in our collection of genotype J (strength of zero) who were hypopigmented, but the next weakest genotype I (TYR protein strength of 1), had the next highest frequency of fair skin (69%), fair blonde hair (29.4%), blue eye colour (71.1%), and highest inner and outer arm reflectance measurements (64.19/51.61). Genotype I had a mean nevi count of 88.2, lower than G and H, which had the highest counts of 116.7 and 100.3 respectively. Genotype I also had low penetrance for olive/dark skin (4.8%, G lowest at 2.2%), and was lowest for black hair (1.5%) and brown eye colour (13.3%). From these results it is clear that the predicted TYR protein strength (Table S3), dependent upon the phase of the S192Y and R402Q polymorphisms, correlates well with the range of human pigmentary traits assessed here.
Table 3. Skin, hair and eye colour distribution for each TYR genotype.
| TYR genotypea | TYR genotype phaseb | TYR Protein phasec | Combined Allelic Strengthd | Skin coloure | Hair colourf | Eye colourg | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Fair % | Medium % | Olive/dark % | N (%) | Red % | Fair/ blond % | Light brown % | Dark brown % | Black % | N (%) | Blue % | Green % | Brown % | ||||
| A | 1-1/1-1 | S-R/S-R | 6 | 502 (13.4) | 33.3 | 44.8 | 21.9 | 711 (13.2) | 7.0 | 12.8 | 32.3 | 38.3 | 9.6 | 172 (9.9) | 32.6 | 31.1 | 36.2 |
| B | 1-1/2-1 | S-R/Y-R | 5 | 884 (23.7) | 40.5 | 45.1 | 14.4 | 1222 (22.6) | 7.4 | 16.8 | 33.2 | 38.2 | 4.3 | 350 (20.1) | 38.3 | 33.7 | 27.9 |
| C | 1-1/1-2 | S-R/S-Q | 4 | 759 (20.4) | 43.2 | 44.8 | 12.0 | 1123 (20.8) | 7.2 | 14.3 | 33.9 | 39.6 | 4.9 | 374 (21.5) | 46.9 | 30.3 | 22.8 |
| E | 2-1/2-1 | Y-R/Y-R | 4 | 410 (11.0) | 40.5 | 49.0 | 10.5 | 602 (11.2) | 6.3 | 19.1 | 36.7 | 34.9 | 3.0 | 193 (11.1) | 45.2 | 29.0 | 25.8 |
| D/F | 1-1/2-2 2-1/1-2 | S-R/Y-Q Y-R/S-Q | 3 | 776 (20.8) | 47.4 | 45.2 | 7.3 | 1126 (20.9) | 6.1 | 17.2 | 36.9 | 35.2 | 4.6 | 400 (22.9) | 49.6 | 26.9 | 23.4 |
| G | 2-1/2-2 | Y-R/Y-Q | 2 | 46 (1.2) | 52.2 | 45.7 | 2.2 | 57 (1.1) | 8.8 | 12.3 | 45.6 | 28.1 | 5.3 | 31 (17.8) | 67.4 | 15.2 | 17.4 |
| H | 1-2/1-2 | S-Q/S-Q | 2 | 305 (8.2) | 54.8 | 40.0 | 5.2 | 482 (8.9) | 6.2 | 22.0 | 35.1 | 33.0 | 3.7 | 190 (10.9) | 60.9 | 20.8 | 18.3 |
| I | 1-2/2-2 | S-Q/Y-Q | 1 | 42 (0.02) | 69.0 | 26.2 | 4.8 | 68 (1.3) | 7.4 | 29.4 | 39.7 | 22.1 | 1.5 | 32 (1.8) | 71.1 | 15.6 | 13.3 |
| J | 2-2/2-2 | Y-Q/Y-Q | 0 | 1 (0.0) | 100 | 0.0 | 0.0 | 2 (0.004) | 0.0 | 100 | 0.0 | 0.0 | 0.0 | 1 (0.057) | 100 | 0 | 0 |
Genotypes designated in Table S3 for rs1042602*C/A and rs1 126809*G/A
Protein phases designated at S192Y-R402Q as 192S = 1, 192Y = 2; 402R = 1, 402Q = 2
Based on a four point scale S-R = 3, Y-R =2, S-Q =1, Y-Q = 0
Sample set of 3725 for skin colour,
Sample set of 5393 for hair colour,
Sample set of 1743 for eye colour in BTNS and/or melanoma study samples in which genotype phase has been determined
Table 4. Mean Skin Reflectance and Nevi count for each TYR genotype.
| TYR genotypea | TYR genotype phaseb | TYR protein phasec | Combined Allelic Strengthd | Skin Reflectancee | Nevif | ||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | Inner arm (SD) % | Outer arm (SD) % | N (%) | Log Count (SD) | Mean Count | ||||
| A | 1-1/1-1 | S-R/S-R | 6 | 242 (12.9) | 61.06 (5.40) | 51.61 (7.291) | 262 (13.2) | 1.9048 (0.3003) | 80.3 |
| B | 1-1/2-1 | S-R/Y-R | 5 | 457 (24.3) | 61.61 (3.92) | 52.69 (5.82) | 480 (24.3) | 1.9406 (0.2457) | 87.2 |
| C | 1-1/1-2 | S-R/S-Q | 4 | 380 (20.3) | 62.02 (3.88) | 53.09 (5.81) | 396 (20.0) | 1.9764 (0.2396) | 94.7 |
| E | 2-1/2-1 | Y-R/Y-R | 4 | 206 (11.0) | 62.04 (3.62) | 53.35 (6.72) | 220 (11.1) | 1.9322 (0.2201) | 85.5 |
| D/F | 1-1/2-2 2-1/1-2 | S-R/Y-Q Y-R/S-Q | 3 | 383 (20.4) | 62.32 (3.25) | 53.17 (5.46) | 406 (20.5) | 1.9500 (0.2446) | 89.1 |
| G | 2-1/2-2 | Y-R/Y-Q | 2 | 19 (1.0) | 64.42 (2.45) | 56.63 (12.32) | 20 (1.0) | 2.0672 (0.2207) | 116.7 |
| H | 1-2/1-2 | S-Q/S-Q | 2 | 167 (8.9) | 63.34 (3.68) | 53.89 | 173 (8.7) | 2.0013 (0.2462) | 100.3 |
| I | 1-2/2-2 | S-Q/Y-Q | 1 | 21 (1.1) | 64.19 (3.10) | 54.05 (7.46) | 22 (1.1) | 1.9457 (0.2604) | 88.2 |
| J | 2-2/2-2 | Y-Q/Y-Q | 0 | 0 (0) | Nil (Nil) | Nil (Nil) | 0 (0) | Nil (Nil) | Nil (Nil) |
Genotypes designated in Table S3 for rs1042602*C/A and rs1 126809*G/A
Protein phases designated at S192Y-R402Q as 192S = 1, 192Y = 2; 402R = 1, 402Q = 2
Based on a four point scale S-R = 3, Y-R =2, S-Q =1, Y-Q = 0
Sample set of 1875 for skin reflectance,
Sample set of 1,979 for nevi counts in BTNS and/or melanoma study samples in which genotype phase has been determined
Geometric mean with standard deviation.
Discussion
Only two common TYR protein polymorphisms, rs1042602*C/A S192Y and rs1126809*G/A R402Q, have been selected within European populations during the evolution of lighter skin colour (Norton et al., 2007) arising on independent haplotypes between 6,100-15,600 and 20,400-29,400 years ago respectively (Hudjashov et al., 2013). The S192Y nonsynonymous change is also found at low frequency in South Asian/Indian populations but is virtually absent in East Asian populations (Chaki et al., 2011; Miyamura et al., 2005; Stokowski et al., 2007). The R402Q allele has been less studied for its population distribution; it appears at a frequency of 21.5% in European samples, and has been reported in Indian population samples at a frequency of 5.3% (Miyamura et al., 2005), but is not present in other Asian and African populations in the HapMap database (Altshuler et al., 2010). Computational approaches to TYR functional activity, based on protein flexibility and dynamic properties applied to amino acid changes associated with albinism, have also included an independent analysis of these two common polymorphisms (K and Purohit, 2013). Based on this quantitative structural assessment and free energy scores, both of these variants were considered to be less stable than the WT TYR molecule, but the effect of having both changes together in the one TYR molecule was not considered. Given the independent evolution of these polymorphisms (Hudjashov et al., 2013) the double variant 192Y-402Q (phase 2-2) protein is thought to derive from a recombinant haplotype occurring at low frequency in Europeans populations.
A confounding issue in the literature is the use of human TYR expression constructs without full consideration of what combination of polymorphisms/haplotypes were being assayed. In this respect, one of the first TYR expression vectors used to map the catalytic activities of human TYR (Tripathi et al., 1992) was pcTYR (Bouchard et al., 1989). The sequence of the full-length clone pBBTY-1, used to construct pcTYR, reveals that it was derived from a 192Y/402R (phase 2-1) allele. In the assay of the pcTYRArg402Gln expression construct (Tripathi et al., 1991), the phase of the TYR polymorphisms was not considered when drawing the conclusion that the 402Q change alone produced a thermolabile variant protein with a reduction to 25% of WT activity when transfected HeLa cells were cultured at 37°C compared to 31°C. The expression construct used in these published experiments was equivalent to a double variant 192Y-402Q (2-2) allele (Fukai et al., 1995), and no comparison with a natural 192S-402Q (1-2) or WT 192S-402R (1-1) TYR allele expression construct was performed at the time, nor has a WT 192S-402R (1-1) TYR expression construct been examined for comparative enzyme or copper binding activity (Spritz et al., 1997). Later work has reported in assays of both tyrosine hydroxylase and DOPAoxidase activities that the 192Y (phase 2-1) allele had only 60% of the activity of the 192S (phase 1-1) allele, possibly due to steric hindrance effects in the TYR protein copper-A catalytic site (Chaki et al., 2011).
The hypopigmented phenotype presenting in the two individuals homozygous for TYR 192Y-402Q allele (2-2:2-2) within our collection suggests that this double variant haplotype, as well as contributing to normal variation (Table 1), does indeed compromise human pigmentation. It may help to explain the conundrum posed in the literature (Oetting et al., 2009; Rooryck et al., 2009; Simeonov et al., 2013): does the TYR 402Q polymorphism contribute to albinism phenotypes? The answer to this perhaps lies in the TYR haplotype being considered. The 192S-402Q (1-2) TYR protein is unlikely to compromise enzyme activity to an extent that it will complement an albinism allele so as to cause complete OCA1A (Oetting et al., 2009). However, the 192Y-402Q (2-2) form of the protein may have such deficient enzyme activity that it would complement an albinism allele and manifest as an incomplete albinism, such as OCA1B (King et al., 2003). The OCA1B phenotype has been broadly defined as ranging from very little cutaneous pigmentation to nearly normal skin and hair pigmentation, indicating that it is produced by mutations that result in a residual amount of TYR activity. Further ophthalmological studies of individuals carrying the 192Y-402Q (2-2) allele need to be performed before this association with albinism can be confirmed.
Autosomal recessive ocular albinism (AROA) is a form of OCA1B that occurs with reduced pigmentation of the retina and iris but near normal skin and hair (Fukai et al., 1995), with the initial report claiming the 402Q was associated with this condition when found with a TYR null allele. Other reports have agreed with this hypothesis (Chiang et al., 2008; Hutton and Spritz, 2008; Rooryck et al., 2008), but evidence using family studies has countered this claim. In the study of Oetting et al., 2009 (Oetting et al., 2009), segregation of the 402Q allele with a pathologic mutation of TYR was examined in the parents of 12 OCA probands, with both parents normal for all pigmentation characteristics. In 9 of these cases the proband had both maternal and paternal TYR mutations identified, thus the 402Q allele was not contributing to OCA in the probands; nor did it cause albinism when in trans with a TYR mutation present in the parents. Surprisingly, in the remaining 3 cases where one parental mutation was not identified, the haplotype is listed as 192Y-402Q (2-2) in the proband (OCA cases 10 and 11 (Oetting et al., 2009)). Rather than concluding that the specific mutation had not been identified, in this instance the data would support the conclusion that the 192Y-402Q (2-2) haplotype is responsible for the albinism condition in the proband. Notably the general population frequency of the 192S-402Q (1-2) haplotype at 30% and 192Y-402Q (2-2) haplotype at 1.9% means that for the most part it will appear as though the 402Q change is not associated with albinism. Follow up haplotype studies will be required in OCA1B patients to provide conclusive proof that 192Y-402Q (2-2) haplotype is acting as a hypopigmentation allele in trans with other TYR albinism mutations.
Several genetic association studies have suggested and quantitated a role for these common TYR polymorphisms in normal variation of human pigmentation traits (Branicki et al., 2011; Candille et al., 2012; Duffy et al., 2010; Gudbjartsson et al., 2008; Hu et al., 2011; Nan et al., 2009; Sulem et al., 2007); however, a large scale association study based on TYR genotypes including phased haplotypes has hitherto not been performed to more precisely define these associations. Based on the results of our biochemical studies performed with genotyped melanocytes we proposed an additive model for the 4 TYR haplotypes (Tables 1 and 2) to explain the penetrance of the 10 TYR genotypes (A-J Table S3) seen for skin, hair, eye colour, skin reflectance and mole count in our populations derived from Southeast Queensland (Duffy et al., 2010; Sturm et al., 2008). As the TYR enzyme is a pivotal component of melanogenesis, it is to be expected that any TYR allele that influences protein stability, activity or location will have major and overall affects on pigmentation independent of the compartment the melanocyte cell is situated, be it cutaneous, follicular or ocular. The complete reliance of melanocytes on TYR catalytic activity to produce melanin is opposed to other pigmentation genes that may be more selective in their influence on melanogenesis depending on the location of the melanocytes in the skin (SLC45A2, SLC24A5) (Sturm and Duffy, 2012), hair (MC1R) (Duffy et al., 2004) or iris (OCA2) (Sturm and Larsson, 2009). Thus the TYR genotypes A to J would be expected to interact epistatically with these other pigmentation genes in determination of general lightening or darkening of skin, hair and eye phenotypes. The linear relationship of TYR genotypes to pigmentation phenotype is evident in the genetic association studies we have presented (Tables 3 and 4), with the exception of nevi count in which this correlation may be more complex.
The temperature dependent activity of the 402Q polymorphism provides some explanation as to why the 192S-402Q (1-2) allele may not be as detrimental to melanogenesis in the body as biochemical assays suggest in vitro. As seen for other temperature sensitive mutations effecting melanocyte function (Florell et al., 2005), external body temperature regulation depends on thermoregulatory and environmental factors. The temperature range in which impaired proteins function coincides with the range of normal fluctuations of cutaneous temperature. Theoretical estimates of temperature gradients found in human skin and subcutaneous tissues (Pal and Pal, 1990), suggest that even hair follicles present within the dermal layer are operating below core body temperature allowing for functional TYR activity. Studies of melanogenesis utilising primary cultures of melanocytes derived from different donors should consider the TYR genotype of their strains as determinants of melanin content (Fuller et al., 2001; Iozumi et al., 1993), the type of melanin produced (Wakamatsu et al., 2006) and other pigmentation gene polymorphisms present when they are derived from populations with European ancestry (Cook et al., 2009). If the 402Q polymorphism is found within clonal cell strains, lowering of cell growth temperature approaching 31°C maybe necessary to obtain a more accurate representation of melanogenesis that occurs in the cutaneous and follicular tissues of the donor.
Finally, the impact of TYR polymorphisms common in European populations investigated using the additive genotype model that we propose here should be taken into account as an important modifier of other pigmentation gene polymorphisms in predictive models of human skin, hair and eye colour.
Materials and Methods
Cell isolation and culture conditions
Human melanoblasts were cultured from neonatal foreskin tissue as previously described (Cook et al., 2003; Cook et al., 2005). All cultures were propagated as melanoblasts, and subsequently differentiated to melanocytes through cultivation in melanocyte growth medium for 7 days as previously described (Cook et al., 2003). All results presented in this manuscript are derived from MB:MC differentiated cells. The study was conducted according to the Declaration of Helsinki Principles, and ascertainment of foreskin tissue samples was approved by the University of Queensland Medical Research Ethics Committee and QIMR Human Research Ethics Committee. Experiments were conducted with parental consent to the use of tissue for research.
Genotyping of foreskin tissue, cultured cells, BTNS and melanoma samples
DNA extraction from foreskin tissue/cultured cells and MC1R gene sequencing was performed as previously described (Leonard et al., 2003). The genotype of polymorphisms SLC45A2 rs16891982*G/C, SLC24A5 rs1426654*G/A and OCA2 rs12913832*C/T was determined using TaqMan single-nucleotide polymorphism genotyping assays as described (Beaumont et al., 2008; Cook et al., 2009). Similar assays were performed for TYR rs1042602*C/A (C_8362862_10) and rs1126809*G/A (custom design AHHSV0M Applied Biosystems). The fourth exon of TYR was sequenced (Johanson et al., 2009) to verify the rs1126809 SNP on several samples using ABI PRISM Big Dye Terminator Sequencing (Applied Biosystems, Foster City, CA), with reactions processed by the Australian Equine Genetics Research Centre (AEGRC, Brisbane, Australia).
The Brisbane Twin Nevus Study (BTNS) collection and melanoma samples, together with the assessment of pigmentary phenotype characteristics, were as described previously (Duffy et al., 2010; Sturm et al., 2008).
Cell extraction and immunoblotting
Melanoblast cell strains were seeded and allowed to adhere into both T75 flasks for protein extraction and T25 flasks for melanin assays. After a minimum of 7 days incubation in melanocyte medium with media change at day 4, cells were first washed in ice cold PBS and total protein lysates then prepared using ice cold 0.1M phosphate buffer (pH6.8)/ 1% Triton X-100 with Roche complete protease inhibitor cocktail. Following a 20min incubation at 4°C, lysates were centrifuged 12000g, 5min at 4°C. BCA assay was used to calculate protein concentration. Western blot analysis was performed as described previously (Cook et al., 2009). Denatured protein (5ug) in sample buffer was resolved on an 8% polyacrylamide gel and transferred to PVDF membrane. Primary antibody dilutions incubated at 4°C overnight included anti-TYR 1:2500 (monoclonal T311, Merck Millipore) and anti-TYRP1 1:2500 (B8G3) (gift from Professor Peter Parsons, QIMR). Protein loading was determined using anti-GAPDH 1:10000 (Bio-Scientific). Secondary antibodies, anti-mouse 1:10000 (Life Technologies) and anti-rabbit 1:7500 (Merck Millipore Vic, Australia), were incubated for 1hr at room temperature. Membranes were visualised using Immobilon western chemiluminescent HRP substrate. Protein expression levels, normalised to GAPDH, were quantified using ImageJ software.
Neonatal foreskin tissue was processed as previously described (Pellegrini et al., 2013; Roberts et al., 2006). Following incubation with Dispase II the epidermal sheets were removed and washed twice in PBS. The samples were centrifuged at 12000g for 5min at 4°C. Sample pellets were resuspended in ice cold 0.1M phosphate buffer (pH 6.8)/1% Triton X-100 and Roche complete protease inhibitor cocktail and incubated with agitation at 4°C overnight. The samples were then sheared through a series of 18G to 27G needles and centrifuged at 12,000g for 5min at 4°C. BCA assay was used to calculate protein concentration, with 10ug used for gel electrophoresis. TYR activity was also measured in duplicate in these samples as described below, except 50ul of sample was combined with 50ul L-DOPA (3mg/ml in 0.1M phosphate buffer, pH 6.8). Where appropriate statistical significance was determined by one way ANOVA followed by Dunnet’s post hoc or by unpaired t-test using Prism v5 (Graphpad software Inc.).
TYR enzyme activity and glycosylation analysis
TYR enzyme activity was assayed using 0.1M phosphate buffer (pH6.8)/1% Triton X-100 total protein lysates prepared as previously described (Newton et al., 2007). DOPA oxidase activity was determined by measuring the OD490/min/mg protein to give a rate of L-DOPA oxidation using 75ul lysate aliquots combined with 75ul L-DOPA (3mg/ml in 0.1M phosphate buffer, pH 6.8), performed in triplicate at 37°C over a 2hr time period. Melanocyte strains were split and cultured in T75 flasks in separate incubators at 37°C or 31°C for a 24hr time period. Cell lysates were prepared using 0.1M phosphate buffer (pH 6.8)/1% Triton X-100 and enzyme digests were performed as previously described (Newton et al., 2007). Lysates (10ug) were digested with either 500U of Endoglycosidase H or PNGaseF in accordance with the conditions specified by the manufacturer, or left untreated in reaction buffer. Sample buffer (5x) was added before analysing by western blot as described.
Immunofluorescent imaging and Melanin Assays
Predifferentiated melanocyte strains were split and grown on coverslips in 12 well plates in separate incubators at 37°C or 31°C for a 24hr time period before fixation in 4% PFA. Cells were washed in PBS then permeablized in 0.1% Triton X-100 for 4min. Indirect immunofluorescence microscopy was then performed as previously described (Beaumont et al., 2011) with anti-TYR 1:700 (T311), anti-TYRP1 1:50 B8G3, and anti-calnexin 1:500. Secondary antibodies were Alexa Fluor 488 donkey anti-rabbit 1:200 and Alexa Fluor 594 donkey anti-mouse 1:200.
Melanin assays were performed on extracts of cells washed in PBS, and then lysed by addition of 1N NaOH followed by collection with a cell scraper. The lysates were heated to 60°C for 30min, and duplicate or triplicate 50uL aliquots made in individual wells of a 96 well plate. Absorbance was read at 405nm and compared to a standard curve generated from serial dilution of synthetic melanin dissolved in 1N NaOH (Cook et al., 2009).
Analysis of pigmentation characteristics in adolescent twin families and melanoma cases
The association between TYR genotype and pigmentation was examined using data from two ongoing genetic epidemiological longitudinal studies (Duffy et al., 2010; Sturm et al., 2008). The Brisbane Twin Nevus Study commenced in 1988, and follows the development of acquired common nevi and other melanoma risk phenotypes in adolescent twins and their siblings from the age of 12 years, up to age 33 in some individuals. Twin and sibling nevus count, freckling, eye hair and skin colour are assessed by a nurse examiner. Skin reflectance at 650nM was measured on sun-exposed and non-exposed sites on the arm. Colouring of parents is measured by self-report questionnaire. The BTNS provided 3836 adolescent twins, their siblings and parents (1155 nuclear families) where the two key variants had been genotyped and phenotypes such as self-reported skin hair and eye colour were available. The Queensland Familial Melanoma Project (QFMP) is a stratified sample of all melanoma cases reported to the Queensland Cancer Registry 1982-1990, along with their first-degree relatives. Pigmentation phenotypes have been recorded at multiple time points by self-completed questionnaire and by telephone interview. This study provided 1211 melanoma cases with complete genotype and phenotype information. In both studies hair colour (current or recalled as at age 20) was recorded on a five point scale (“fair”, “light brown”, “red”, “dark brown”, “black”), eye colour on a three point scale (“blue or grey”, “green or hazel”, “brown or black”), skin colour on a three point scale (“light”, “medium”, “dark”). The sample is overwhelmingly (>95%) of northern European origin (mainly Anglo-Celtic). Standard statistical analyses have been carried out in the R statistical package. Maximum likelihood based haplotype association analysis was performed using the MENDEL program version 12 (Lange et al., 2001; Lange et al., 2005) which can efficiently take advantage of our familial data.
Supplementary Material
Significance.
The contribution to normal variation in human pigmentation of two common TYR polymorphisms, S192Y and R402Q, found in populations of European origin has been investigated. A linear progression series from highest for WT/WT to lowest penetrance for homozygous 192Y-402Q/192Y-402Q genotypes, correlated well with darker skin, hair and eye colour, lower skin reflectance and nevi counts. TYR genotype is likely to be a significant modifier of other pigmentation gene polymorphisms in predictive models of human skin, hair and eye colour. A rare double variant haplotype may explain the contribution of the 402Q polymorphism to hypopigmented phenotypes.
Acknowledgments
Imaging was performed in the Australian Cancer Research Foundation (ACRF)/Institute for Molecular Bioscience Dynamic Imaging Facility for Cancer Biology, which was established with the support of the AGRF. The in vitro cell culture and biochemical studies were funded by Australian Research Council (ARC) Discovery Project Grant DP1094964. The in vivo studies were supported by the National Institutes of Health (NIH)/National Cancer Institute (CA88363, P.I. Prof. N.K. Hayward), the National Health and Medical Research Council of Australia (NHMRC), and an Australian Research Council Linkage grant (LP LP110100121) which is part funded by Identitas USA Inc. within the framework of the international Visigen consortium. D.L.D., G.W.M., J.L.S. and R.A.S are supported by the NHMRC Fellowships scheme. We thank Marlene Grace for phenotype collection, Leanne Wallace for sample processing, and the twins, their families, and melanoma patients for their cooperation in our research.
References
- Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, Yu F, Bonnen PE, De Bakker PI, Deloukas P, Gabriel SB, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–8. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont KA, Hamilton NA, Moores MT, Brown DL, Ohbayashi N, Cairncross O, Cook AL, Smith AG, Misaki R, Fukuda M, et al. The recycling endosome protein Rab17 regulates melanocytic filopodia formation and melanosome trafficking. Traffic. 2011;12:627–43. doi: 10.1111/j.1600-0854.2011.01172.x. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Shekar SN, Cook AL, Duffy DL, Sturm RA. Red hair is the null phenotype of MC1R. Hum Mutat. 2008;29:E88–94. doi: 10.1002/humu.20788. [DOI] [PubMed] [Google Scholar]
- Berson JF, Frank DW, Calvo PA, Bieler BM, Marks MS. A common temperature-sensitive allelic form of human tyrosinase is retained in the endoplasmic reticulum at the nonpermissive temperature. J Biol Chem. 2000;275:12281–9. doi: 10.1074/jbc.275.16.12281. [DOI] [PubMed] [Google Scholar]
- Bouchard B, Fuller BB, Vijayasaradhi S, Houghton AN. Induction of pigmentation in mouse fibroblasts by expression of human tyrosinase cDNA. The Journal of experimental medicine. 1989;169:2029–42. doi: 10.1084/jem.169.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branicki W, Liu F, Van Duijn K, Draus-Barini J, Pospiech E, Walsh S, Kupiec T, Wojas-Pelc A, Kayser M. Model-based prediction of human hair color using DNA variants. Hum Genet. 2011;129:443–54. doi: 10.1007/s00439-010-0939-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candille SI, Absher DM, Beleza S, Bauchet M, Mcevoy B, Garrison NA, Li JZ, Myers RM, Barsh GS, Tang H, et al. Genome-wide association studies of quantitatively measured skin, hair, and eye pigmentation in four European populations. PLoS One. 2012;7:e48294. doi: 10.1371/journal.pone.0048294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki M, Sengupta M, Mondal M, Bhattacharya A, Mallick S, Bhadra R, Ray K. Molecular and functional studies of tyrosinase variants among Indian oculocutaneous albinism type 1 patients. J Invest Dermatol. 2011;131:260–2. doi: 10.1038/jid.2010.274. [DOI] [PubMed] [Google Scholar]
- Chiang PW, Drautz JM, Tsai AC, Spector E, Clericuzio CL. A new hypothesis of OCA1B. Am J Med Genet A. 2008;146A:2968–70. doi: 10.1002/ajmg.a.32539. [DOI] [PubMed] [Google Scholar]
- Cook AL, Chen W, Thurber AE, Smit DJ, Smith AG, Bladen TG, Brown DL, Duffy DL, Pastorino L, Bianchi-Scarra G, et al. Analysis of cultured human melanocytes based on polymorphisms within the SLC45A2/MATP, SLC24A5/NCKX5, and OCA2/P loci. J Invest Dermatol. 2009;129:392–405. doi: 10.1038/jid.2008.211. [DOI] [PubMed] [Google Scholar]
- Cook AL, Donatien PD, Smith AG, Murphy M, Jones MK, Herlyn M, Bennett DC, Leonard JH, Sturm RA. Human melanoblasts in culture: expression of BRN2 and synergistic regulation by fibroblast growth factor-2, stem cell factor, and endothelin-3. J Invest Dermatol. 2003;121:1150–9. doi: 10.1046/j.1523-1747.2003.12562.x. [DOI] [PubMed] [Google Scholar]
- Cook AL, Smith AG, Smit DJ, Leonard JH, Sturm RA. Co-expression of SOX9 and SOX10 during melanocytic differentiation in vitro. Exp Cell Res. 2005;308:222–35. doi: 10.1016/j.yexcr.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Box NF, Chen W, Palmer JS, Montgomery GW, James MR, Hayward NK, Martin NG, Sturm RA. Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum Mol Genet. 2004;13:447–61. doi: 10.1093/hmg/ddh043. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Zhao ZZ, Sturm RA, Hayward NK, Martin NG, Montgomery GW. Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. J Invest Dermatol. 2010;130:520–8. doi: 10.1038/jid.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–8. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- Florell SR, Meyer LJ, Boucher KM, Hart M, Cannon-Albright LA, Harris RM, Grossman D, Samlowski WE, Zone JJ, Brinton JP, et al. Nevus distribution in a Utah melanoma kindred with a temperature-sensitive CDKN2A mutation. J Invest Dermatol. 2005;125:1310–2. doi: 10.1111/j.0022-202X.2005.23945.x. [DOI] [PubMed] [Google Scholar]
- Fukai K, Holmes SA, Lucchese NJ, Siu VM, Weleber RG, Schnur RE, Spritz RA. Autosomal recessive ocular albinism associated with a functionally significant tyrosinase gene polymorphism. Nat Genet. 1995;9:92–5. doi: 10.1038/ng0195-92. [DOI] [PubMed] [Google Scholar]
- Fuller BB, Spaulding DT, Smith DR. Regulation of the catalytic activity of preexisting tyrosinase in black and Caucasian human melanocyte cell cultures. Exp Cell Res. 2001;262:197–208. doi: 10.1006/excr.2000.5092. [DOI] [PubMed] [Google Scholar]
- Giebel LB, Spritz RA. RFLP for MboI in the human tyrosinase (TYR) gene detected by PCR. Nucleic acids research. 1990;18:3103. doi: 10.1093/nar/18.10.3103-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel LB, Strunk KM, Spritz RA. Organization and nucleotide sequences of the human tyrosinase gene and a truncated tyrosinase-related segment. Genomics. 1991;9:435–45. doi: 10.1016/0888-7543(91)90409-8. [DOI] [PubMed] [Google Scholar]
- Gudbjartsson DF, Sulem P, Stacey SN, Goldstein AM, Rafnar T, Sigurgeirsson B, Benediktsdottir KR, Thorisdottir K, Ragnarsson R, Sveinsdottir SG, et al. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat Genet. 2008;40:886–891. doi: 10.1038/ng.161. [DOI] [PubMed] [Google Scholar]
- Halaban R, Cheng E, Zhang Y, Moellmann G, Hanlon D, Michalak M, Setaluri V, Hebert DN. Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells. Proc Natl Acad Sci U S A. 1997;94:6210–5. doi: 10.1073/pnas.94.12.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R, Svedine S, Cheng E, Smicun Y, Aron R, Hebert DN. Endoplasmic reticulum retention is a common defect associated with tyrosinase-negative albinism. Proc Natl Acad Sci U S A. 2000;97:5889–94. doi: 10.1073/pnas.97.11.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HH, Guedj M, Descamps V, Jouary T, Bourillon A, Ezzedine K, Taieb A, Bagot M, Bensussan A, Saiag P, et al. Assessment of tyrosinase variants and skin cancer risk in a large cohort of French subjects. J Dermatol Sci. 2011;64:127–33. doi: 10.1016/j.jdermsci.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PLoS One. 2013;8:e74307. doi: 10.1371/journal.pone.0074307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton SM, Spritz RA. A comprehensive genetic study of autosomal recessive ocular albinism in Caucasian patients. Investigative ophthalmology & visual science. 2008;49:868–72. doi: 10.1167/iovs.07-0791. [DOI] [PubMed] [Google Scholar]
- Iozumi K, Hoganson GE, Pennella R, Everett MA, Fuller BB. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J Invest Dermatol. 1993;100:806–11. doi: 10.1111/1523-1747.ep12476630. [DOI] [PubMed] [Google Scholar]
- Jin Y, Ferrara T, Gowan K, Holcomb C, Rastrou M, Erlich HA, Fain PR, Spritz RA. Next-Generation DNA Re-Sequencing Identifies Common Variants of TYR and HLA-A that Modulate the Risk of Generalized Vitiligo via Antigen Presentation. J Invest Dermatol. 2012;132:1730–3. doi: 10.1038/jid.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson HC, Hyland V, Wicking C, Sturm RA. DNA elution from buccal cells stored on Whatman FTA Classic Cards using a modified methanol fixation method. BioTechniques. 2009;46:309–11. doi: 10.2144/000113077. [DOI] [PubMed] [Google Scholar]
- K B, Purohit R. Mutational analysis of TYR gene and its structural consequences in OCA1A. Gene. 2013;513:184–95. doi: 10.1016/j.gene.2012.09.128. [DOI] [PubMed] [Google Scholar]
- King RA, Pietsch J, Fryer JP, Savage S, Brott MJ, Russell-Eggitt I, Summers CG, Oetting WS. Tyrosinase gene mutations in oculocutaneous albinism 1 (OCA1): definition of the phenotype. Hum Genet. 2003;113:502–13. doi: 10.1007/s00439-003-0998-1. [DOI] [PubMed] [Google Scholar]
- King RA, Townsend D, Oetting W, Summers CG, Olds DP, White JG, Spritz RA. Temperature-sensitive tyrosinase associated with peripheral pigmentation in oculocutaneous albinism. The Journal of clinical investigation. 1991;87:1046–53. doi: 10.1172/JCI115064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K, Cantor R, Horvath S, Perola M, Sabatelli C, Sinsheimer J, Sobel E. MENDEL version 4.0: A complete package for the exact genetic analysis of discrete traits in pedigree and population data sets. Am J Hum Genet. 2001;69:504. [Google Scholar]
- Lange K, Sinsheimer JS, Sobel E. Association testing with Mendel. Genetic epidemiology. 2005;29:36–50. doi: 10.1002/gepi.20073. [DOI] [PubMed] [Google Scholar]
- Leonard JH, Marks LH, Chen W, Cook AL, Boyle GM, Smit DJ, Brown DL, Stow JL, Parsons PG, Sturm RA. Screening of human primary melanocytes of defined melanocortin-1 receptor genotype: pigmentation marker, ultrastructural and UV-survival studies. Pigment Cell Res. 2003;16:198–207. doi: 10.1034/j.1600-0749.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- Miyamura Y, Verma IC, Saxena R, Murase A, Kono M, Suzuki T, Yasue S, Shibata S, Sakakibara A, Tomita Y. Establishment of tyrosinase sequence database in normally pigmented Indians and Japanese for rapid determination of novel mutations. J Dermatol Sci. 2005;39:167–73. doi: 10.1016/j.jdermsci.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Nan H, Kraft P, Hunter DJ, Han J. Genetic variants in pigmentation genes, pigmentary phenotypes, and risk of skin cancer in Caucasians. Int J Cancer. 2009;125:909–17. doi: 10.1002/ijc.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RA, Cook AL, Roberts DW, Leonard JH, Sturm RA. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J Invest Dermatol. 2007;127:2216–27. doi: 10.1038/sj.jid.5700840. [DOI] [PubMed] [Google Scholar]
- Norton HL, Kittles RA, Parra E, Mckeigue P, Mao X, Cheng K, Canfield VA, Bradley DG, Mcevoy B, Shriver MD. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol Biol Evol. 2007;24:710–722. doi: 10.1093/molbev/msl203. [DOI] [PubMed] [Google Scholar]
- Oetting WS, Pietsch J, Brott MJ, Savage S, Fryer JP, Summers CG, King RA. The R402Q tyrosinase variant does not cause autosomal recessive ocular albinism. Am J Med Genet A. 2009;149A:466–9. doi: 10.1002/ajmg.a.32654. [DOI] [PubMed] [Google Scholar]
- Pal DS, Pal S. Prediction of temperature profiles in the human skin and subcutaneous tissues. Journal of mathematical biology. 1990;28:355–64. doi: 10.1007/BF00178783. [DOI] [PubMed] [Google Scholar]
- Pellegrini C, Zulian A, Gualandi F, Manzati E, Merlini L, Michelini ME, Benassi L, Marmiroli S, Ferlini A, Sabatelli P, et al. Melanocytes--a novel tool to study mitochondrial dysfunction in Duchenne muscular dystrophy. J Cell Physiol. 2013;228:1323–31. doi: 10.1002/jcp.24290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnazhagan S, Hou L, Kwon BS. Structural organization of the human tyrosinase gene and sequence analysis and characterization of its promoter region. J Invest Dermatol. 1994;102:744–8. doi: 10.1111/1523-1747.ep12376924. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Newton RA, Beaumont KA, Helen Leonard J, Sturm RA. Quantitative analysis of MC1R gene expression in human skin cell cultures. Pigment Cell Res. 2006;19:76–89. doi: 10.1111/j.1600-0749.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- Rooryck C, Morice F, Didier L, Taieb A, Arveiler B. Genetic basis of oculocutaneous albinism. Expert Rev Dermatol. 2009;4:611–622. [Google Scholar]
- Rooryck C, Morice-Picard F, Elcioglu NH, Lacombe D, Taieb A, Arveiler B. Molecular diagnosis of oculocutaneous albinism: new mutations in the OCA1-4 genes and practical aspects. Pigment Cell Melanoma Res. 2008;21:583–7. doi: 10.1111/j.1755-148X.2008.00496.x. [DOI] [PubMed] [Google Scholar]
- Shriver MD, Parra EJ, Dios S, Bonilla C, Norton H, Jovel C, Pfaff C, Jones C, Massac A, Cameron N, et al. Skin pigmentation, biogeographical ancestry and admixture mapping. Hum Genet. 2003;112:387–99. doi: 10.1007/s00439-002-0896-y. [DOI] [PubMed] [Google Scholar]
- Simeonov DR, Wang X, Wang C, Sergeev Y, Dolinska M, Bower M, Fischer R, Winer D, Dubrovsky G, Balog JZ, et al. DNA variations in oculocutaneous albinism: an updated mutation list and current outstanding issues in molecular diagnostics. Hum Mutat. 2013;34:827–35. doi: 10.1002/humu.22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz RA, Ho L, Furumura M, Hearing VJ., Jr Mutational analysis of copper binding by human tyrosinase. J Invest Dermatol. 1997;109:207–12. doi: 10.1111/1523-1747.ep12319351. [DOI] [PubMed] [Google Scholar]
- Stokowski RP, Pant PV, Dadd T, Fereday A, Hinds DA, Jarman C, Filsell W, Ginger RS, Green MR, Van Der Ouderaa FJ, et al. A genomewide association study of skin pigmentation in a South Asian population. Am J Hum Genet. 2007;81:1119–1132. doi: 10.1086/522235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm RA. Molecular genetics of human pigmentation diversity. Hum Mol Genet. 2009;18:R9–17. doi: 10.1093/hmg/ddp003. [DOI] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL. Human pigmentation genes under environmental selection. Genome Biol. 2012;13:248. doi: 10.1186/gb-2012-13-9-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL, Zhao ZZ, Leite FP, Stark MS, Hayward NK, Martin NG, Montgomery GW. A single SNP in an evolutionary conserved region within intron 86 of the HERC2 gene determines human blue-brown eye color. Am J Hum Genet. 2008;82:424–31. doi: 10.1016/j.ajhg.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm RA, Larsson M. Genetics of human iris colour and patterns. Pigment Cell Melanoma Res. 2009;22:544–62. doi: 10.1111/j.1755-148X.2009.00606.x. [DOI] [PubMed] [Google Scholar]
- Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, Manolescu A, Karason A, Palsson A, Thorleifsson G, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39:1443–1452. doi: 10.1038/ng.2007.13. [DOI] [PubMed] [Google Scholar]
- Tripathi RK, Giebel LB, Strunk KM, Spritz RA. A polymorphism of the human tyrosinase gene is associated with temperature-sensitive enzymatic activity. Gene expression. 1991;1:103–10. [PMC free article] [PubMed] [Google Scholar]
- Tripathi RK, Hearing VJ, Urabe K, Aroca P, Spritz RA. Mutational mapping of the catalytic activities of human tyrosinase. J Biol Chem. 1992;267:23707–12. [PubMed] [Google Scholar]
- Wakamatsu K, Kavanagh R, Kadekaro AL, Terzieva S, Sturm RA, Leachman S, Abdel-Malek Z, Ito S. Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin. Pigment Cell Res. 2006;19:154–62. doi: 10.1111/j.1600-0749.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Wang N, Hebert DN. Tyrosinase maturation through the mammalian secretory pathway: bringing color to life. Pigment Cell Res. 2006;19:3–18. doi: 10.1111/j.1600-0749.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Braakman I, Matlack KE, Helenius A. Quality control in the secretory pathway: the role of calreticulin, calnexin and BiP in the retention of glycoproteins with C-terminal truncations. Mol Biol Cell. 1997;8:1943–54. doi: 10.1091/mbc.8.10.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


