Abstract
Transporter associated with antigen processing 1 (TAP1) I333V gene polymorphism has been suggested to be associated with type 1 diabetes mellitus (T1DM) susceptibility. However, the results from individual studies are inconsistent. To explore the association of TAP1 I333V gene polymorphisms with T1DM, a meta-analysis involving 2246 cases from 13 individual studies was conducted. The pooled odd ratios (ORs) and their corresponding 95% confidence intervals (95% CIs) were evaluated by a fixed-effect model. A significant relationship was observed between TAP1 I333V gene polymorphism and T1DM in allelic (OR: 1.35, 95% CI: 1.08–1.68, P = 0.007), dominant (OR: 1.462, 95% CI: 1.094–1.955, P = 0.010), homozygous (OR: 1.725, 95% CI: 1.082–2.752, P = 0.022), heterozygous (OR: 1.430, 95% CI: 1.048–1.951, P = 0.024) and additive (OR: 1.348, 95% CI: 1.084–1.676, P = 0.007) genetic models. No significant association between TAP1 I333V gene polymorphism and T1DM was detected in a recessive genetic model (OR: 1.384, 95% CI: 0.743–2.579, P = 0.306) in the entire population, especially among Caucasians. No significant association between them was found in an Asian or African population. TAP1 I333V gene polymorphism was significantly associated with increased T1DM risk. V allele carriers might be predisposed to T1DM susceptibility.
Keywords: Transporter associated with antigen processing 1, I333V, polymorphism, type 1 diabetes mellitus
Introduction
Diabetes mellitus (DM) is a common dysmetabolic syndrome that causes internal insulin insufficiency or insulin resistance, and contributes to the glucose and lipid metabolism disturbance led by the combined action of genetic and environmental factors. Type 1 DM (T1DM), also known as insulin-dependent DM (IDDM), is an organ-specific autoimmune disease characterized by selective destruction of the insulin-producing islet beta cells of the pancreas [1]. Type 1 diabetes mellitus patients account for 7–10% of DM patients. The clinical symptoms of T1DM are relatively serious, and harm the physical and psychological health of patients. The pathogenesis of T1DM is highly complex. The β cell autoantigens, cells from the autoimmune system (such as T cells, B cells, macrophagocytes and dendritic cells), and cytokines and radicals produced by these cells have a critical function in the progression of T1DM.
Human leucocyte antigen (HLA) region, also known as major histocompatibility complex (MHC) region, located in 6p21, are the major gene regions for T1DM. Approximately 60% of T1DM susceptibility is attributed to HLA gene, in which class II region is most strongly associated with T1DM. A total of 42% of T1DM susceptibility comes from this region. Transporter associated with antigen processing (TAP) gene is located in the class II region between DQB1 and DPB1. The TAP1 gene product is a transporter associated with the endogenous antigen processing and presentation. The TAP1 can transport peptides into the endoplasmic reticulum, where they combine with MHC I molecules. The MHC I molecules and antigen peptide compounds are subsequently expressed in the cytomembrane and recognized by the CD8+ T cell receptor, thereby stimulating a cytotoxic lymphocyte protective immune response [2]. In 1992, Van Kaer et al. found that MHC I molecules are significantly reduced and depleted of CD8+ T cells in transgenic mice with a disrupted TAP1 gene [3]. TAP1 gene deficiency and mutation might lead to the endogenous antigen processing and transportation barrier, which results in such autoimmune disease as T1DM. The 1207th base adenine (A) of the TAP1 gene is substituted by guanine (G), which results in the wild-type isoleucine (Ile, I) being replaced by valine (Val, V) at 333rd amino acid.
Although some studies on the relationship between TAP1 I333V gene polymorphism and T1DM exist, the results from individual studies remain controversial. In 1994, Cucca et al. reported that no primary association exists between TAP1 I333V gene polymorphism and IDDM in Italy [4]. Analogously, Nakanishi et al. found that no significant difference existed in the distribution of TAP1 I333V alleles between IDDM patients and normal controls, and they concluded that TAP1 I333V gene polymorphism did not exhibit a primary association with Japanese IDDM [5]. In contrast, Jackson et al. determined the relative risk of TAP1 I333V gene polymorphism and T1DM using single-stranded conformation polymorphism in the United States [6]. In 2004, Sheng et al. reported that TAP1 333V allele was the susceptibility allele for T1DM in a Chinese population [7].
In this study, a meta-analysis involving 1140 T1DM patients and 1108 controls was conducted to determine the relationship between TAP1 I333V gene polymorphism and T1DM.
Materials and methods
Publication search and inclusion criteria
The terms ‘Transporter associated with antigen processing 1’, ‘I333V’, ‘T1DM’ and ‘polymorphism’ were adopted to search the electronic databases of PubMed, Embase, Web of Science, China National Knowledge Infrastructure and China Biological Medicine Database. The last research was updated on 31 October 2013, and the included publication years ranged from 1992 to 2007.
The chosen studies had to be in accordance with the following criteria: (i) evaluation of the TAP1 I333V gene polymorphism and T1DM; (ii) T1DM diagnosis and classification criteria were presented by the American Diabetes Association and revised by the World Health Organization in 1997; (iii) case–control or cohort study published in an official journal; and (iv) the study should follow the Hardy–Weinberg equilibrium (HWE).
Data extraction
Data were abstracted according to a standard protocol. Studies that did not meet the inclusion criteria, were repeated publications, or provided deficient data were eliminated. If similar data appeared in different studies, the data were only used once. The abstracted data contained the following items: the first author*s name, publication year, region, number of genotypes, genotyping, study design, matching criteria, and total number of cases and controls.
Statistical analyses
Six genetic models, namely, the allelic (distribution of V allelic frequency of TAP1 I333V gene polymorphism), recessive (VV versus IV+II), dominant (IV+VV versus II), homozygous (VV versus II), heterozygous (IV versus II) and additive (V versus I) genetic models, were adopted. The relationship between TAP1 I333V gene polymorphism and T1DM was compared using odds ratio (OR) and its corresponding 95% confidence interval (CI). Chi-squared-based Q-test was used to calculate the heterogeneity between the individual studies, and significance was set at P < 0.05 [8]. If heterogeneity was observed among the individual studies, the pooled OR was estimated using a random-effect model (DerSimonian and Laird method) [9]. Otherwise, a fixed-effect model was used (Mantel-Haenszel method) [10]. The pooled OR was determined by Z-test, and significance was also set at P < 0.05.
Hardy–Weinberg equilibrium was assessed by Fisher*s exact test, and significance was set at P < 0.05. The funnel plot was used to estimate the potential publication bias. Egger*s linear regression test on the natural logarithm scale of the OR was used to assess funnel plot asymmetry, and significance was set at P < 0.05 [11]. STATA 11.0 software was used for statistical analyses (StataCorp, College Station, TX, USA).
Results
Studies and populations
A total of 25 studies were searched, among which 13 papers fulfilled the inclusion criteria. Data were abstracted from 1140 T1DM cases and 1106 controls (Table 1) [4–7,12–20]. The nine study regions were China, Italy, Denmark, France, Japan, United States, Germany, Senegal and Finland. These countries belong to four continents, namely, Asia, America, Europe and Africa. The populations from America and Europe belonged to the Caucasian subgroup. The remaining studies belonged to the Asian and African subgroups. Among the 12 excluded studies, five were reviews [21–25], and seven studies were not associated with TAP1 I333V gene polymorphism or T1DM [26–32]. No study was discarded for deviating from HWE.
Table 1.
Characteristics of the investigated studies of the association of the TAP1 I333V gene polymorphism and T1DM
| T1DM | Control | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Region | Ethnicity | II | IV | VV | II | IV | VV | Geno-typing | Matching criteria | Sample size (T1DM/control) |
| Colonna [12] | 1992 | Italy | Caucasian | 29 | 17 | 4 | 27 | 10 | 0 | PCR-SSCP | Ethnicity | 50/37 |
| Caillat-Zucman [13] | 1993 | France | Caucasian | 74 | 35 | 3 | 66 | 31 | 1 | PCR-SSOM | Ethnicity | 112/98 |
| Jackson [6] | 1993 | USA | Caucasian | 147 | 75 | 19 | 143 | 54 | 4 | PCR-SSCP | Ethnicity | 241/201 |
| Cucca [4] | 1994 | Italy | Caucasian | 99 | 26 | 4 | 52 | 25 | 2 | ARMS-PCR | Ethnicity | 129/99 |
| Kawaguchi [14] | 1994 | Japan | Asia | 36 | 9 | 0 | 45 | 7 | 1 | PCR-RFLP | Age, sex, ethnicity | 45/53 |
| Nakanishi [5] | 1994 | Japan | Asia | 77 | 18 | 0 | 59 | 15 | 1 | PCR-SSOM | Ethnicity | 95/75 |
| van Endert [15] | 1994 | Denmark | Caucasian | 19 | 27 | 1 | 40 | 21 | 1 | PCR-SSOM | Ethnicity | 47/62 |
| Chauffert [16] | 1997 | Senegal | Africa | 55 | 34 | 3 | 79 | 36 | 2 | PCR-RFLP | Sex, ethnicity | 92/117 |
| Ma [17] | 1997 | Finland | Caucasian | 77 | 39 | 3 | 66 | 23 | 3 | ARMS-PCR | Age, sex, ethnicity | 119/92 |
| Rau [18] | 1997 | German | Caucasian | 40 | 54 | 4 | 82 | 68 | 11 | PCR-SSCP | Ethnicity | 98/161 |
| Yan [19] | 1997 | USA | Caucasian | 36 | 10 | 3 | 21 | 7 | 2 | PCR-RFLP | Ethnicity | 49/30 |
| Sheng [7] | 2004 | China | Asia | 12 | 35 | 0 | 36 | 13 | 4 | PCR | Sex, ethnicity | 47/53 |
| Zhai [20] | 2007 | China | Asia | 10 | 3 | 3 | 40 | 7 | 1 | PCR-RFLP | Sex, ethnicity | 16/48 |
T1DM: type 1 diabetes mellitus; PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphism; ARMS-PCR: amplification refractory mutation system PCR; PCR-SSOM: PCR sequence specific oligonucleotide method; PCR-SSCP: PCR-single-strand conformation polymorphism; NP: not provided. Case–control study design was used in all of the above studies.
Pooled analyses
A significant relationship was observed between TAP1 I333V gene polymorphism and T1DM in allelic (OR: 1.35, 95% CI: 1.08–1.68, P = 0.007), dominant (OR: 1.462, 95% CI: 1.094–1.955, P = 0.010), homozygous (OR: 1.725, 95% CI: 1.082–2.752, P = 0.022), heterozygous (OR: 1.430, 95% CI: 1.048–1.951, P = 0.024) and additive (OR: 1.348, 95% CI: 1.084–1.676, P = 0.007) genetic models. No significant association between TAP1 I333V gene polymorphism and T1DM was detected in a recessive genetic model (OR: 1.384, 95% CI: 0.743–2.579, P = 0.306) in the entire population, especially among Caucasians. No significant association was found between them among the Asian or African population (Figs 1–4, Table 2).
Fig. 1.
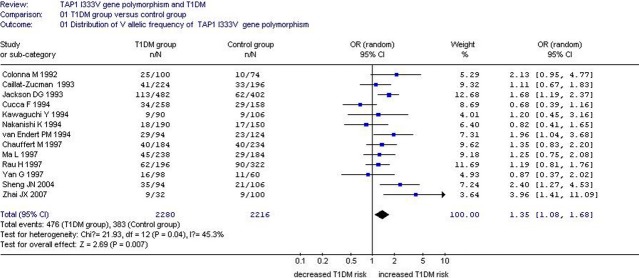
Forest plot of T1DM associated with TAP1 I333V gene polymorphism under an allelic genetic model (distribution of V allelic frequency of TAP1 gene).
Fig. 4.
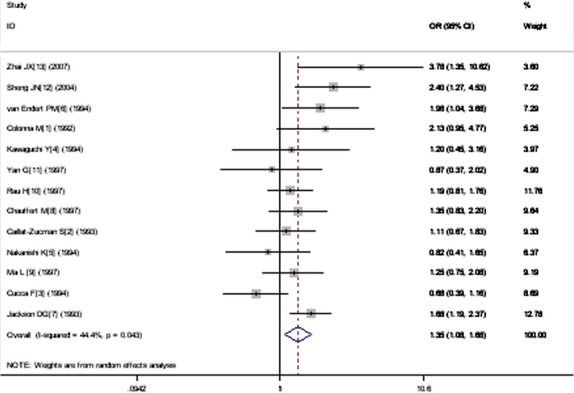
Forest plot of T1DM associated with TAP1 I333V gene polymorphism under an additive genetic model (V versus I).
Table 2.
Summary of meta-analysis of association of TAP1 I333V gene polymorphism and T1DM
| Genetic model | Pooled OR (95% CI) | P value | Literature number | T1DM size | Control size | Pheterogeneity (I2%) |
|---|---|---|---|---|---|---|
| Allelic genetic model | 1.35 (1.08–1.68) | 0.007* | 13 | 1140 | 1106 | 0.04* (44.4) |
| Subgroup 1: before 1995 | 1.26 (0.90–1.75) | 0.17 | 7 | 719 | 605 | 0.04* (53.7) |
| Subgroup 2: after 1996 | 1.39 (1.11–1.74) | 0.004* | 6 | 421 | 501 | 0.13 (40.8) |
| Recessive genetic model | 1.384 (0.743–2.579) | 0.306 | 13 | 1140 | 1106 | 0.202 (23.9) |
| Dominant genetic model | 1.462 (1.094–1.955) | 0.010* | 13 | 1140 | 1106 | 0.007* (55.9) |
| Subgroup 1: IV1 < 30 | 1.289 (0.781–2.126) | 0.320 | 7 | 431 | 384 | 0.034* (56.1) |
| Subgroup 2: IV1 > 30 | 1.620 (1.143–2.296) | 0.007* | 6 | 709 | 722 | 0.041* (56.9) |
| Homo genetic model | 1.725 (1.082–2.752) | 0.022* | 13 | 1140 | 1106 | 0.324 (12.0) |
| Hetero genetic model | 1.430 (1.048–1.951) | 0.024* | 13 | 1140 | 1106 | 0.004* (58.4) |
| Subgroup 1:II1 < 40 | 2.183 (1.148–4.151) | 0.017* | 6 | 254 | 283 | 0.037* (57.8) |
| Subgroup 2: II1 ≥ 40 | 1.191 (0.963–1.472) | 0.106 | 7 | 886 | 823 | 0.190 (31.2) |
| Additive genetic model | 1.348 (1.084–1.676) | 0.007* | 13 | 1140 | 1106 | 0.043 (44.4) |
| Subgroup 1:II1 < 40 | 1.867 (1.361–2.561) | <0.001* | 6 | 254 | 283 | 0.262 (22.8) |
| Subgroup 2: II1 ≥ 40 | 1.218 (1.023–1.450) | 0.027* | 7 | 886 | 823 | 0.147 (36.8) |
P < 0.05. T1DM: type 1 diabetes mellitus; CI: confidence interval; OR: odds ratio; T1DM size: the total number of T1DM cases; control size: the total number of control group; homo genetic model: homozygous genetic model; hetero genetic model: heterozygous genetic model.
Fig. 2.
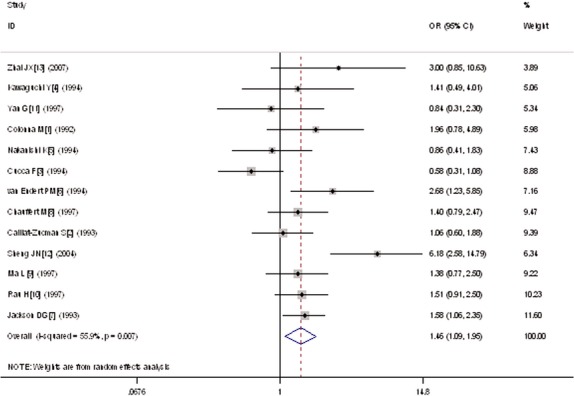
Forest plot of T1DM associated with TAP1 I333V gene polymorphism under a dominant genetic model (VV+ IV versus II).
Fig. 3.
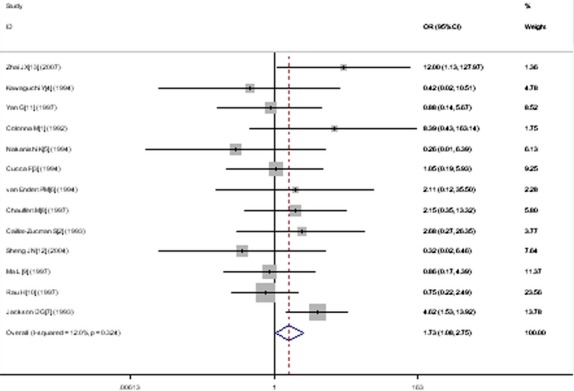
Forest plot of T1DM associated with TAP1 I333V gene polymorphism under a homozygous genetic model (VV+ IV versus II).
Significant heterogeneity was observed in the allelic (Pheterogeneity = 0.04), dominant (Pheterogeneity = 0.007), heterozygous (Pheterogeneity = 0.004) and additive (Pheterogeneity = 0.043) genetic models. The following meta-regression was performed to explore the source of heterogeneity. In the allelic genetic model, the heterogeneity could only be explained by the publication year (P = 0.026). The entire population was separated into two subgroups based on publication year. The manuscripts published before 1995 belonged to subgroup 1, and the manuscripts published after 1996 were classified into subgroup 2. In subgroup analysis stratified by publication year, significantly increased T1DM risk was detected in subgroup 2 (OR: 1.39, 95% CI: 1.11–1.74, P = 0.004), whereas no significant increase in T1DM risk was found in subgroup 1 (OR: 1.26, 95% CI: 0.90–1.75, P = 0.17). Heterogeneity was observed in subgroup 1 (Pheterogeneity = 0.04, I2 = 53.7%), but not in subgroup 2 (Pheterogeneity = 0.13, I2 = 40.8%). These results indicate that heterogeneity mainly existed in the studies published before 1995, and the conclusions were relatively consistent in the studies published after 1996.
In the dominant genetic model, heterogeneity could be explained by publication year (P = 0.039), II genotype number of T1DM group sample size (II1, P = 0.003), IV genotype number of T1DM group sample size (IV1, P = 0.001) and IV genotype number of control group sample size (IV0, P = 0.023). All the individual studies were classified into two subgroups according to IV1. The studies with IV1 < 30 were grouped into subgroup 1, whereas the studies with IV1 > 30 were grouped into subgroup 2. In the subsequent subgroup analysis stratified by IV1, significantly increased T1DM risk was only found in subgroup 2 (OR: 1.620, 95% CI: 1.143–2.296, P = 0.007). In subgroup 1, no significant increase in T1DM risk was detected (OR: 1.289, 95% CI: 0.781–2.126, P = 0.320). Heterogeneity decreased in two subgroups but remained significant (subgroup 1: Pheterogeneity = 0.034, I2 = 56.1%; subgroup 2: Pheterogeneity = 0.041, I2 = 56.9%).
In the heterozygous genetic model, heterogeneity could be explained by II1 (P = 1.0 × 10−10), IV0 (P = 0.005), total sample size of control group (T0, P = 0.028) and weight (P = 0.002). All the individual studies were divided into two subgroups according to II1. Subgroup 1 was defined as II1 < 40, and subgroup 2 was defined as II1 ≥ 40. In the subsequent subgroup analysis stratified by II1, significantly increased T1DM risk was detected in subgroup 1 (OR: 2.183, 95% CI: 1.148–4.151, P = 0.017, Pheterogeneity = 0.037). In subgroup 2, no significant increase in T1DM risk was found (OR: 1.191, 95% CI: 0.963–1.472, P = 0.106, Pheterogeneity = 0.190).
In the additive genetic model, heterogeneity could be explained by II1 (P = 0.003), VV genotype number of T1DM group sample size (VV1, P = 0.027) and VV genotype number of control group sample size (VV0, P = 0.027). Individual studies were separated into two subgroups according to II1, which was similar to that in the heterozygous genetic model. In the following subgroup analysis stratified by II1, significantly increased T1DM risk was detected in both subgroups (subgroup 1: OR: 1.867, 95% CI: 1.361–2.561, P < 0.001; subgroup 2: OR: 1.218, 95% CI: 1.023–1.450, P = 0.027). The heterogeneity disappeared in both subgroups (subgroup 1: Pheterogeneity = 0.262, I2 = 22.8%; subgroup 2: Pheterogeneity = 0.147, I2 = 36.8%).
Logistic regression was performed on multivariable-adjusted risks, such as age, sex, region and ethnicity. These risk factors had no effect on the association between TAP1 I333V gene polymorphism and T1DM (OR = 1).
In the subgroup analysis stratified by ethnicities among the Caucasian population, a significant association between TAP1 I333V gene polymorphism and T1DM was found in allelic (OR: 1.30, 95% CI: 1.09–1.55, P = 0.004), dominant (OR: 1.330, 95% CI: 1.079–1.640, P = 0.008), homozygous (OR: 1.854, 95% CI: 1.082–3.176, P = 0.022), heterozygous (OR: 1.351, 95% CI: 0.889–2.054, P = 0.029) and additive genetic models (OR: 1.297, 95% CI: 1.088–1.548, P = 0.004). No significant association between TAP1 I333V gene polymorphism and T1DM was detected in a recessive genetic model (OR: 1.643, 95% CI: 0.971–2.780, P = 0.064).
In the Asian subgroup, no significant association between TAP1 I333V gene polymorphism and T1DM was found in the allelic (OR: 1.69, 95% CI: 0.86–3.34, P = 0.13), recessive (OR: 0.928, 95% CI: 0.222–3.873, P = 0.918), dominant (OR: 2.133, 95% CI: 0.815–5.583, P = 0.123), homozygous (OR: 1.235, 95% CI: 0.293–5.211, P = 0.773) and heterozygous genetic models (OR: 2.141, 95% CI: 0.773–6.248, P = 0.164). A significant association was found between them in a additive genetic model (OR: 1.614, 95% CI: 1.095–2.378, P = 0.015; Table 3).
Table 3.
Summary of meta-analysis of association of TAP1 I333V gene polymorphism and T1DM stratified by ethnicities
| Genetic model | Pooled OR (95% CI) | P value | Study number | T1DM size | Control size | Pheterogeneity (I2%) |
|---|---|---|---|---|---|---|
| Allelic genetic model | 1.35 (1.08–1.68) | 0.007* | 13 | 1140 | 1106 | 0.04* (44.4) |
| Caucasian subgroup | 1.30 (1.09–1.55) | 0.004* | 8 | 845 | 760 | 0.09 (43.3) |
| Asian subgroup | 1.69 (0.86–3.34) | 0.13 | 4 | 203 | 229 | 0.04* (64.2) |
| African subgroup | 1.35 (0.83–2.20) | 0.23 | 1 | 92 | 117 | NA |
| Recessive genetic model | 1.384 (0.743–2.579) | 0.306 | 13 | 1140 | 1106 | 0.202 (23.9) |
| Caucasian subgroup | 1.643 (0.971–2.780) | 0.064 | 8 | 845 | 760 | 0.301 (16.4) |
| Asian subgroup | 0.928 (0.222–3.873) | 0.918 | 4 | 203 | 229 | 0.071 (57.3) |
| African subgroup | 0.900 (0.578–1.399) | 0.639 | 1 | 92 | 117 | NA |
| Dominant genetic model | 1.462 (1.094–1.955) | 0.010* | 13 | 1140 | 1106 | 0.007* (55.9) |
| Caucasian subgroup | 1.330 (1.079–1.640) | 0.008* | 8 | 845 | 760 | 0.074 (45.8) |
| Asian subgroup | 2.133 (0.815–5.583) | 0.123 | 4 | 203 | 229 | 0.007 (75.0) |
| African subgroup | 1.399 (0.792–2.470) | 0.248 | 1 | 92 | 117 | NA |
| Homozygous genetic model | 1.725 (1.082–2.752) | 0.022* | 13 | 1140 | 1106 | 0.324 (12.0) |
| Caucasian subgroup | 1.854 (1.082–3.176) | 0.025* | 8 | 845 | 760 | 0.349 (10.5) |
| Asian subgroup | 1.235 (0.293–5.211) | 0.773 | 4 | 203 | 229 | 0.129 (47.1) |
| African subgroup | 3.136 (0.509–19.315) | 0.218 | 1 | 92 | 117 | NA |
| Heterzygous genetic model | 1.430 (1.048–1.951) | 0.024* | 13 | 1140 | 1106 | 0.004* (58.4) |
| Caucasian subgroup | 1.351 (0.889–2.054) | 0.029* | 8 | 845 | 760 | 0.081 (44.7) |
| Asian subgroup | 2.141 (0.733–6.248) | 0.164 | 4 | 203 | 229 | 0.004* (77.2) |
| African subgroup | 1.975 (1.119–3.486) | 0.019* | 1 | 92 | 117 | NA |
| Additive genetic model | 1.348 (1.084–1.676) | 0.007* | 13 | 1140 | 1106 | 0.043* (44.4) |
| Caucasian subgroup | 1.297 (1.088–1.548) | 0.004* | 8 | 845 | 760 | 0.09 (43.3) |
| Asian subgroup | 1.614 (1.095–2.378) | 0.015* | 4 | 203 | 229 | 0.044* (62.9) |
| African subgroup | 1.347 (0.827–2.196) | 0.232 | 1 | 92 | 117 | NA |
NA: not applicable.
P < 0.05.
The haplotypes from TAP1 I333V and Asp637Gly (A637G) gene polymorphisms were chosen, constructed and analysed. The three haplotypes, namely, TAP1A (333I-637A), TAP1B (333V-637G) and TAP1C (333V-637A) haplotypes, were constructed and analysed. In the following meta-analysis, the TAP1A haplotype could decrease T1DM risk (OR: 0.58, 95% CI: 0.44–0.76, P < 0.001). The other two haplotypes, namely, TAP1B and TAP1C, had no association with T1DM risk (TAP1B: OR: 1.12, 95% CI: 0.56–2.23, P = 0.75; TAP1C: OR: 1.79, 95% CI: 0.43–7.53, P = 0.43; Fig. 5, Table 4).
Fig. 5.
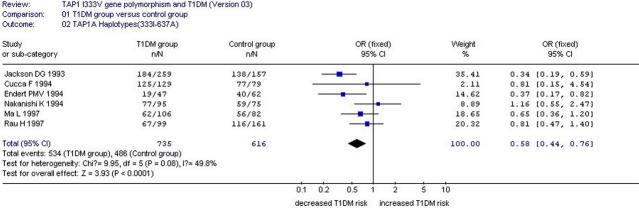
Forest plot of T1DM associated with TAP1A haplotype (333I-637A).
Table 4.
Summary of meta-analysis of association of TAP1 I333V and Asp637Gly (A637G) haplotypes
| Genetic model | Pooled OR (95% CI) | P value | Study number | T1DM size | Control size | Pheterogeneity (I2%) |
|---|---|---|---|---|---|---|
| TAP1A (333I-637A) | 0.58 (0.44–0.76) | <0.001* | 6 | 735 | 616 | 0.08 (49.8) |
| TAP1B (333V-637G) | 1.12 (0.56–2.23) | 0.75 | 5 | 847 | 685 | 0.009 (70.5) |
| TAP1C (333V-637A) | 1.79 (0.43–7.53) | 0.43 | 3 | 635 | 507 | 0.007* (80.0) |
P < 0.05.
Bias diagnostics
The funnel plot and Egger*s test were used to evaluate the publication bias of the individual studies. No publication bias was observed by visual inspection of the Begg*s funnel plot in the additive genetic model (Fig. 6). No significant difference in Egger*s test was observed, which implies low publication bias in this meta-analysis (additive genetic model, T = 0.90, P = 0.386).
Fig. 6.
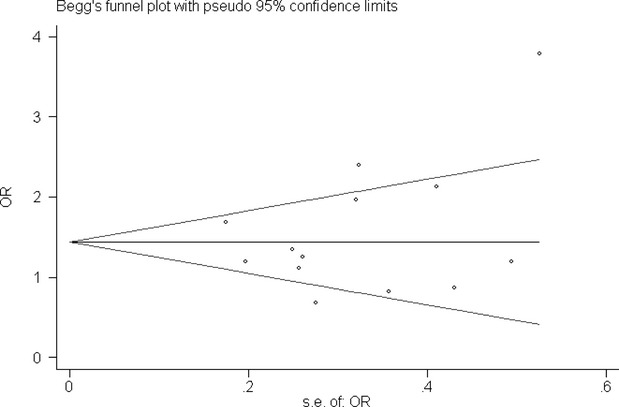
Begg*s funnel plot for studies of the association of T1DM associated with TAP1 I333V gene polymorphism under an additive genetic model (V versus I). The horizontal and vertical axis correspond to the OR and confidence limits. OR: odds ratio; SE: standard error.
Discussion
In this meta-analysis, a significant relationship was observed between TAP1 I333V gene polymorphism and T1DM in the allelic (OR: 1.35), dominant (OR: 1.462), homozygous (OR: 1.725), heterozygous (OR: 1.430) and additive (OR: 1.348) genetic models, especially among Caucasians. No positive association between TAP1 I333V gene polymorphism and T1DM was observed in a recessive genetic model (OR: 1.384), which could be associated with the less VV genotype number in the general population. Table 1 showed that no VV genotype was present in several studies. Thus, TAP1 333V allele possibly increased T1DM risk, i.e. V allele possibly conferred T1DM susceptibility to humans. No significant association was found between TAP1 I333V gene polymorphism and T1DM in an Asian or African population. The different results from the different populations were probably associated with the different ethnicities. Moreover, the relatively few papers on Asian and African populations possibly led to the negative results, which implied that large-scale studies should be performed in future.
Given that heterogeneity existed in the allelic and heterozygote genetic models, meta-regression was performed to determine the source of heterogeneity. In the subsequent heterogeneity source analysis, publication year, IV1 and II1 were suggested to be the central confounding factors to explain the source of heterogeneity in allelic, dominant, heterozygous and additive genetic models (P < 0.05).
Transporter associated with antigen processing 1 A637G is another common TAP1 gene polymorphism associated with T1DM. In the TAP1 gene, 2120th base adenine (A) was replaced by guanine (G), which resulted in the corresponding amino acid aspartate (Asp, A) being substituted by glycine (Gly, G) at 637th amino acid, of which 637G allele possibly increased the T1DM risk. Thus, analysis of the TAP1 gene 333IV-637AG haplotypes was performed [6,7]. The results from haplotype analysis showed that TAP1A (333I-637A) haplotype had a protective effect for T1DM, which was in agreement with the aforementioned meta-analysis results. The negative association between the TAP1B (333V-637G) and TAP1C (333V-637A) haplotypes and T1DM was possibly associated with the limited number of studies because only five and three papers were included respectively. Thus, the results need to be further verified by more studies.
Transporter associated with antigen processing 1 gene polymorphisms result in different recognition and transport affinity to the same endogenous antigen peptide, and cause different bodies to show various immune responses to the same endogenous antigen, including protective immunity, immune tolerance and autoimmune tendency [33]. The mechanism of the association between TAP1 I333V gene polymorphism and T1DM remains unclear. In 1998, Quadri et al. reported that TAP1 333I allele specifically enhances translocation of model peptides containing basic C-terminal amino acid residue. However, TAP1 333V allele did not show specificity for the peptide with basic amino acid residue C-terminus, which indicated that the TAP1 I333V mutation could lead to the change in specificity of the transported peptides. Some peptides transported by mutated TAP1 gene products might cause the expression deficiency of MHC I molecules, which resulted in the spontaneous termination of immune tolerance, launched the autoimmunity process and caused the destruction of pancreas islet β cells [34]. In the present meta-analysis, TAP1 333V allele increased the T1DM risk, which could be associated with the differences in peptide transport. However, this result is still a hypothesis that needs confirmation by future experiments.
In 2004, Sheng et al. found that the IV genotype of TAP1 gene locus 333 was a susceptible gene for T1DM that had a possible function in the production of autoantibodies, such as insulin autoantibody, islet cell antibody and glutamic acid decarboxylase antibody. Thus, the autoimmune response and destruction in the pancreas islet β cells resulted in T1DM. However, the concrete mechanisms by which the TAP1 333 IV genotype promoted autoantibody production remained unclear [7].
The following limitations were noted in the present meta-analysis. Large-scale studies on the association of T1DM with TAP1 I333V gene polymorphism are still insufficient. The TAP1 expression level was influenced not only by TAP1 I333V gene polymorphism but also by other genetic and environmental factors, such as ethnicity, inflammation state and other immune system diseases. Given that T1DM is a multigenic heredity disease, TAP1 I333V gene polymorphism might be associated with the gene linkage disequilibrium, such as TAP1 Val458Leu, Asp637Gly and Arg648Gln, which increase T1DM susceptibility [35].
Transporter associated with antigen processing 1 I333V gene polymorphism was positively associated with increased T1DM risk. Patients with the V allele may be susceptible to T1DM. Our results may help in establishing individual T1DM therapy strategies. In consideration of the limitations, more large-scale studies are needed to elucidate the significance of our conclusions.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (NSFC 81100073 to Dr. Yan-Yan Li), Excellent Young and Middle-Aged Teachers Assistance Program of Nanjing Medical University for Dr. Yan-Yan Li (2013–2015, JX2161015034) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Thank all our colleagues working in the Department of geriatrics, the First Affiliated Hospital of Nanjing Medical University.
Disclosure
The authors confirm that there are no conflicts of interest.
Conflicts of interest
None.
Author contribution
Conceived and designed the experiments: YL. Performed the experiments: YL, WG, SP. Analysed the data: YL, LW, XM. Contributed reagents/material/analysis tools: YL, XW. Wrote the manuscript: YL, XL, HW. Reference collection and data management: YL, YQ, CZ, ZY. Statistical analyses and paper writing: YL, JX, JW. Study design: YL, AC.
References
- 1.Axelsson S, Chéramy M, Hjorth M, et al. Long-lasting immune responses 4 years after GAD-alum treatment in children with type 1 diabetes. PLoS ONE. 2011;6:e29008. doi: 10.1371/journal.pone.0029008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahram S, Arnold D, Bresnahan M, et al. Two putative subunits of a peptide pump encoded in the human major histocompatibility complex class II region. Proc Natl Acad Sci USA. 1991;88:10094–8. doi: 10.1073/pnas.88.22.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Kaer L, Ashton-Rickardt PG, Ploegh HL, et al. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992;71:1205–14. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 4.Cucca F, Congia M, Trowsdale J, et al. Insulin-dependent diabetes mellitus and the major histocompatibility complex peptide transporters TAP1 and TAP2: no association in a population with a high disease incidence. Tissue Antigens. 1994;44:234–40. doi: 10.1111/j.1399-0039.1994.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi K, Kobayashi T, Murase T, et al. Lack of association of the transporter associated with antigen processing with Japanese insulin-dependent diabetes mellitus. Metabolism. 1994;43:1013–7. doi: 10.1016/0026-0495(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 6.Jackson DG, Capra JD. TAP1 alleles in insulin-dependent diabetes mellitus: a newly defined centromeric boundary of disease susceptibility. Proc Natl Acad Sci USA. 1993;90:11079–83. doi: 10.1073/pnas.90.23.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheng JN, Zhu ZO, Wang Z, et al. Study on the association of transporter associated with antigen processing gene with type - 1 diabetes mellitus and autoantibodies. Anh Med J. 2004;25:117–9. [Google Scholar]
- 8.Cochran WG. The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics. 1968;24:295–313. [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colonna M, Bresnahan M, Bahram S, et al. Allelic variants of the human putative peptide transporter involved in antigen processing. Proc Natl Acad Sci USA. 1992;89:3932–6. doi: 10.1073/pnas.89.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caillat-Zucman S, Bertin E, Timsit J, et al. Protection from insulin-dependent diabetes mellitus is linked to a peptide transporter gene. Eur J Immunol. 1993;23:1784–8. doi: 10.1002/eji.1830230808. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi Y, Ikegami H, Fukuda M, et al. Absence of association of TAP and LMP genes with type 1 (insulin-dependent) diabetes mellitus. Life Sci. 1994;54:2049–53. doi: 10.1016/0024-3205(94)00713-6. [DOI] [PubMed] [Google Scholar]
- 15.van Endert PM, Liblau RS, Patel SD, et al. Major histocompatibility complex-encoded antigen processing gene polymorphism in IDDM. Diabetes. 1994;43:110–7. doi: 10.2337/diab.43.1.110. [DOI] [PubMed] [Google Scholar]
- 16.Chauffert M, Cissé A, Chevenne D, et al. Susceptibility to type 1 diabetes in the Senegalese population is linked to HLA-DQ and not TAP and LMP genes. Diabetes Care. 1997;20:1299–303. doi: 10.2337/diacare.20.8.1299. [DOI] [PubMed] [Google Scholar]
- 17.Ma L, Penfornis A, Wang X, et al. Evaluation of TAP1 polymorphisms with insulin dependent diabetes mellitus in Finnish diabetic patients. The Childhood Diabetes in Finland (DiMe) Study Group. Hum Immunol. 1997;53:159–66. doi: 10.1016/s0198-8859(97)00030-x. [DOI] [PubMed] [Google Scholar]
- 18.Rau H, Nicolay A, Donner H, et al. Polymorphisms of TAP1 and TAP2 genes in German patients with type 1 diabetes mellitus. Eur J Immunogenet. 1997;24:229–36. doi: 10.1111/j.1365-2370.1997.00266.x. [DOI] [PubMed] [Google Scholar]
- 19.Yan G, Fu Y, Faustman DL. Reduced expression of Tap1 and Lmp2 antigen-processing genes in the nonobese diabetic (NOD) mouse due to a mutation in their shared bidirectional promoter. J Immunol. 1997;159:3068–80. [PubMed] [Google Scholar]
- 20.Zhai JX, Shen C, Ye DQ. The polymorphism of transporter associated with antigen processing genes among type1 diabetes mellitus. Chin J Prev Contr Chron Dis. 2007;15:28–31. [Google Scholar]
- 21.Li YJ, Zheng YQ, Zhang JY, et al. Progress in research on susceptibility genes of type 1 diabetes mellitus. Chin J Biologicals. 2011;24:241–4. [Google Scholar]
- 22.Zhao LJ, Xiang AX, Dong YH. Progress in study on childhood diabetes mellitus. Sect Mat Chi Heal Foreign Med Sci. 2005;16:219–21. [Google Scholar]
- 23.Chen Y, Wang J. Invariant natural killer T cells mediate regulation in type 1 diabetes mellitus. J Med Postgra. 2011;24:1306–8. [Google Scholar]
- 24.Huang XY, Liu S, Yan DQ, et al. The role of cytokines in the pathogenesis of type 1 diabetes. Prog Mod Biom. 2011;24:5147–51. [Google Scholar]
- 25.Shen J, Gu W. New genes associated with type 1 diabetes. Intern J Endocrino Metab. 2006;26:40–2. [Google Scholar]
- 26.Grallert H, Dupuis J, Bis JC, et al. Eight genetic loci associated with variation in lipoprotein-associated phospholipase A2 mass and activity and coronary heart disease: meta-analysis of genome-wide association studies from five community-based studies. Eur Heart J. 2012;33:238–51. doi: 10.1093/eurheartj/ehr372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson DG, Capra JD. TAP2 association with insulin-dependent diabetes mellitus is secondary to HLA-DQB1. Hum Immunol. 1995;43:57–65. doi: 10.1016/0198-8859(94)00124-9. [DOI] [PubMed] [Google Scholar]
- 28.Esposito L, Lampasona V, Bosi E, et al. HLA DQA1-DQB1-TAP2 haplotypes in IDDM families: no evidence for an additional contribution to disease risk by the TAP2 locus. Diabetologia. 1995;38:968–74. doi: 10.1007/BF00400587. [DOI] [PubMed] [Google Scholar]
- 29.Caillat-Zucman S, Daniel S, Djilali-Saiah I, et al. Family study of linkage disequilibrium between TAP2 transporter and HLA class II genes. Absence of TAP2 contribution to association with insulin-dependent diabetes mellitus. Hum Immunol. 1995;44:80–7. doi: 10.1016/0198-8859(95)00062-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XM, Wang HY, Luo YY, et al. HLA-DQ, DR allele polymorphism of type 1 diabetes in the Chinese population: a meta-analysis. Chin Med J. 2009;122:980–6. [PubMed] [Google Scholar]
- 31.Bodmer JG, Marsh SG, Albert ED, et al. Nomenclature for factors of the HLA system, 1995. Tissue Antigens. 1995;46:1–18. doi: 10.1111/j.1399-0039.1995.tb02470.x. [DOI] [PubMed] [Google Scholar]
- 32.Powis SH, Tonks S, Mockridge I, et al. Alleles and haplotypes of the MHC-encoded ABC transporters TAP1 and TAP2. Immunogenetics. 1993;37:373–80. doi: 10.1007/BF00216802. [DOI] [PubMed] [Google Scholar]
- 33.Mandel I, Paperna T, Glass-Marmor L, et al. Tight junction proteins expression and modulation in immune cells and multiple sclerosis. J Cell Mol Med. 2012;16:765–75. doi: 10.1111/j.1582-4934.2011.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan L, Cai MY, Ding HL, et al. Contribution of transporter associated with antigen processing gene polymorphism to predisposition to type 1 diabetes: a preliminary study. Chin J Endocrinol Metab. 2003;19:40–2. [Google Scholar]
- 35.Quadri SA, Singal DP. Peptide transport in human lymphoblastoid and tumor cells: effect of transporter associated with antigen presentation (TAP) polymorphism. Immunol Lett. 1998;61:25–31. doi: 10.1016/s0165-2478(97)00157-0. [DOI] [PubMed] [Google Scholar]


