Abstract
Background:
The association of Epstein-Barr virus (EBV) with Burkitt's lymphoma (BL) is variable in different geographic regions. In developing countries, the association of EBV with BL is regarded to be of an endemic-type in equatorial Africa (> 95%) and sporadic-type in the developed countries (15-30%). The purpose of this study is to assess the frequency of EBV infection in BL, in Iran. The study also aims to compare Ribonucleic acid (RNA) in situ hybridization (RISH), the standard diagnostic method, with the polymerase chain reaction (PCR)-based method for diagnosing BL.
Materials and Methods:
In this epidemiological study, the paraffinized specimens of 18 cases of BL were selected. Next, the ISH of EBV-encoded RNA (EBER-RISH) and PCR assays that were based on Epstein Barr Nuclear Antigen 2 (EBNA2) amplification were used. The EBV strain was determined by PCR. The data were analyzed using the SPSS10 software and by performing Pearson correlation coefficient formula at a significant level of 0.05.
Results:
EBV RNA was detected in 50% of the BL specimens. Type 1 and 2 accounted for 70 and 30% of the cases, respectively. Regarding RISH as the standard method for EBV diagnosis, the PCR assays showed a sensitivity and specificity of 100 and 88.9%, respectively.
Conclusion:
According to the obtained findings, the frequency of EBV in BL was 50% and PCR and RISH showed high concordance and sensitivity in EBV detection. Therefore, PCR can be used as a faster method for EBV detection in high-risk geographical regions.
Keywords: Burkitt's lymphoma, Epstein-Barr virus, in situ hybridization, polymerse chain reaction
INTRODUCTION
About half of the childhood cancers in equatorial Africa are due to Burkitt's lymphoma (BL)
(i.e., endemic or African BL), while it occurs at a much lower rate in the rest of the world (i.e., sporadic or American BL).[1]
The endemic type is associated with the Epstein-Barr virus (EBV) in almost all cases (DNA virus is present in more than 95% of the cases).[2,3] The association of BL with EBV is much less common in non-endemic areas. In the United States of America (USA), the association of BL with EBV is only 30%, while it is intermediate between endemic and sporadic types in developing countries,[4] ranging from 55% in Taiwan,[2] 80% in Pakistan,[5] 27% in China,[6] and 13% in Japan.[7] The BL-type associated with the acquired immunodeficiency syndrome has also been introduced, in which the virus genome is detected in approximately 30 to 50% of the patients.[8]
The EBV, a wide-spread human herpes virus, has a high affinity to B-cells.[9] The first exposure to the virus depends on the socioeconomic conditions and usually occurs in the first decade of life. In fact, more than 90% of the adults are infected with the virus. Initial infection with the virus leads to a persistent, latent, and asymptomatic infection, which remains in a limited amount in the memory B-cells.[10,11]
In recent years, the serological and molecular assessments have evidenced that EBV is associated with a number of malignancies.[12] Thus, a deep understanding of this relationship can be useful for clinical and therapeutic interventions.[13,14]
To identify EBV infection, the following methods are used: In situ hybridization (ISH), southern blotting, and polymerase chain reaction (PCR).[15] As a high amount of EBV-transcripts are expressed in the latently infected cells, the RNA-ISH (RISH) method is used as the standard method for diagnosing infected cells, to detect EBV-encoded RNA (EBER).[16,17] The PCR-based methods are also used to determine the strain of virus (1 or 2).[18]
In this research, we studied the prevalence of EBV in BL, in Iran, and also compared the sensitivity and specificity of PCR, as an easier and faster method for EBV detection, with RISH as the standard method.
MATERIALS AND METHODS
Case selection
In this epidemiological study, 18 cases of BL were studied. These cases had been diagnosed at Al-Zahra Hospital and the Dental School of Medical Sciences (Isfahan, Iran) from 2008 to 2013. The specimens were formalin-fixed, paraffin-embedded tissues. The cases were intra-abdominal biopsies (six cases), head and neck region biopsies, including jaws, brain, nose, scalp, submandibular region, and cervical lymph nodes (ten cases), and thorax and pelvis (one case each). Other clinical information including age and sex was also available.
To begin with, the histopathological diagnoses were reviewed by two independent pathologists, according to the Revised European–American Lymphoma Classification (REAL).[19]
Immunohistochemical staining, which was used as a criterion for entrance, was carried out in all the cases by the standard avidin-biotin-peroxidase technique. The antibody panel included CD20, CD10, and CD3 (DAKO, Carpintaria, CA).[20]
Finally, EBV detection was performed by the two methods, namely, RISH and PCR.
EBER-1 RNA in situ hybridization
To detect EBER-1, an EBV transcript expressed actively in latently infected cells, based on Zytovision (Bremerhaven, Germany) protocol, RISH with a biotinylated probe was done.[21,22]
Briefly, 5 μm tissue sections were prepared on poly-D-lysine-treated glass slides and incubated at 70°C for a day. Then, the sections were deparaffinized and 3% solution of H2O2 was used to block the endogenous peroxidase. Enzymatic digestion of the sections was done with protease, followed by dehydration in ethanol and drying at room temperature. The slides were incubated in pre-hybridization solution for 20 minutes at 95°C. After washing with phosphate buffer saline (PBS), an EBER-1 biotinylated probe was added and incubated overnight (Invitrogen, Germany). The slides were washed again with PBS and placed in the washing solution (PBS) for 10 minutes at 25°C. Next, the slides were placed in AG solution, after that in the DAB solution, and finally counterstained with hematoxylin and mounted with Entellan.
The cells that presented with dark brown to black nuclear staining were considered as infected. Positive control was a case previously diagnosed as an EBV-positive BL.
Polymerase chain reaction amplification
Sections of 5 μm were used to extract high molecular weight DNA from tissue samples by using the 5-Prime kit (5 prime, Hamburg, Germany). During sectioning the samples, care was taken to avoid cross-contamination between them. A spectrophotometer was used to check the amount and purity of the DNA.[23]
EBNA2 primers have been described previously.[24] In this study, two primers were used for EBV detection and typing [Table 1].
Table 1.
Primers used for EBNA2 pcr typing

Briefly, the reaction mixture consisted of 10 mM TRIS-HCl (PH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM of the dNTp mix, gelatin 0.001%, 0.3 μM of each primer, 1 U of Taq DNA polymerase, and 1 μg of the DNA template or 5 μl of 1:10 diluted lysate.
The reaction tubes were placed in a thermo cycler (Applied Biosystem, Germany) and 35 cycles of amplification (30 seconds at 96°C, 30 seconds at 60°C, and one minute at 72°C) were conducted. After the last cycle, the process was followed by an extension step for 10 minutes. The amplified DNA was analyzed by an Applied Biosystem.
After the data were collected, the Pearson correlation coefficient was used in the SPSS10 software (SPSS Inc., Chicago, IL, USA) to analyze the data. The significance level was determined to be 0.05.
RESULTS
Patient characteristics
The clinical data of the BL cases are concisely presented. There were 13 males and five females, aged between two and 81 years, with a mean of 21 years.
Regarding the site of tumor involvement, the head and neck region was the most frequent (ten cases) followed by intra-abdominal organs (six cases), and two cases of thorax and pelvis.
Immunohistochemical findings
B-cell origin was documented by expressing CD10 and CD20 in all the cases of BL.
RISH and PCR assays
All samples were evaluated by RISH and PCR. The findings are summarized in Table 2. EBV RNA was detected in nine out of 18 BL cases by RISH [Figure 1] and 10 out of 18 cases by PCR. The discordant result was one PCR positive/ISH negative. As ISH is considered the standard test for EBV detection, 100% sensitivity and 88.9% specificity were obtained for the PCR assay. Type 1 EBV was identified in six out of nine cases (70%), while type 2 was found in three samples (30%).
Table 2.
Detection of ebv by RISH and PCR
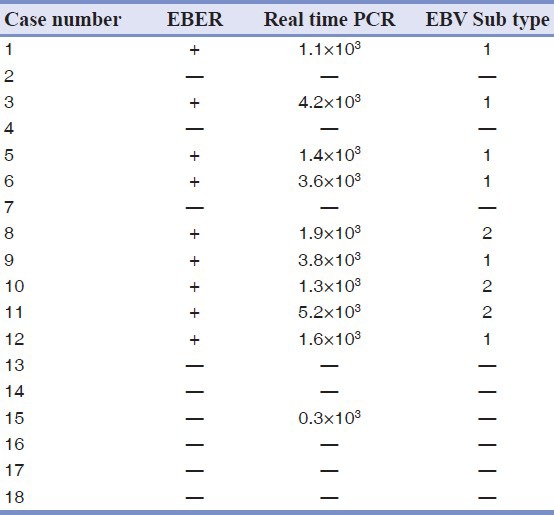
Figure 1.
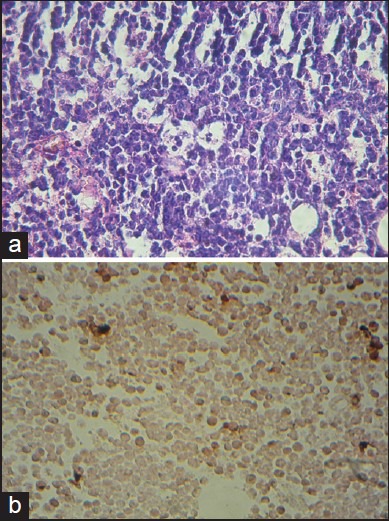
Burkitt's lymphoma. (a) Histological section (H and E, ×400) showing diffuse infiltration of medium-sized, non-cleaved lymphoid cells, with interspersed macrophages presenting a ‘starry-sky’ appearance. (b) Dark brown RNA in situ hybridization signals by EBV-encoded RNA (400×)
DISCUSSION
The incidence rate of BL shows considerable geographic variations. In equatorial Africa, the annual incidence is between five and ten per 100,000 children younger than 15 years old, whereas, in USA it occurs at a rate of about two per million persons.[4,25] Patients with acquired immunodeficiency syndrome (AIDS) are at higher risk of developing BL. The exact reasons for these differences are unclear, although environmental (e.g., malarial infection) and genetic factors, as also immunosuppression may play a role.[6]
In Iran, BL is the second most common high grade B-cell lymphoma.[26] In a study conducted in Fars province (in Southern part of Iran), 10.8% of the lymphoid malignancies in children under 15 years were BL.[27] In another study in Mashhad (in Northeast of Iran), BL accounted for 0.5% of all lymphoid malignancies in adults.[28]
Jaw lesions are the most characteristic manifestations of the disease and are commonly seen in 50 to 70% of the patients with endemic BL, while in the sporadic type, the most common site of involvement is the abdomen.[5] In this study, the major site of involvement has been the head and neck region (55.5% of the cases) followed by intra-abdominal organs (11.1% of the cases).
Among other differences between endemic and sporadic types, the mean age at the time of diagnosis is noticeable. In the endemic type in Africa, it is 6.09, while for the sporadic type it is 19.2 years.[1] In this study, the patients’ age range was 2-81 years, with a mean of 21 years.
One of the most obvious differences between endemic and sporadic types is their different associations with EVB in various geographical regions.[29] In tropical Africa, EBV is present in almost all the cases, whereas, in the sporadic form, in developed countries, it turns up in 15 to 30% of the samples. Also, in developing countries, the association is intermediate between endemic and sporadic types.[9]
Although the precise role of EBV in the BL pathogenesis is unclear even after several decades, it seems EBV has an initiator role and malaria has a promoter role in tumor development in Africa.[1]
Today, in EBV-positive lymphomas, EBV is considered as a therapeutic target in the new therapeutic interventions. The focus on this issue has resulted in the improvement of EBV detection methods.[9]
According to the studies conducted in Asian countries, the prevalence of EBV in BL was reported to be 80% in Pakistan,[5] 55% in Taiwan,[2] 27% in China,[6] and 13% in Japan.[7]
The standard diagnostic method to assay EBV-infection in tumor cells is ISH, which detects EBER. PCR-based methods are applied to determine the virus subtype. Although due to its simplicity, PCR is regarded as the first-line method for virus detection, its high sensitivity may cause false-positive results. This high sensitivity can be accounted for by the fact that memory cells and non-tumor bystander lymphocytes may also be traced.[10,11,17]
In this regard, Klumb et al. observed a high concordance (more than 90%) between EBV detected by ISH and by PCR in the BL samples. They also introduced PCR as a suitable method for diagnosing EBV infection in the pathological samples of non-Hodgkin B-cell lymphoma.[4] Hassan et al. also found high sensitivity and concordance (more than 96%) between ISH and PCR procedures in tracing EBV in BL samples. They further suggested that EBV detection could be done with PCR as a more rapid and simpler method, followed by RISH for confirmation.[9]
In the present study, by regarding ISH as the standard test for EBV diagnosis, an incidence rate of 50% (nine of 18 samples) was found, which is higher than that in the developed countries and is intermediate between the endemic and sporadic types.
The comparison of EBV detection through PCR and RISH procedures indicated high sensitivity (100%) and specificity (88.9%) for PCR assessment. Furthermore, a correlation coefficient of 88.9% (PV < 001) was achieved between PCR and RISH.
The results of this study and previous studies can validate that the PCR assay detects EBV in BL samples. The PCR method can have an important role in diagnosing EVB in lymphoma samples, because in those care centers that serve large populations, ISH is conducted as the second or third method. Note that ISH is used only after histopathological and immunohistochemical diagnosis. The financial cost of ISH in low-income communities is also a limiting factor. Therefore, PCR can be performed as a first-line procedure, followed by ISH for confirmation.[4,9,17]
Previous studies have identified the subtype 2 virus as the predominant type in Africa and subtype 1 as the predominant one in other regions.[30,31,32] However, in Egypt, Anwar et al. has observed that subtype 1 virus is predominant on the contrary to the endemic type. This difference could be due to such environmental factors as malaria and AIDS, which are predisposing risk factors in BL, but observed less commonly in Egypt than in other parts of Africa.[3]
In this study, determining the virus subtype by PCR, revealed sub type 1 in 70% and subtype 2 in 30% of the samples. This is consistent with the previous studies. Accordingly, we can conclude that BL in Iran has a lower association with environmental risk factors such as malaria and AIDS than Africa.
CONCLUSION
The current study shows that both the PCR and ISH methods have high concordance and sensitivity in EBV detection. Therefore, it is suggested that PCR can be used for EBV diagnosis, followed by RISH confirmation.
Identifying the subtype 1 virus as the dominant type in 70% of the samples, being similar to the sporadic type in Asian countries, probably confirms that the environmental predisposing risk factors such as malaria and AIDS have a less significant role in BL pathogenesis in Iran.
ACKNOWLEDGMENTS
This article was based on a thesis submitted to the School of Dentistry, Isfahan University of Medical Sciences, in partial fulfillment of the requirements for an MSc degree in Oral and Maxillofacial Pathology. This study was supported by the Isfahan University of Medical Sciences Grant # 391154. The authors also thank Miss Mahmoodi for preparing the manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Shapira J, Peylan-Ramu N. Burkitt's lymphoma. Oral Oncol. 1998;34:15–23. doi: 10.1016/s1368-8375(97)00041-9. [DOI] [PubMed] [Google Scholar]
- 2.Chao TY, Wang TY, Lee WH. Association between Epstein-Barr virus and Burkitt's lymphoma in Taiwan. Cancer. 1997;80:121–8. doi: 10.1002/(sici)1097-0142(19970701)80:1<121::aid-cncr16>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Anwar N, Kingma DW, Bloch AR, Mourad M, Raffeld M, Franklin J, et al. The investigation of Epstein-Barr viral sequence in 41 cases of Burkitt's lymphoma from Egypt. Cancer. 1995;76:1245–52. doi: 10.1002/1097-0142(19951001)76:7<1245::aid-cncr2820760723>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Klumb CE, Hassan R, Oliveria DE, De Resende LM, Carrico MK, Dobbin JD, et al. Geographic Variation in Epstein-Barr virus-associated Burkitt's lymphoma in children from Brazil. Int J Cancer. 2004;108:66–70. doi: 10.1002/ijc.11443. [DOI] [PubMed] [Google Scholar]
- 5.Mansoor A, Stevenson S, Li RZ, Frekko K, Weiss W, Ahmad M. Prevalence of Epstein-Barr viral sequences and EBV LMP1 oncogen deletions in Burkitt's lymphoma from Pakistan: Epidemiological correlations. Hum Pathol. 1997;28:283–8. doi: 10.1016/s0046-8177(97)90125-8. [DOI] [PubMed] [Google Scholar]
- 6.Chan JK, Tsang WY, NG CS, Wong CS, Lo ES. A study of the association of Epstein-Barr virus with Burkitt's lymphoma occuring in a Chinese population. Histopathology. 1995;26:239–45. doi: 10.1111/j.1365-2559.1995.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 7.Miyoshi I. Burkitt's lymphoma in Japan. IARC Sci Publ. 1985;60:135–47. [PubMed] [Google Scholar]
- 8.Hamilton-Dutoit SJ, Rea D, Raphael M, Sandvej K, Delecluse HJ, Gisselbrecht C, et al. Epstein-Barr virus-latent gene expression and tumor cell phenotype in acquired immunodeficiency syndrome-related non-Hodgkin's lymphoma. Correlation of lymphoma phenotype with three distinct patterns of viral latency. Am J Pathol. 1993;143:1072–85. [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan R, White LR, Stefanoff CG, Oliveria DE, Felisbino FE, klumb CE, et al. Epstein- Barr Virus (EBV) detection and typing by PCR: A contribution to diagnostic screening of EBV-positive Burkitt's lymphoma. Diagn Pathol. 2006;1:17. doi: 10.1186/1746-1596-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tierney RJ, Steven N, Young LS, Rickinson AB. Epstein-Barr virus latency in blood mononuclear cells: Analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–85. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decker LL, Klaman LD, Thorley-Lawson DA. Detection of the latent form of Epstein-Barr virus DNA in peripheral blood of healthy individuals. J Virol. 1996;70:3286–99. doi: 10.1128/jvi.70.5.3286-3289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu JL, Glaser SL. Epstein-Barr virus-associated malignancies: Epidemiologic patterns and etiologic implications. Crit Rev Oncol Hematol. 2000;34:27–53. doi: 10.1016/s1040-8428(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 13.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–68. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 14.Rao CR, Guttierrez M, Bhatia K, Fend F, Franklin J, Appaji L, et al. Association of Burkitt's lymphoma with the Epstein-Barr virus in two developing countries. Leuk Lymphoma. 2000;39:329–37. doi: 10.3109/10428190009065832. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchiya S. Diagnosis of Epstein-Barr virus-associated diseases. Crit Rev Oncol Hematol. 2002;44:227–38. doi: 10.1016/s1040-8428(02)00114-2. [DOI] [PubMed] [Google Scholar]
- 16.Ambinder RF, Mann RB. Detection and characterization of Epstein-Barr virus in clinical specimens. Am J Pathol. 1994;145:239–52. [PMC free article] [PubMed] [Google Scholar]
- 17.Magrath IT, Sareban E. Clinical features of Burkitt's lymphoma in the U. S. A. In: Lenoir GM, O’Conor GE, Olweny CL, editors. Burkitt's lymphoma: A human cancer model. IARC Scientific Pub. No.60. Lyon: International Agency for Research on Cancer; 1985. pp. 119–27. [Google Scholar]
- 18.Araujo L, Foss HD, Bittencourt A, Hummel M, Demel G, Mendonca N, et al. Expression of Epstein-Barr virus-gene products in Burkitt's lymphoma in Northeast Brazil. Blood. 1996;87:5279–86. [PubMed] [Google Scholar]
- 19.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European–American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–92. [PubMed] [Google Scholar]
- 20.Jannings CD, Foon KA. Recent advances in flow cytometry: Application to the diagnosis of hematologic malignancies. Blood. 1997;90:2863–92. [PubMed] [Google Scholar]
- 21.Glickman JN, Howe JG, Steiz JA. Structural analyses of EBER1 and EBER2 ribonucleoprotein particles present in Epstein-Barr virus infected cells. J Virol. 1988;62:902–11. doi: 10.1128/jvi.62.3.902-911.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss LM, Chen YY, Liu XF, Shibata D. Epstein-Barr virus and Hodgkin's disease. A correlative in situ hybridization and polymerase chain reaction study. Am J Pathol. 1991;139:1259–65. [PMC free article] [PubMed] [Google Scholar]
- 23.Stefanoff CG, Hassan R, Gonzalez AC, Andrade LA, Tabak D, Romano S, et al. Laboratory strategy for efficient handling of paraffin-embedded tissues for molecular detection of clonality in non-Hodgkin lymphomas. Diagn Mol Pathol. 2003;12:79–87. doi: 10.1097/00019606-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 24.van Baarle D, Hovenkamp E, Kersten MJ, Klein MR, Miedema F, van Oers MH. Direct Epstein-Barr virus (EBV) typing on peripheral Blood mononuclear cells: No association between EBV type 2 infection or superinfection and the development of acquired immunodeficiency syndrome–related non-Hodgkin's lymphoma. Blood. 1999;93:3949–55. [PubMed] [Google Scholar]
- 25.Rabkin CS, Ward MH, Maans A, Blattner WA. Epidemiology of non-Hodgkin's lymphomas. In: Magrath I, editor. The non-Hodgkin's lymphoma. 2nd ed. London: Edward Arnold; 1997. pp. 171–86. [Google Scholar]
- 26.Hashemi M, Sarafzadeh AR. Epstein-Barr virus and Burkitt's lymphoma. Pejouhandeh. 2003;35:339–41. [Google Scholar]
- 27.Karimi M, Mehrabani D, Yarmohammadi H, Jahromi FS. The prevalence of signs and symptoms of childhood leukemia and lymphoma in Fars Province, Southern Iran. Cancer Detect Prev. 2008;32:178–83. doi: 10.1016/j.cdp.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Mozaheb Z, Aledavood A, Farzad F. Distributions of major sub-types of lymphoid malignancies among adults in Mashhad, Iran. Cancer Epidemiol. 2011;35:26–9. doi: 10.1016/j.canep.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Hsu JL, Glaser SL. Epstein-Barr virus-associated malignancies: Epidemiologic patterns and etiologic implications. Crit Rev Oncol Hematol. 2000;34:27–53. doi: 10.1016/s1040-8428(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 30.Cardy AH, Sharp L, Little J. Burkitt's lymphoma: A review of the epidemiology. Kuwait Med J. 2001;33:293–306. [Google Scholar]
- 31.Biggar RJ, Henle W, Fleisher G, Böocker J, Lennette ET, Henle G. Primary Epstein-Barr virus infections in African infants. I. Decline of maternal antibodies and time of infection. Int J Cancer. 1978;22:329–43. doi: 10.1002/ijc.2910220304. [DOI] [PubMed] [Google Scholar]
- 32.Tao Q, Robertson KD, Manns A, Hildeshein A, Ambinder RF. Epstein-Barr virus (EBV) in endemic Burkitt's lymphoma: Molecular analysis of primary tumor tissue. Blood. 1998;91:1373–81. [PubMed] [Google Scholar]


