Abstract
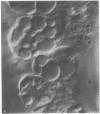
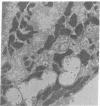
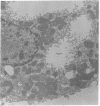
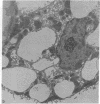
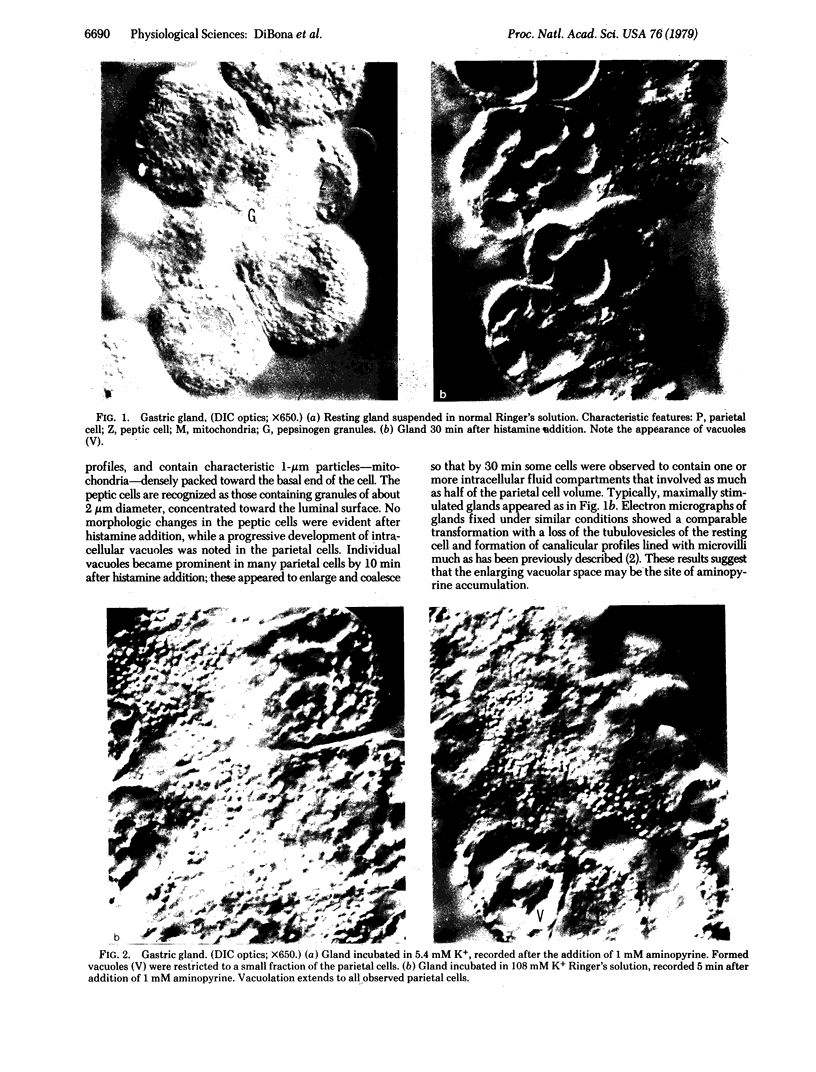
Isolated gastric glands of the rabbit were examined both with differential interference-contrast microscopy and with electron microscopy to describe the morphologic correlates of acid secretion. Stimulation of the glands with histamine resulted in the development of intracellular spaces within the parietal cells. A similar transformation was produced by addition of 1 mM aminopyrine, whether the weak base was added in the presence of normal-K+ (5.4 mM) or high-K+ (108 mM) solutions. The intracellular space was compatible with the expanded canaliculus described in stimulated parietal cells. Confirmation that the space produced by histamine is the site of acid secretion was gained by combining fluorescence and interference-contrast methods in the presence of the dye acridine orange, which displays a pH-dependent metachromasia in its emission spectrum. Human gastrin I resulted in an observable discharge of peptic granules.
Full text
PDF
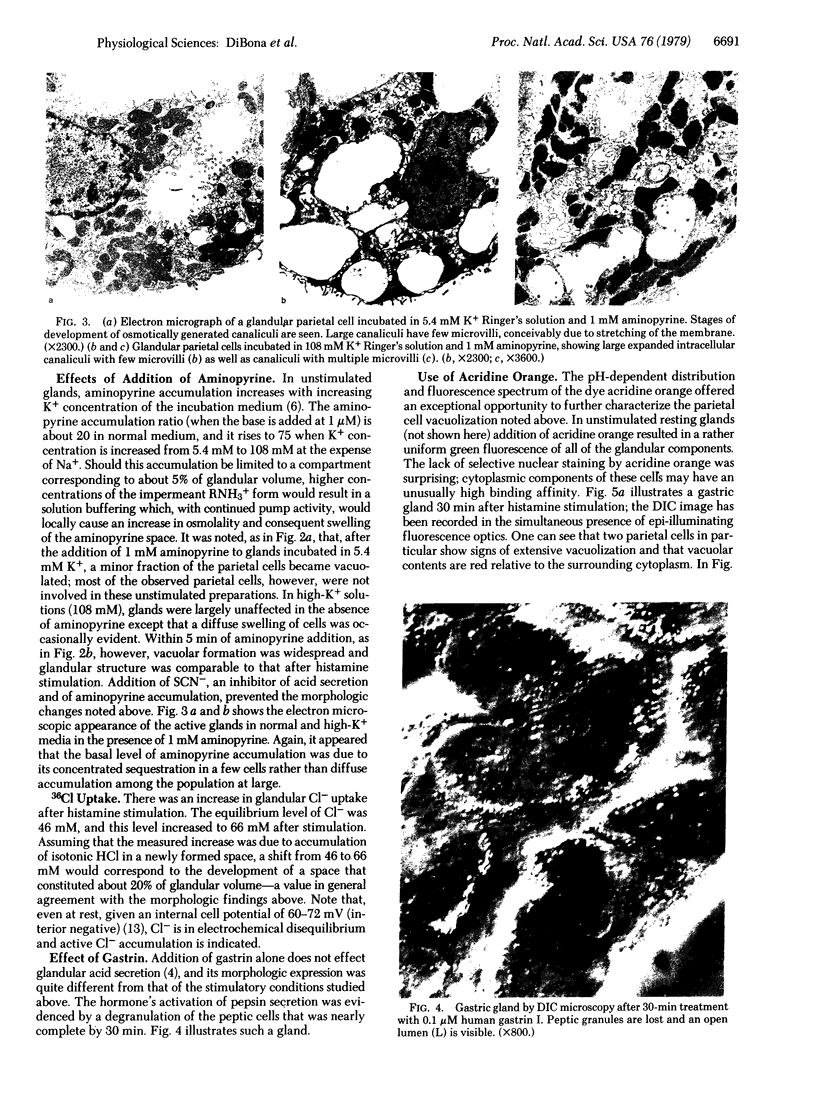
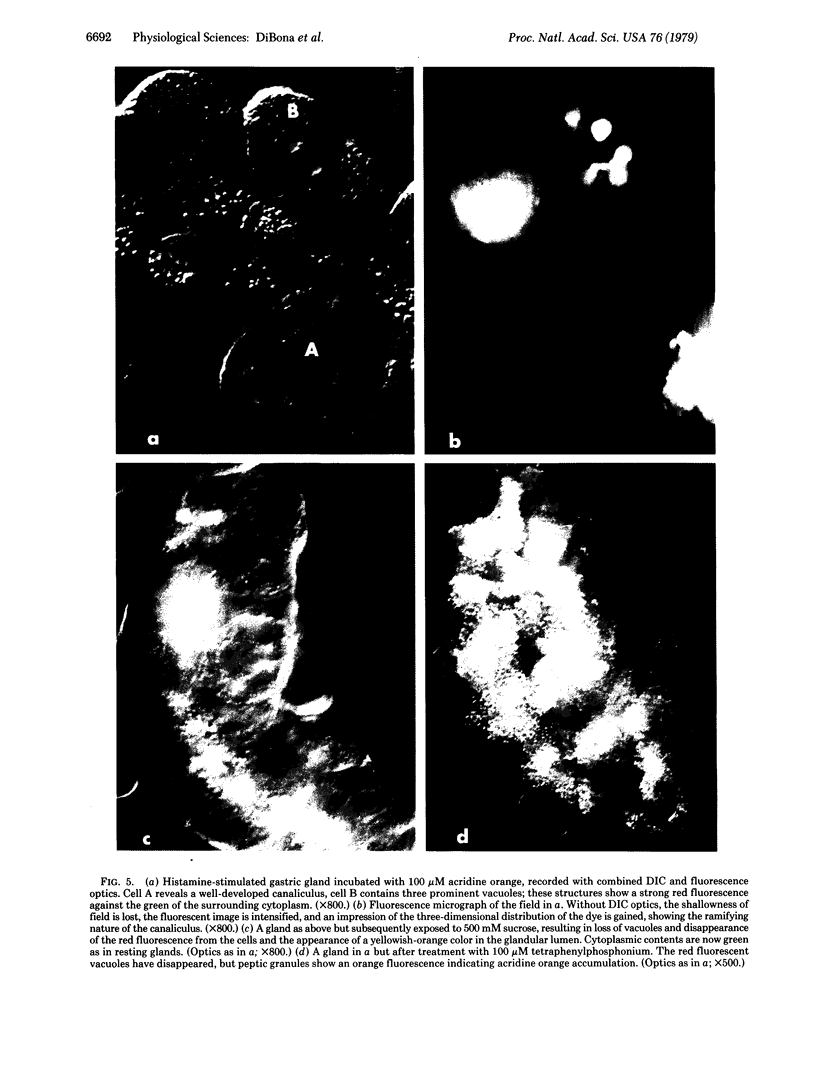

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., David G. B., Nomarski G. The zeiss-Nomarski differential interference equipment for transmitted-light microscopy. Z Wiss Mikrosk. 1969 Nov;69(4):193–221. [PubMed] [Google Scholar]
- Berglindh T., Obrink K. J. A method for preparing isolated glands from the rabbit gastric mucosa. Acta Physiol Scand. 1976 Feb;96(2):150–159. doi: 10.1111/j.1748-1716.1976.tb10184.x. [DOI] [PubMed] [Google Scholar]
- Blum A. L., Shah G. T., Wiebelhaus V. D., Brennan F. T., Helander H. F., Ceballos R., Sachs G. Pronase method for isolation of viable cells from Necturus gastric mucosa. Gastroenterology. 1971 Aug;61(2):189–200. [PubMed] [Google Scholar]
- Dell'Antone P., Colonna R., Azzone G. F. The membrane structure studied with cationic dyes. 1. The binding of cationic dyes to submitochondrial particles and the question of the polarity of the ion-translocation mechanism. Eur J Biochem. 1972 Jan 21;24(3):553–565. doi: 10.1111/j.1432-1033.1972.tb19718.x. [DOI] [PubMed] [Google Scholar]
- DiBona D. R. Direct visualization of epithelial morphology in the living amphibian urinary bladder. J Membr Biol. 1978;40(Spec No):45–70. doi: 10.1007/BF02025998. [DOI] [PubMed] [Google Scholar]
- HARRIS J. B., FRANK H., EDELMAN I. S. Effect of potassium on ion transport and bioelectric potentials of frog gastric mucosa. Am J Physiol. 1958 Nov;195(2):499–504. doi: 10.1152/ajplegacy.1958.195.2.499. [DOI] [PubMed] [Google Scholar]
- Lichtshtein D., Kaback H. R., Blume A. J. Use of a lipophilic cation for determination of membrane potential in neuroblastoma-glioma hybrid cell suspensions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):650–654. doi: 10.1073/pnas.76.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabon E., Chang H., Sachs G. Quantitation of hydrogen ion and potential gradients in gastric plasma membrane vesicles. Biochemistry. 1978 Aug 8;17(16):3345–3353. doi: 10.1021/bi00609a027. [DOI] [PubMed] [Google Scholar]
- Rehm W. S. Some aspects of the problem of gastric hydrochloric acid secretion. Arch Intern Med. 1972 Feb;129(2):270–278. [PubMed] [Google Scholar]
- Sachs G., Chang H. H., Rabon E., Schackman R., Lewin M., Saccomani G. A nonelectrogenic H+ pump in plasma membranes of hog stomach. J Biol Chem. 1976 Dec 10;251(23):7690–7698. [PubMed] [Google Scholar]
- Sedar A. W. Fine structure of the stimulated oxyntic cell. Fed Proc. 1965 Nov-Dec;24(6):1360–1367. [PubMed] [Google Scholar]