Abstract
Pyogenic granuloma (PG) is a well-known benign non-neoplastic overgrowth. It is a response to mild irritation and might be related to hormonal factors and certain kinds of drugs. PG preferentially affects the gingiva, but can be found extragingivally with varying clinical features. The most common treatment is surgical excision. This article describes a case of extragingival PG occurring on the tongue with unusual presentation, with emphasis on non-surgical treatment. Since surgical management had not been successful, an alternative approach was taken. We illustrated how the lesion was successfully treated with a series of intralesional corticosteroid injections.
Keywords: Drug-induced, pregnancy tumor, pyogenic granuloma
INTRODUCTION
Pyogenic granuloma (PG) is a common benign non-neoplastic mucocutaneous lesion.[1] It is usually defined as a reactive tumor like overgrowth in oral cavity following irritation or minor trauma.[1,2] Other factors which are considered etiological are hormonal factors and certain kinds of drugs.[3,4] Actually, some drugs such as cyclosporine have an important role in the genesis of PG. In some articles, four cases of oral PG were reported in chronic graft-versus-host diseases in patients who were receiving cyclosporine.[5,6]
Clinically, PG is an exophytic lesion with a smooth or lobulated surface that is usually hemorrhagic. It may develop as a small, erythematous papule or nodule.[7,8,9] The size varies in diameter from a few millimeters to several centimeters.[1,2] Rarely does PG exceed 2.5 cm in size and it usually reaches its full size within weeks or months, and remains indefinitely thereafter.[10] Clinical development of the lesion is slow, asymptomatic, and painless, but it may also grow rapidly.[1,2,11] The surface is characteristically ulcerated and friable which may be covered by a yellow fibrinous membrane. Its color ranges from pink to red to purple, depending on the age of the lesion.[2,12] Young PGs are highly vascular in appearance.[1] PG in oral cavity is known to involve gingiva commonly, accounting for 75% of all cases although extragingival lesions on the lips, tongue, oral mucosa, and palate have been also reported.[13,14,15]
PG may occur at all ages, but a higher frequency of PG is observed in the second decade of life in young adults, especially among women, possibly because of the vascular effects of female hormones.[1,2,8,15]
Microscopically, PG is characterized by highly vascular proliferation amidst granulation tissue and chronic inflammatory cell infiltrate.[1,2] The blood vessels often show a clustered or medullary pattern separated by less vascular fibrotic septa.[10]
When a mass is found in oral cavity, it is important to formulate a differential diagnosis since this would help further evaluation of the condition and management of the patient.[16] In view of its clinical characteristics, the differential diagnosis of PG includes peripheral giant cell granuloma, peripheral ossifying fibroma, metastases from malignant tumors, hemangioma, inflammatory hyperplasia, Kaposi's sarcoma, angiosarcoma, and non-Hodgkin's lymphoma. Although metastatic tumors to oral region are uncommon, attached gingiva is the most commonly affected soft tissue followed by tongue. The clinical appearance of metastatic oral lesion in most cases resemble hyperplastic or reactive lesions such as PG. Kaposi's sarcoma can mimic a number of intraoral lesions such as PG. Non-Hodgking's lymphoma is usually found to be an asymptomatic gingival enlargement or mass resembling a PG.[17,18,19,20] The established diagnosis depends on biopsy.[1,2]
Although many treatment techniques have been described for PG, when it is large or occurs in a surgically difficult area, choosing an appropriate treatment modality can be difficult.[21] Surgical excision is the most common treatment. However, this may result in scars. When the procedure would produce marked deformity, incisional biopsy is mandatory.[12,14,22] Recently, the use of more conservative treatments, such as cryosurgery,[23,24] laser surgery, injection of absolute ethanol, and sodium tetradecyl sulfate (STS) sclerotherapy have been proposed.[22,25,26,27] Other investigators also used a series of intralesional corticosteroid injections for treatment of PG, especially for highly recurrent lesions. In spite of these treatments, recurrence is not infrequent. After excision, recurrence occurs in up to 16% of cases.[28] It should be emphasized that gingival cases show a much higher recurrence rate than lesions on other oral mucosal sites.[29]
This article describes a case of extragingival PG occurring on the tongue with unusual presentation, with emphasis on the diagnosis and treatment.
CASE REPORT
A 45-year-old man was referred to the department of Oral Medicine of the Isfahan University of Medical Sciences, Isfahan, Iran. He was complaining of exophytic painless mass in dorsal surface of his tongue. He expressed that the lesion had appeared 2 years ago and lasting ever since. The mass had negligible growth rate when he first noticed, but had grown rapidly over the past 2 months to attain the present size. The patient's chief complaint was his fear of speaking because it might make the relatives shocked and wonder and also his phobia of transmission to others or whether it might have a cancerous nature.
Medical history of the patient showed history of epilepsy begining at age 10, taking anti-seizure medications since childhood, and his dental history showed multiple chronic oral ulcers diagnosed as drug-induced lichenoid reaction which were presumed to be related to anti-seizure medications. The list of medications included phenytoin sodium (300 mg/d) and carbamazepine (600 mg/d). It seemed that the patient was of a low socioeconomic status and had not followed his treatment and was taking his medicaments irregularly. Evaluating his laboratory tests revealed anemia, leucopenia, transient thrombocytopenia, folic acid deficiency, and increased liver enzymes. In the study of peripheral blood smear and bone marrow aspiration, mild relative erythroid hyperplasia was diagnosed for him. Symptoms of hirsutism due to phenytoin were evident in his head and face. Despite of long-term phenytoin consumption, there was no gingival hyperplasia.
Clinical examination revealed a reddish-yellow exophytic, pedunculated mass which centered on the dorsal surface of tongue, measuring 4 × 3 × 1 cm3 in size. The lesion was lobulated with a smooth surface. The base of the lesion was so tightly stuck to the bed that the interface was always slightly bloody [Figure 1]. Neither any traumatic factor in the oral cavity, nor palpable cervical lymph nodes were detected. The diagnostic hypotheses were hemangioma, angiosarcoma, inflammatory hyperplasia, and PG.
Figure 1.
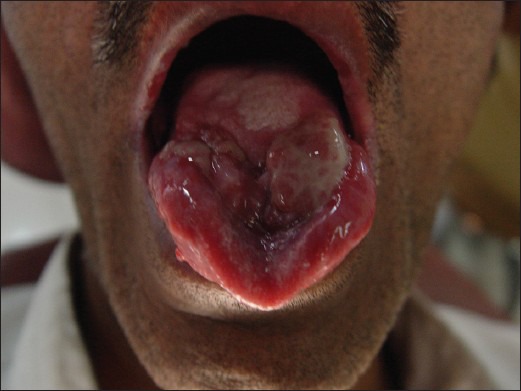
The pyogenic granuloma over the dorsal surface of the tongue
An excisional biopsy of one of the masses was taken. Hematoxylin-eosin stained section showed [Figure 2] hyperplastic stratified squamous parakeratinized epithelium covered with a serofibrinous membrane. The underlying fibrovascular stroma showed a large number of budding capillaries, plump fibroblasts, and areas of extravasated blood and a dense acute and chronic inflammatory cell infiltrate. These findings were consistent with a histopathological diagnosis of PG.
Figure 2.
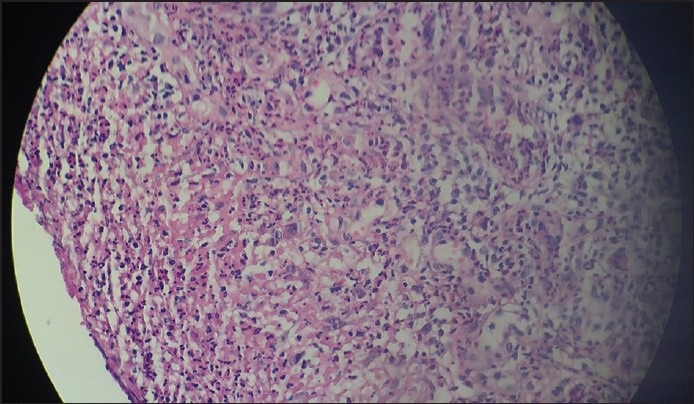
Hematoxylin-eosin stained section of the lesion
Although the lesion had been removed excisionally, it relapsed in 3 months. At first, we referred the patient to a hematologist for evaluating the problems of his medical history. According to the hematologist, his main blood problems and increased hepatic enzymes were side effects of his medications. We searched for the side effects of his medications and found PG as a possible carbamazepine side effect in very rare cases.[30,31] Then, he was referred to a neurologist for changing of his medications. Finally, Gabapentine took the place of carbamazepine.
Regarding the recurrence of the lesion, we preferred intralesional injection of corticosteroids. A solution was prepared by diluting 0.1 ml of triamcinolone 40 mg/ml with 0.5 ml of 0.5% lidocaine, and 0.1 ml of the mixture was injected into the lesion. The injections were given weekly for 16 weeks. Local antifungal treatment was prescribed simultaneously to prevent fungal superinfection, Significant improvement of the lesion at each visit was noted. At 20 week follow up, the lesion was 90% resolved, with some residual erythema and swelling [Figure 3]. The residual lesion was removed by cryosurgery. The patient was monitored for a year and no relapse was noted [Figure 4].
Figure 3.
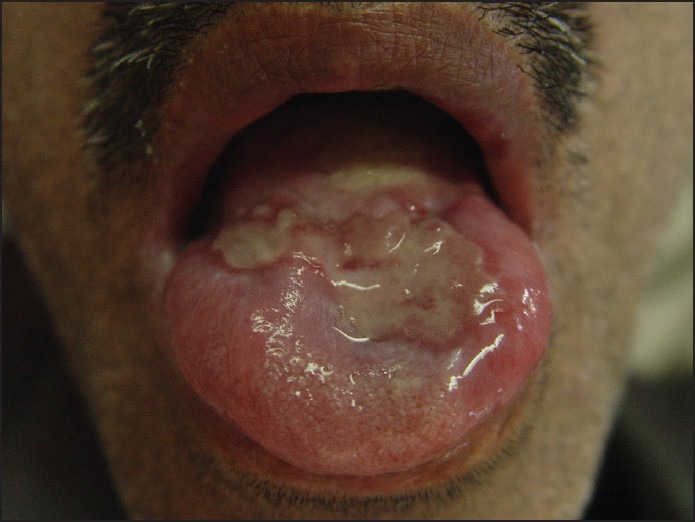
Primary after treatment
Figure 4.
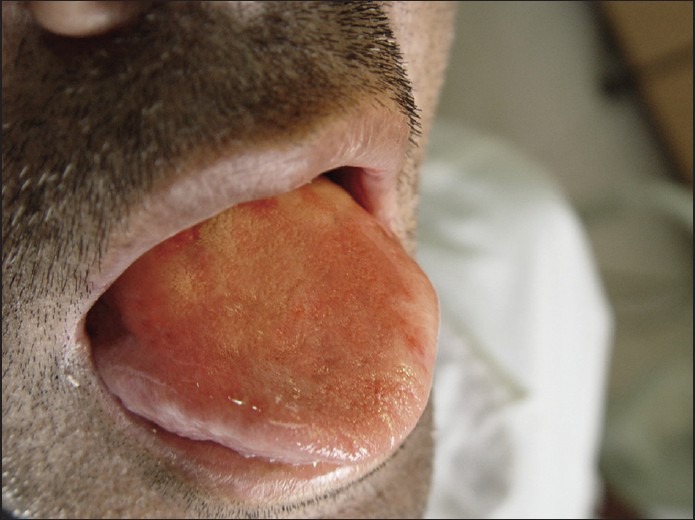
One year after treatment
DISCUSSION
Although PG is a non-neoplastic growth in oral cavity, proper diagnosis, prevention, management, and treatment of the lesion are very important. Poncet and Dor in 1897 first described PG.[7,32] PG of oral cavity is known to usually involve the gingiva, accounting for 75% of all cases, but its occurrence on tongue seems to be relatively rare.[1,13,22] The size of the lesion usually ranges between 0.5 and 2 cm,[33] but in this case the lesion was much larger.
Such atypical presentation can be blended with features of various conditions. Thus, the clinical diagnosis of an extragingival PG can be challenging. Biopsy must be taken from all clinically suspected PGs to minimize the risk of misdiagnosis. The histopathological picture of the extragingival PG is quite similar to gingival or any part of the body.
Certain kinds of medications are considered to be the etiology of PG. Some medications such as cyclosporine have an important role in the genesis of PG. Bachymer et al.[5] and Lee et al.[6] reported four cases of oral PG in chronic graft-versus-host disease in patients who were receiving cyclosporine. Here, the patient was taking carbamazepine and phenytoin because of seizure for 35 years. Carbamazepine (CBZ) is an anticonvulsant medication used in the treatment of epilepsy which is FDA-approved. In a study of PG among patients, who were receiving carbamazepine up to Apr, 18, 2012, 11,798 individuals reported to have side effects. Among them, two patients (0.02%) had PG. All subjects who had PG like our patient were male and the chief condition they were involved was convulsion.[30] A physician from Canada in 2003 reported PG as a possible carbamazepine side effect in a 15-year-old boy.[31]
This patient showed many side effects of carbamazepine and phenytoin including: anemia, leucopenia, transient thrombocytopenia, folic acid deficiency, increased liver enzymes, and hirsutism. According to the hematologist, his main blood problems and increased hepatic enzymes were side effects of his medications. Both carbamazepine and phenytoin lead to lichenoid reactions (DILRs) and these medications were the cause of the ulcers in his mouth.[12] Another interesting point was that despite of taking phenytoin for many years, there was no evidence of gingival hyperplasia. Phenytoin-induced gingival enlargement affects approximately 50% of patients who receive the drug for longer than 3 months.[12]
We cannot certainly claim that the lesion was due to long-term carbamazepine intake. But considering no relapse after replacing carbamazepine by Gabapentine reinforces the hypothesis in this case that PG may be associated with long-term carbamazepine intake.
There are numerous treatment plans for PGs and the conservative surgical excision is the most chosen technique which is usually curative. Although these are reactive hyperplasias, they have a relatively high rate of recurrence after surgical removing alone, especially in pregnant patients. A recurrence rate of 15% has been reported.[34] Recurrences after surgical removal of extragingival PGs is however uncommon.[34] In the present case, the lesion had relapsed after surgery (excisional biopsy), so we choose the intra lesional injection. Parisi et al. used a series of intralesional corticosteroid injections for treatment of PG, particularly for highly recurrent lesions.[11] We believed the lesion would respond to the anti-inflammatory and vasoconstrictive actions of the corticosteroids, and might prevent or suppress the release of angiogenic factors. Intralesional steroid injections have been used to treat many oral mucosal diseases, specifically vesiculobullous lesions. It is important to inject the exact appropriate amount of steroid to prevent tissue necrosis. With proper indications and careful injection, this method is able to be an alternative treatment modality for PG, particularly for highly recurrent lesions. The present case was monitored for 1 year after complementary excision. The wound healing was complete and no recurrence was noted.
CONCLUSION
Oral PGs are well documented in the literature. However, their occurrence in extragingival sites, in head and neck regions, is rare and often has atypical presentation. Eyes cannot see what the mind does not know! It is true when a clinician is not cognizant of the possibility of occurrence of this reactive lesion on an unusual site may misdiagnose as a more serious disease. This can be easily overcome by histopathological diagnosis which confirms its innocuous nature.
This report emphasizes the complexity of the diagnosis of oral lesions and leads the dentist to consider different diagnostic methods for distinct lesions. We call attention to the uncommon mucocutaneous labial PG and to the fact that excisional biopsy is the safest method for diagnosis and treatment of PG on the lip, even when involving the mucosa and skin.
To minimize the risk of misdiagnosis, biopsy should be taken to confirm the diagnosis. PG may be confused with various benign and malignant conditions. Intralesional steroid injections have been used to treat many oral mucosal diseases, specifically vesiculobullous lesions. It is important to inject the appropriate amount of steroid to prevent tissue necrosis. With proper case selection and careful injection, this method can be an alternative treatment modality for PG, particularly for highly recurrent lesions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Neville BW, Damm DD, Allen CM, Bouquot JE. 2nd ed. Philadelphia: WB Saunders; 2002. Oral and maxillofacial pathology; pp. 437–95. [Google Scholar]
- 2.Regezi JA, Sciubba JJ, Jordan RCK. 4th ed. Philadelphia: WB Saunders; 2003. Oral pathology: Clinical pathologic considerations; pp. 115–6. [Google Scholar]
- 3.Mussalli NG, Hopps RM, Johnson NW. Oral pyogenic granuloma as a complication of pregnancy and the use of hormonal contraceptives. Int J Gynaecol Obstet. 1976;14:187–91. doi: 10.1002/j.1879-3479.1976.tb00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller RA, Ross JB, Martin J. Multiple granulation tissue lesions occurring in isotretinoin treatment of acne vulgaris – successful response to topical corticosteroid therapy. J Am Acad Dermatol. 1985;12:888–9. doi: 10.1016/s0190-9622(85)80126-2. [DOI] [PubMed] [Google Scholar]
- 5.Bachmeyer C, Devergie A, Mansouri S, Dubertret L, Aractingi S. Pyogenic granuloma of the tongue in chronic graft versus host disease. Ann Dermatol Venereol. 1996;123:552–4. [PubMed] [Google Scholar]
- 6.Lee L, Miller PA, Maxymiw WG, Messner HA, Rotstein LE. Intraoral pyogenic granuloma after allogeneic bone marrow transplant. Report of three cases. Oral Surg Oral Med Oral Pathol. 1994;78:607–10. doi: 10.1016/0030-4220(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 7.Graham RM. Pyogenic granuloma: An unusual presentation. Dent Update. 1996;23:240–1. [PubMed] [Google Scholar]
- 8.Eversole LR. 3rd ed. Hamilton: BC Decker; 2002. Clinical outline of oral pathology: Diagnosis and treatment; p. 113. [Google Scholar]
- 9.Wood NK, Goaz PW. 5th ed. Missouri: Mosby; 1996. Differential diagnosis of oral and maxillofacial lesions; pp. 549–50. [Google Scholar]
- 10.Bouquot JE, Nikai H. Lesions of the oral cavity. In: Gnepp DR, editor. Diagnostic surgical pathology of the head and neck. 3rd ed. Philadelphia: WB Saunders; 2002. pp. 141–233. [Google Scholar]
- 11.Parisi E, Glick PH, Glick M. Recurrent intraoral pyogenic granuloma with satellitosis treated with corticosteroids. Oral Dis. 2006;12:70–2. doi: 10.1111/j.1601-0825.2005.01158.x. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg MS, Glick M. 10th ed. Vol. 141. Hamilton: BC Decker; 2003. Burket's oral medicine: Diagnosis and treatment; p. 2. [Google Scholar]
- 13.Laskaris G. 4th ed. New York: Thieme Publishers; 1997. Color atlas of oral diseases; pp. 400–1. [Google Scholar]
- 14.Shafer WG, Hine MK, Levy BM. 4th ed. Philadelphia: WB Saunders; 1983. A textbook of Oral Pathology; pp. 359–60. [Google Scholar]
- 15.Lin RL, Janniger CK. Pyogenic granuloma. Cutis. 2004;74:229–33. [PubMed] [Google Scholar]
- 16.Willies-Jacobo LJ, Isaacs H, Jr, Stein MT. Pyogenic granuloma presenting as a congenital epulis. Arch Pediatr Adolesc Med. 2000;154:603–5. doi: 10.1001/archpedi.154.6.603. [DOI] [PubMed] [Google Scholar]
- 17.Enzinger FM, Weiss SW. 3rd ed. St Louis: Mosby; 1995. Soft tissue tumors; p. 600. [Google Scholar]
- 18.Calonje E, Wilson-Jones E. Vascular tumors: Tumors and tumor-like conditions of blood vessels and lymphatics. In: Elder D, Elenitsas R, Jaworsky C, Johnson B Jr, editors. Lever's histopathology of the skin. Philadelphia: Lippincott-Raven; 1997. p. 895. [Google Scholar]
- 19.Raut A, Huryn J, Pollack A, Zlotolow I. Unusual gingival presentation of post-transplantation lymphoproliferative disorder: A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:436–41. doi: 10.1067/moe.2000.107446. [DOI] [PubMed] [Google Scholar]
- 20.Jafarzadeh H, Sanatkhani M, Mohtasham N. Oral pyogenic granuloma: A review. J Oral Sci. 2006;48:167–75. doi: 10.2334/josnusd.48.167. [DOI] [PubMed] [Google Scholar]
- 21.Sills ES, Zegarelli DJ, Hoschander MM, Strider WE. Clinical diagnosis and management of hormonally responsive oral pregnancy tumor (pyogenic granuloma) J Reprod Med. 1996;41:467–70. [PubMed] [Google Scholar]
- 22.Ichimiya M, Yoshikawa Y, Hamamoto Y, Muto M. Successful treatment of pyogenic granuloma with injection of absolute ethanol. J Dermatol. 2004;31:342–4. doi: 10.1111/j.1346-8138.2004.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 23.Ishida CE, Ramos-e-Silva M. Cryosurgery in oral lesions. Int J Dermatol. 1998;37:283–5. doi: 10.1046/j.1365-4362.1998.00426.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang SQ, Goldberg LH. Treatment of recurrent pyogenic granuloma with excision and frozen section for margin control. Dermatol Surg. 2008;34:1115–6. doi: 10.1111/j.1524-4725.2008.34222.x. [DOI] [PubMed] [Google Scholar]
- 25.Galeckas KJ, Uebelhoer NS. Successful treatment of pyogenic granuloma using a 1,064-nm laser followed by glycerin sclerotherapy. Dermatol Surg. 2009;35:530–4. doi: 10.1111/j.1524-4725.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- 26.Powell JL, Bailey CL, Coopland AT, Otis CN, Frank JL, Meyer I. Nd:YAG laser excision of a giant gingival pyogenic granuloma of pregnancy. Lasers Surg Med. 1994;14:178–83. doi: 10.1002/1096-9101(1994)14:2<178::aid-lsm1900140211>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Moon SE, Hwang EJ, Cho KH. Treatment of pyogenic granuloma by sodium tetradecyl sulfate sclerotherapy. Arch Dermatol. 2005;141:644–6. doi: 10.1001/archderm.141.5.644. [DOI] [PubMed] [Google Scholar]
- 28.Taira JW, Hill TL, Everett MA. Lobular capillary hemangioma (pyogenic granuloma) with satellitosis. J Am Acad Dermatol. 1992;27:297–300. doi: 10.1016/0190-9622(92)70184-h. [DOI] [PubMed] [Google Scholar]
- 29.Vilmann A, Vilmann P, Vilmann H. Pyogenic granuloma: Evaluation of oral conditions. Br J Oral Maxillofac Surg. 1986;24:376–82. doi: 10.1016/0266-4356(86)90023-9. [DOI] [PubMed] [Google Scholar]
- 30.Ehealthme carbamazepine side effect. [Last cited on 2012 Apr 18]. Available from: http://www.ehealthme.com/drug-interactions/carbamazepineside effect .
- 31.Palmero ML, Pope E. Eruptive pyogenic granulomas developing after drug hypersensitivity reaction. J Am Acad Dermatol. 2009;60:855–7. doi: 10.1016/j.jaad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Bjork K, Hoede N, Korting GW, Burgdorf WH, Young SK. Philadelphia: WB Saunders; 1996. Diseases of the oral mucosa and the lips; pp. 229–30. [Google Scholar]
- 33.Sapp JP, Eversole LR, Wysocki GP. 2nd ed. Missouri: Mosby; 2004. Contemporary oral and maxillofacial pathology; pp. 318–9. [Google Scholar]
- 34.Pierson JC, Pierson DM. Pyogenic Granuloma (Lobular Capillary Hemanigioma) [Last cited on 2005 Nov 12]. Available from: http://www.emedicine.com/derm/topic368.htm .


