Abstract
Background:
It is known that dental unit waterline can be a source of infection. The aim of this study was to evaluate the efficacy of a mouthwash, chlorhexidine, in controlling microbial and fungal contamination of dental unit waterlines.
Materials and Methods:
In the present experimental study, the water in high-speed handpieces and air/water syringes of 35 dental units in a dental school was investigated microbiologically. Five of the units and one tap water served as controls; 100-200-mL water samples were collected aseptically in sterile containers in the morning after a 2-min purge. Water reservoir bottles were emptied and 50 mL of 0.2% chlorhexidine mouthwash was introduced into the tank. Then the water syringe was used to flush the waterline until the pink-colored chlorhexidine was observed to flow from the water syringe. Before the next day's session and before the students used the unit, two water samples from the water syringe and water turbine was collected. The samples were transferred to the laboratory. After 48 h at 37°C, the microbial colonies were counted. The number of these colonies was evaluated using colony forming unit CFU. Data were analyzed with Mann — Whitney U test and SPSS 13.5 statistical program. The statistical significance was defined at P ≤ 0.05.
Results:
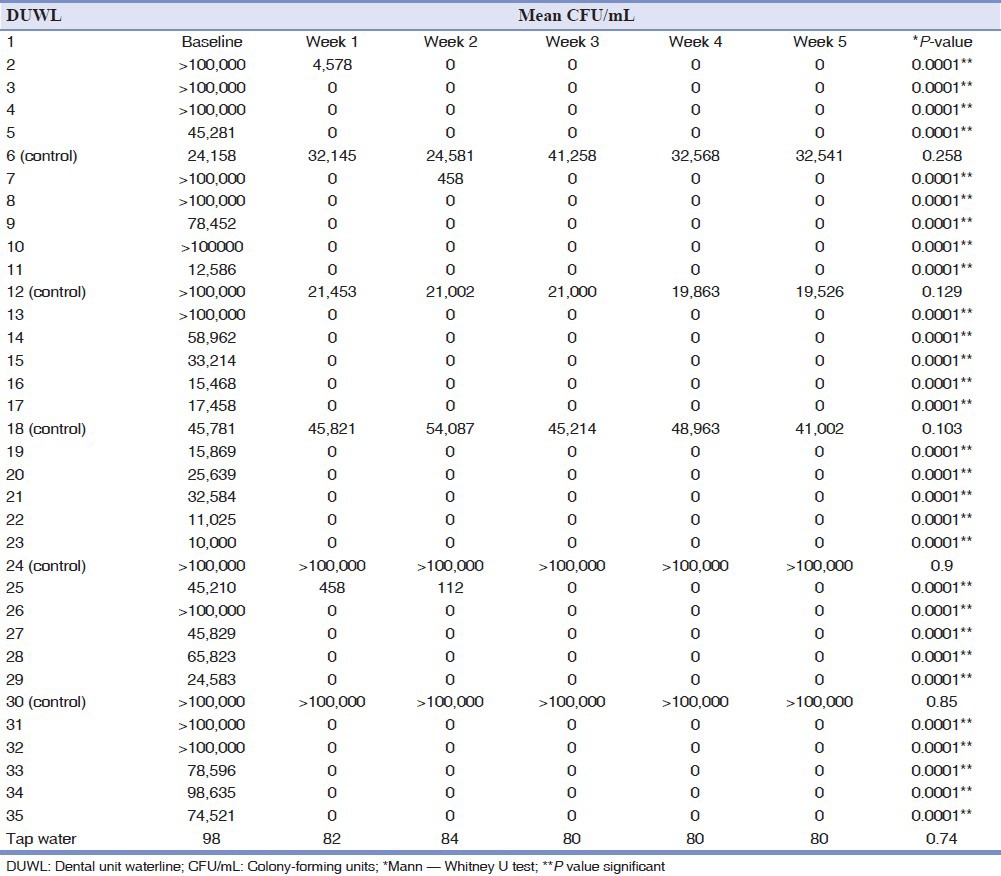
All 35 units were contaminated before chlorhexidine use; no contamination was detected after adding chlorhexidine to the waterlines of the units. After week 1, 28 of the 30 treated dental unit waterlines (DUWLs) had values of CFU/mL less than 200.
Conclusion:
The present study showed that the use of chlorhexidine could reduce microbial counts in dental unit waterlines.
Keywords: Antibacterial, antifungal, chlorhexidine, infection control, dental
INTRODUCTION
Infection control has been one of the controversial issues in dentistry for several years and undoubtedly dental professionals agree that studying infection transmission routes has an important role in preventive methods and infection control.[1] It is known that dental unit waterline is one of the infection sources. Water is used in almost all dental procedures. For example, water is used as a coolant and temperature controller in handpieces and high-speed or even low-speed turbines, water syringes, or in scaling procedures.[2,3]
Water is sprayed into the patient's mouth and as it is not sterile and is contaminated, it contains a large number of micro-organisms. Moreover, aerosols are created during dental procedures, and water laden with micro-organisms is considered an infection risk factor for both dental staff and patients with competent or compromised immune systems. Presence of Legionella pneumophila antibodies with a high prevalence rate in dental students in comparison with other students is good evidence for this contamination.[2,4]
Bacterial infection in dental water system was detected around 40 years ago. Although most studies show more contamination in water samples from dental schools, current research in the UK and USA shows high contamination rates in dental offices, too. Nowadays, with high-risk infections such as HIV, HVB, and HVC, serious measures should be applied to study the potential micro-organisms, and solutions should be introduced to protect patients with compromised immune systems, pregnant women, the elderly, smokers, organ transplant patients, or patients who receive chemotherapy and radiotherapy.[5,6]
Despite serious efforts and studies, even in developed countries, no final and clear-cut solution has been introduced yet. Some researchers recommend use of distilled water and some solvents such as hydrogen peroxide and sodium hypochlorite. As mentioned above, although several studies have been carried out, prevention effectiveness is not clear. Level of contamination is high and it seems dental practitioners do not take this issue seriously.[4,7,8]
Many studies have suggested treatment with various disinfectant solutions, including hydrogen peroxide,[9] chlorhexidine gluconate,[10] sodium hypochlorite,[11] chlorine dioxide,[12] povidone-iodine,[13] Listerine mouthwash,[14] and electrochemically activated water.[15] Meiller et al. showed that sodium hypochlorite, Cavicide, glutaraldehyde, Listerine Antiseptic, Peridex, and Sterilex Ultra are potentially useful in the management of dental unit waterline biofilm.[16] These have been developed and implemented in many dental practices with mixed long-term results. Also, Kettering et al. showed that using tap water alone or tap water with hydrogen peroxide did not improve water quality in dental units.[17] The effects of anti-microbial agents on unit waterline contamination have not been studied in international levels. Thus, it seems that it is important to study the effects of disinfectants such as chlorhexidine (used as a mouthwash by most patients). Studies show that 0.2% chlorhexidine has a significant antiseptic effect;[18,19,20] therefore, the aim of this study was to evaluate the efficacy of a mouthwash, chlorhexidine, in controlling microbial and fungal contamination of dental unit waterlines.
MATERIALS AND METHODS
In this experimental study, 35 dental units that used a self-contained water system were selected randomly from the 97 units available in the Dental School of Kerman University of Medical Sciences. Five of these units were selected as controls in which no periodic disinfection procedures were carried out. A tap water source, too, was used as a study control.
A unit selection system using a closed water tank was installed by the medical equipment technician. The investigators collected a total of 35 water samples for baseline measurements before the study began, and 35 samples were collected once a week for the next 5 weeks. The study went on during the school weekdays.[7] Startup was initiated by treating the dental unit waterlines (DUWLs) with 0.2% chlorhexidine mouthwash for three consecutive nights. Routine weekly treatment protocols were then implemented as described below.
Waterlines were flushed for 30 s, and 10-mL samples were subsequently collected from the air-water syringe in sterile test tubes. Samples for microbial assays were taken at a worst-case point immediately before the chemical treatment.[7] Water reservoir bottles were emptied and 50 mL of 0.2% chlorhexidine mouthwash was introduced into the tank; according to research results chlorhexidine has antibacterial effects in this concentration.[10,12,13] Chlorhexidine is easy to mix and has the advantage of being pink, which enables the operator to easily see that it is in the water tank, which is evident in the effluent water.
Then the system was charged to clear the system when the unit was flushed.[7] Then the water syringe was used to flush the waterline until the pink-colored chlorhexidine was observed to flow from the water syringe. Then chlorhexidine was maintained in the units overnight. Before the next day's session and before the students used the unit, two water samples from the water syringe and water turbine were collected after 20 s of flushing and refilled with tap water; the lines were flushed for 60 s.[7]
Samples were collected according to this method for five consecutive weeks; finally, there were 20 samples collected from each unit.
An external control was provided from the hand washbasin taps adjacent to each unit, the water supply of which was derived from the main water supply. Water samples were prepared in completely sterile conditions and transferred to the microbiology laboratory.[7] In the laboratory, all the samples were passed through 0.35% millipore filters. Then 1 mL of each of the diluted samples was spread-plated on medium S (Sabvrd) for mold growth and on blood agar, SS (Salmonella, Shigella), EMB (Eosin methylene blue) to grow aerobic bacteria; thioglycolate broth was used for review and identification of anaerobic species, including Actinomyces israelii. The samples were placed at temperatures of 25°C and 37°C for growth of fungi and bacteria, respectively, and to ultimately determine bacterial and fungal species and the number of colony forming units (CFUs). CFU counts more than 200 were considered severe contamination.[7,21,22,23] All the experimental procedures in the present study were carried out by two dental students. Finally, data were analyzed with Mann — Whitney U test using SPSS 13.5(SPSS Inc., Chicago, IL, USA). The statistical significance was defined at P ≤ 0.05.
RESULTS
In this study, four samples were prepared from each study unit at each week interval (two samples before chlorhexidine use and two samples after its incorporation). Therefore, from 30 study units in each week interval, 120 samples were prepared and at the end of week 5, 600 samples were taken and from the control units 100 samples were prepared. Based on research findings all the samples of high-speed handpieces and air/water syringes (case and control units) were contaminated before chlorhexidine use. The following micro-organisms were the most frequently occurring bacteria present in all the operative sites: Staphylococcus aureus, Escherichia coli, Klebsiella, Klarosporium, Pseudomonas, Penicillium, and Candida albicans.
In this research, Pseudomonas aeruginosa was detected in 27% of DUWL samples. The DUWL water contaminated with this bacterial species showed a significantly higher total number of bacteria in comparison to DUWL free from it. There were no significant differences between the levels of contamination in the air/water syringes and high-speed handpieces (P = 0.09). The concentration of total bacteria isolated from one site was 134,056 CFU/mL on the average; the minimum and maximum were 10,000 CFU/mL and 720,000 CFU/mL, respectively. Table 1 shows that CFU counts varied considerably at baseline, with the vast majority far exceeding 200 CFU/mL. After one week and incorporation of chlorhexidine, however, the counts for only two of the 30 study units were above 200 CFU/mL (P = 0.0001), and by week 4, all the units had counts well below 200 CFU/mL (P = 0.0001). Control units continued to yield high concentrations of environmental organisms throughout the 5 weeks of the study period. This study showed no significant differences between the control DUWLs (P = 0.124); however, significant differences were observed between the disinfectant-treated waterlines over the 5 weeks of the study period (P = 0.001) [Table 2].
Table 1.
Average concentrations (CFU/mL) and proportions of particular genera/species of microorganisms in water samples from dental unit reservoirs
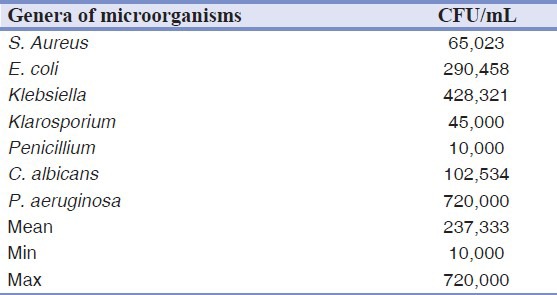
Table 2.
Reduction mean CFU/mL in DUWL based on treatment with chlorhexidine mouthwash from week 1 to week 5
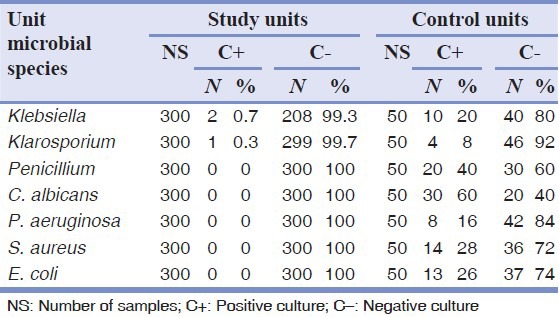
The numbers of bacterial and fungal contamination cases before and after adding chlorhexidine are shown in Tables 3 and 4.
Table 3.
The results of bacterial and fungal cultures before adding chlorhexidine
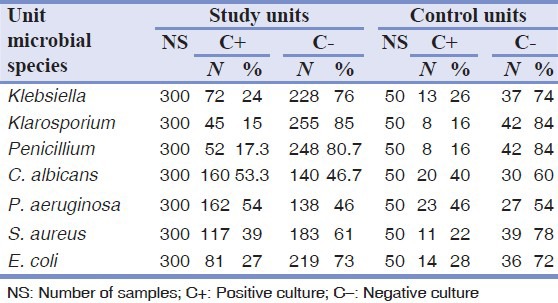
Table 4.
The results of bacterial and fungal culture after adding chlorhexidine
DISCUSSION
Microbial control of DUWLs minimizes the risk of exposure to potential pathogens and creates a safe working environment for treatment of patients. Two major sources of contamination are (1) oral microbial flora in patients because of the suction and backflow of patient saliva from the saliva ejector and possibility of entering the water system, and (2) biofilm in waterlines.[1,24].
Determination of concentration and composition of microflora in the unit waterlines is the basis for evaluation of DUWL microbial contamination. In 1963, Blacke was the first to discuss DUWL microbial contamination.[25]
In this research, P. aeruginosa was detected in 27% of DUWL samples. There were no significant differences between the levels of contamination in the air/water syringes and high-speed handpieces. However, in some studies bacterial counts of water samples from high-speed handpieces had higher means in comparison to air/water syringes.[2,5]
study showed that only a small percentage of the total isolated bacterial species were of Streptococcus and Staphylococcus genera, which form the physiological flora of the oral cavity.[26] They were present in DUWL probably as a result of backflow of fluids from patients’ oral cavities, and subsequent multiplication in the unit reservoirs, which might be a potential source of cross-infections. The presence of Pseudomonas aeruginosa in DUWL has been confirmed by some other studies.[27,28,29,30,31] It should be noted that the bacterial genus of Pseudomonas includes species that are potentially pathogenic for immunocompromised individuals.
have been several attempts to reduce the microbial contamination of dental unit waterlines, including autoclaving of handpieces, handpiece replacement between patients, flushing of the unit prior to use, “anti-contamination” devices to prevent retrograde aspiration of oral secretions into the waterline, connection to a separate water supply, ultra-violet radiation disinfection and the use of in-line water filters. Many studies have suggested treatment with various disinfectant solutions, including hydrogen peroxide, glutaraldehyde, sodium hypochlorite, chlorine dioxide, povidoone–iodine, Listerine mouthwash, Bilpron, and electrochemically activated water. Studies have shown that these materials often do not have proper performance or their effects are temporary and insignificant. Meanwhile, a number of expensive materials, on economic terms, are not cost-effective. Some of these materials also have toxic effects and might pose dangers or reactions in the human mouth or corrosion; a decrease in enamel and dentin bond strengths for adhesive restorative dental materials has been reported for some disinfectants. Flushing the handpiece is the most common and safest procedure to lower bacterial counts.[27]
Today, research on materials with minimal cytotoxic effects and high efficacy to remove micro-organisms is ongoing. In this study, chlorhexidine was able to completely inhibit the growth of S. aureus, Penicillium fungus, E. coli, P. aeruginosa and C. albicans. Chlorhexidine is a chemical antiseptic, which is effective on both Gram-positive and Gram-negative bacteria. It is also useful against fungi and enveloped viruses, though this has not been extensively investigated. It is often used as an active ingredient in mouthwashes designed to reduce dental plaque and oral bacterial counts. It has been shown to have an immediate bactericidal action and a prolonged bacteriostatic effect due to adsorption onto the pellicle-coated enamel surfaces.[28,29]
This study showed that after 1 week and incorporation of chlorhexidine, the counts for only 2 of the 30 study units were above 200 CFU/mL, and by week 4, all the units had counts well below 200 CFU/mL. Also, this study proved the efficacy of chlorhexidine in eradicating microbial contamination from dental unit waterlines and in controlling bacterial counts of several bacterial species. The dental units selected for the purpose of this study were several years old and had a significant degree of microbial contamination. The units were located in a busy emergency clinic within a dental educational center to closely simulate usage patterns in a general dental facility.
Chlorhexidine gluconate, povidone iodine, sodium hypochlorite, hydrogen peroxide have been employed to variable effects to remove the biofilm and eliminate the planktonic bacterial count. Liaqat and Sabri found that, overall, combination of chlorhexidine with povidone iodine was very effective in eliminating/reducing the biofilm bacteria at 1000 μg/mL as compared to other combinations.[30]
Other studies assessed water samples from a hospital dental clinic to determine whether a disinfectant/coolant irrigant containing chlorhexidine affects the presence of microbial organisms in dental unit waterlines.[31] In addition, Kettering et al. and Epstein et al. showed that after treatment of dental unit water with disinfectant/coolant irrigants containing chlorhexidine (Lines) no organisms were detected in waterline discharge.[17,19]
CONCLUSION
This study showed that bacterial concentration in dental unit reservoirs reaches excessive values, with the bacterial flora composed of the bacteria characteristic for water supply systems, opportunistic pathogens, and the bacteria of the oral cavity flora. Overall, in the 30 samples of high-speed handpieces and air/water syringes bacterial and fungal contamination was almost non-existent after adding 0.2% chlorhexidine. The results of the present study showed that chlorhexidine reduces microbial counts in dental unit waterlines.
ACKNOWLEDGMENTS
This study was supported by Kerman Dental and Oral Diseases Research Center and by a grant from the Kerman University of Medical Sciences Vice Chancellor for Research (86/11).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.ADA Council on Scientific Affairs. Dental unit waterlines: Approaching the year 2000. J Am Dent Assoc. 1999;130:1653–64. [PubMed] [Google Scholar]
- 2.Walker JT, Bradshaw DJ, Finney M, Fulford MR, Frandsen E, OStergaard E, et al. Microbiological evaluation of dental unit water systems in general dental practice in Europe. Eur J Oral Sci. 2004;112:412–8. doi: 10.1111/j.1600-0722.2004.00151.x. [DOI] [PubMed] [Google Scholar]
- 3.Szymanska J, Wdowiak L, Puacz E, Stojek NM. Microbial quality of water in dental unit reservoirs. Ann Agric Environ Med. 2004;11:355–8. [PubMed] [Google Scholar]
- 4.Walker JT, Bradshaw DJ, Fulford MR, Marsh PD. Microbiological evaluation of a range of disinfectant products to control mixed species biofilm contamination in a laboratory model of the dental unit water system. Appl Eniviron Microbiol. 2003;69:3327–32. doi: 10.1128/AEM.69.6.3327-3332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams HN, Johnson A, Kelley JI, Baer ML, King TS, Mitchell B, Hasler JF. Bacterial contamination of the water supply in newly installed dental units. Quintessence Int. 1995;26:331–7. [PubMed] [Google Scholar]
- 6.Forde A, O’Reilly P, Fitzgerald G, O’Mullane D, Burke FM, O’Sullivan M. Microbial contamination of dental unit water systems. J Ir Dent Assoc. 2005;51:115–8. [PubMed] [Google Scholar]
- 7.Linger JB, Molinari JA, Forbes WC, Farthing CF, Winget WJ. Evaluation of a hydrogen peroxide disinfectant for dental unit waterlines. Am Dent Assoc. 2001;132:1287–91. doi: 10.14219/jada.archive.2001.0374. [DOI] [PubMed] [Google Scholar]
- 8.Kellet M, Holbrook WP. Bacterial contamination of dental handpieces. J Dent. 1980;8:249–53. doi: 10.1016/0300-5712(80)90076-7. [DOI] [PubMed] [Google Scholar]
- 9.Douglas CWI, Rothwell PS. Evaluation of a dental unit with a built in decontamination system. Quintessence Int. 1991;22:721–6. [PubMed] [Google Scholar]
- 10.Kim PJ, Cederberg RA, Puttaiah R. A pilot study of two methods for control of dental unit biofilms. Quintessence Int. 2000;31:41–8. [PubMed] [Google Scholar]
- 11.Abel IC, Miler RL, Micik RE, Ryge G. Studies on dental aerobiology.IV. Bacterial contamination of water delivered by dental units. J Dent Res. 1971;50:1567–9. doi: 10.1177/00220345710500063601. [DOI] [PubMed] [Google Scholar]
- 12.Smith AJ, Bagg J, Hood J. Use of chlorine dioxide to disinfect dental unit water lines. J Hosp Inf. 2001;49:285–8. doi: 10.1053/jhin.2001.1085. [DOI] [PubMed] [Google Scholar]
- 13.Mills SE, Lauderdale PW, Mayhew RB. Reduction of microbial contamination in dental units with povidone-iodine 10% J Am Dent Assoc. 1986;113:280–4. doi: 10.14219/jada.archive.1986.0178. [DOI] [PubMed] [Google Scholar]
- 14.Meiller T, Baqui A, DePaola L, Overholser CD. Disinfection of dental unit waterlines using Listerine antiseptic. J Dent Res. 1995;74:153. [Google Scholar]
- 15.Marais JT, Brozel VS. Electro-chemically activated water in dental unit water lines. Br Dent J. 1999;187:154–8. doi: 10.1038/sj.bdj.4800228. [DOI] [PubMed] [Google Scholar]
- 16.Meiller TF, Kelley JI, Baqui AA, DePaola LG. Laboratory evaluation of anti-biofilm agents for use in dental unit waterlines. J Clin Dent. 2001;12:97–103. [PubMed] [Google Scholar]
- 17.Kettering JD, Stephens JA, Munoz-Viveros CA, Naylor WP. Reducing bacterial counts in dental unit waterlines: Tap water versus distilled water. J Contemp Dent Pract. 2002;15(3):1–9. [PubMed] [Google Scholar]
- 18.Tuttlebee CM, O’Donnell MJ, Keane CT, Russell RJ, Sullivan DJ, Falkiner F, et al. Effective control of dental chair unit waterline biofilm and marked reduction of bacterial contamination of output water using two peroxide-based disinfectants. J Hosp Infect. 2002;52:192–205. doi: 10.1053/jhin.2002.1282. [DOI] [PubMed] [Google Scholar]
- 19.Epstein JB, Dawson JR, Buivids IA, Wong B, Le ND. The effect of a disinfectant/coolant irrigant on microbes isolated from dental unit water lines. Spec Care Dentist. 2002;22:137–41. doi: 10.1111/j.1754-4505.2002.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 20.Fogarty LR, Hack SK, Wolcott MJ, Whitman PL. Abundance and characteristics of the recreational water quality indicator bacteria Escherichia coli and enterococci in gulli faces. J Appl Microbiol. 2003;94:865–78. doi: 10.1046/j.1365-2672.2003.01910.x. [DOI] [PubMed] [Google Scholar]
- 21.Almas K, Shaug N, Ahmad I. An in vitro antimicrobial comparison of miswak extract with commercially available non alcohol mouth rinses. Int J Dent. 2005;3:18–24. doi: 10.1111/j.1601-5037.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- 22.Mcbain A, Bartolo R, Catrenich C, Charbonneau D, Ledder R, Gilbert P. Effects of a chlorhexidine gluconate-containing mouth wash on the vitality and antimicrobial susceptibility of in vitro oral bacterial ecosystems applied and environmental. Microbial J. 2003;69:4770–6. doi: 10.1128/AEM.69.8.4770-4776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayo JA, Oertling KM, Andrieu SC. Bacterial biofilm: A source of dental air water syringes contamination. J Clin Prev Dent. 1990;12:13–20. [PubMed] [Google Scholar]
- 24.Fayle SA, Pollard MA. Decontamination of dental units water systems: A review of current recommendations. Br Dent J. 1996;181:369–372. doi: 10.1038/sj.bdj.4809262. [DOI] [PubMed] [Google Scholar]
- 25.Barbeau J, Gauthier C, Payment P. Biofilms, infectious agents, and dental unit waterlines: A review. Can J Microbiol. 1998;44:1019–28. doi: 10.1139/cjm-44-11-1019. [DOI] [PubMed] [Google Scholar]
- 26.Kathryn L, Ruoff Miscellaneous catalase-negative, gram-positive cocci: Emerging opportunists. J Clin Micro. 2002;40:1129–33. doi: 10.1128/JCM.40.4.1129-1133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AJ, McHugh S, Aitken I, Hood J. Evaluation of the efficacy of Alpron disinfectant for dental unit waterlines. Br Dent J. 2002;193:593–6. doi: 10.1038/sj.bdj.4801635. [DOI] [PubMed] [Google Scholar]
- 28.Kuyyakanond T, Quesnel LB. The mechanism of action of chlorhexidine. FEMS Microbiol Lett. 1993;79:211–215. doi: 10.1111/j.1574-6968.1992.tb14042.x. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins S, Addy M, Wade W. The mechanism of action of chlorhexidine. A study of plaque growth on enamel inserts in vivo. J Clin Periodontol. 1998;15:415–24. doi: 10.1111/j.1600-051x.1988.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 30.Liaqat I, Sabri AN. In vitro efficacy of biocides against dental unit water line (DUWL) biofilm bacteria. Asian J Exp Sci. 2009;1:67–75. [Google Scholar]
- 31.Szymanska R. Control methods of the microbial water quality in dental unit water lines. Ann Agric Environ Med. 2003;10:1–4. [PubMed] [Google Scholar]


