Abstract
Central blood pressure is a predictor of the risk of cardiovascular disease (CVD), and the effects of resistance training (RT) on central blood pressure are largely unknown. This study explored the effects of high-intensity RT on central blood pressure, indices of arterial stiffness and wave reflection and inflammatory/atherogenic markers in overweight or obese, sedentary young men. Thirty-six participants were randomized to RT (12 weeks of training, 3/wk, n = 28) or control groups (C, 12 weeks of no training, n = 8) and assessed for changes in central and brachial blood pressures, augmentation index (AIx), carotid–femoral pulse wave velocity (cfPWV), carotid intima-media thickness (cIMT), body composition, lipids and inflammatory/atherogenic markers. High-intensity RT resulted in decreased central and brachial systolic/diastolic blood pressures (all P≤0.03), despite not altering AIx (P = 0.34) or cfPWV (P = 0.43). The vascular endothelial growth factor increased (P = 0.03) after RT, without any change in cIMT, C-reactive protein, oxidized LDL (oxLDL) or other inflammatory markers (all P≥0.1). Changes in the central systolic blood pressure (cSBP) were positively correlated with changes in oxLDL (r = 0.42, P = 0.03) and soluble E-selectin (r = 0.41, P = 0.04). In overweight/obese young men, high-intensity RT decreases cSBP, independently of weight loss and changes in arterial stiffness. The cardioprotective effects of RT may be related to effects on central blood pressure.
Keywords: arterial stiffness, wave reflection, blood pressure, strength training, exercise
INTRODUCTION
Physical inactivity and obesity are major risk factors for cardiovascular disease (CVD), including the development of atherosclerosis.1 Healthy lifestyle choices, such as regular exercise, represent the first line of defense against the disease and its comorbidities. Establishing healthy lifestyle choices early on in life is crucial, especially in young adults, for whom engaging in physical activity predicts both physical activity level2 and CVD risk3 later in life.
Resistance training (RT) may be an effective lifestyle approach for the prevention and treatment of CVD and has gained considerable attention for its capacity to improve body composition and glucose tolerance.4 However, the effects of RT on arterial stiffness (an independent determinant of cardiovascular mortality5) and inflammation are poorly understood. Previous studies have demonstrated that RT increases arterial stiffness,6–8 whereas others suggest no change.9,10 One hypothesis is that high-intensity and -volume RT might lead to increased arterial stiffness.10 The effects of RT in a young adult population classified as both sedentary and overweight/obese are unknown, as most studies have enrolled normal-weight individuals. In addition, recent data suggest that central pressures may more accurately reflect CVD risk and outcome profiles, compared with peripheral pressures.11,12 At present, little is known about the effects of RT on central blood pressure. In addition, obesity, inflammatory cytokines and elevated cell adhesion molecules are linked with vascular dysfunction, increased central systolic blood pressure (cSBP) and atherosclerotic disease.13,14 For example, young adults aged 20–40 years with a BMI >30 had higher arterial stiffness (by pulse wave velocity), compared with lean subjects. However, data on the effects of RT on these CVD risk factors are limited. Intervention studies have noted no changes in interleukin-6,15,16 tumor necrosis factor-α,15 and cell adhesion molecules,14 whereas others suggest that RT may reduce16–18 or have no effect8,15 on C-reactive protein (CRP).
The present study was designed to clarify the relationships between RT, central blood pressure and several indices of arterial function, and explores their relation to systemic inflammation. We evaluated the effects of a high-intensity RT intervention (12 weeks, three times per week) on central and brachial blood pressures, indices of arterial stiffness, as well as inflammatory and atherogenic markers in sedentary, overweight/obese young men. We hypothesized that RT, independent of weight loss, would improve central blood pressure without increasing arterial stiffness.
METHODS
Study participants
Participants were sedentary (participated in light-intensity physical activities ≤2 times per week) males and did not exhibit any other overt chronic disease symptoms, as indicated by their comprehensive histories and physical examinations at their baseline visit, which occurred between May 2009 and February 2010; the follow-up occurred from August 2009 until May 2010. Following their preintervention assessment, participants were randomized by the study coordinator using a permuted block design with a block size of four and a random number generator into one of the two groups at a control (C, n = 8) to resistance training (RT, n = 28) ratio of 1:3. As a pilot study, this ratio was chosen to maximize the number of participants involved in the RT intervention while providing an adequate control group. Other than the study coordinators, all study personnel were blinded to the randomization. Both groups were instructed to maintain their normal ad libitum diets and the normal activities of daily life. Participants randomized to the C group completed a 12-week control period without RT. Pre- and post-intervention assessments were made at weeks 0 and 13, respectively. Potential participants who had (1) documented CVD, cardiac surgery or any heart arrhythmia found using an electrocardiogram reading; (2) participated in a structured exercise, nutrition or weight loss program within the previous 6 months or (3) used tobacco products or medications that influence cardiovascular function, body composition or insulin indices in the previous 6 months, were excluded from the study. All of the study protocols were approved by the University of California, Los Angeles Institutional Review Board, and were performed according to the Declaration of Helsinki.
Muscular strength testing and resistance training intervention
All training occurred at the John Wooden Recreation Center. Maximal strength testing consisted of 1-repetition maximum (1RM) lifts for the barbell bench press, 45° incline leg press and machine-seated row-assessed changes in participants’ strength. Participants first warmed up each muscle group by performing 8–10 repetitions with weights equivalent to 40–60% of their estimated 1RM. The weight was progressively increased, whereas the repetitions were decreased until participants could safely attempt an estimated 1RM for each exercise. A successful 1RM occurred on the penultimate set, after the participants failed their last set. Participants were allowed 3–4 min of rest between all sets. All participants performed two maximal strength tests—one immediately preceding the first training session and the second immediately preceding their penultimate training session. The control group performed maximal strength tests at times corresponding to those of the RT group without related training sessions.
Participants in the RT group completed a total of 12 weeks of RT, with three supervised sessions per week and each session lasting ~1 h. The training overload was modified using a linear periodization model with three phases. During phase 1 (weeks 1–2), participants completed two sets of exercises, with 12–15 repetitions of each exercise, at 100% of their approximated 12–15 RM (that is, participants were motivated to reach volitional fatigue/failure within 15 repetitions). In phase 2 (weeks 3–7), participants completed three sets of exercises, with 8–12 repetitions, at 100% of their 8–12 RM, and in phase 3 (weeks 8–12), they completed 6–8 repetitions, at 100% of their 6–8 RM. As participants adapted to the training overload, the weight was increased to maintain the prescribed training intensity. All participants trained on three nonconsecutive days per week, cycling between the following two workout regimens: (1) dumbbell (DB) squat, cable row, DB front lunge, DB row, barbell deadlift, DB triceps extension and DB bicep curl and (2) DB step-up, barbell chest press, machine squat, DB overhead press, DB incline chest press, DB side raise, DB reverse fly and abdominal crunches. Two exercises were grouped and performed back-to-back with a 60–90 s rest period occurring after the completion of each superset. A certified personal trainer led all training sessions with a maximum participant-to-trainer ratio of 3:1.
Outpatient visit procedures
Measurements of participants were taken at baseline (pre-test) and on week 13 (post-test), with each test period consisting of two outpatient visits. The first visit took place on a weekday at the UCLA Clinical and Translational Research Center (CTRC), followed by a same-week saturday visit at the Gonda (Goldschmied) Diabetes Center. To control for any acute effects of the training program, the post-test CTRC outpatient visit and the Saturday visit occurred approximately 72 h and 96–120 h, respectively, after the last training session. Before each visit, participants were reminded to avoid all moderate-to-vigorous physical activity 24 h prior to testing and to abstain from all food and drink (except water) for ~12 h prior to each visit. Verbal confirmation of adherence to the aforementioned criteria was obtained immediately prior to all testing.
Outpatient visit, day 1
The outpatient procedures at the CTRC began at 0730 hours and typically lasted 3.5 h. A 12-lead electrocardiogram was administered as a safety measure and checked by a physician before allowing any participation in exercise testing/intervention. Waist circumference was measured in duplicate in all participants. Fasting blood samples were collected from the median cubital vein, and serum was separated and stored at −80 °C until assayed.
Outpatient visit, day 2
Upon arrival, height and weight were measured in duplicate, and body composition was determined using a dual-energy X-ray absorptiometry scan (Hologic QDR4500 Fan Beam X-ray Densitometer, Hologic, Waltham, MA, USA). Next, arterial tonometry and carotid ultrasound were assessed.
Arterial tonometry
Assessment of central blood pressure, wave reflection and arterial stiffness was conducted noninvasively using the SphygmoCor system (AtCor Medical, Sydney, Australia).5 All measurements were taken in the supine position in a quiet, temperature-controlled (23–25 °C) room. During 10 min of rest, two blood pressure measurements (that is, brachial systolic and diastolic pressure) were taken from the right arm using an automated oscillometric cuff (Prevention DS2200 Ultima). The two values were averaged and used for subsequent analysis.
Pulse wave analysis
Pulse wave analysis measurements were taken at the right radial artery using applanation tonometry with the help of a high-fidelity micromanometer (Millar Instruments, Houston, TX, USA). After 20 sequential waveforms had been acquired, a validated generalized transfer function was used to generate the corresponding central aortic pressure waveform yielding central systolic (cSBP) and central diastolic (cDBP) pressures.19,20 Radial pressure waveforms were calibrated against brachial SBP and DBP. A forward wave (P1) caused by stroke volume ejection at the aorta and a reflected wave (P2) were derived from this waveform. Augmentation index (AIx) was defined as the augmented pressure (P2–P1) expressed as a percentage of central pulse pressure. In addition, because AIx is influenced by heart rate, an index normalized for a heart rate of 75 beats per minute (b.p.m.) was used.21 The subendocardial viability ratio was defined as the ratio of the pressure–time integral during diastole (diastolic pressure–time index) to the pressure–time integral during systole (tension time index) and used as an index of cardiac perfusion. Pulse pressure amplification was defined as the ratio of brachial pulse pressure to central pulse pressure. Only high-quality recordings (operator index ≥80%) were included in the analysis. The intra-operator variability for cSBP in this study was 0.71%.
Pulse wave velocity
Carotid–femoral pulse wave velocity (cfPWV) was determined in duplicate using the SphygmoCor system by sequentially recording electrocardiographic-gated carotid and femoral artery waveforms using applanation tonometry. Distances from the carotid sampling site to the suprasternal notch and from the suprasternal notch to the femoral artery site were measured as straight lines between respective points on the body surface using a tape measure. The time interval (t, in seconds) between the onset of femoral and carotid waveforms was determined as the mean from 10 consecutive cardiac cycles. High-quality measurements were confirmed using the standard deviation of time intervals corresponding to the patient’s electrocardiogram and femoral and carotid artery waveforms. Standard deviations greater than 10% of the cfPWV value were not accepted. The cfPWV was calculated using the distance between measurement points (D, in meters) and the measured time delay between the peak of the electrocardiogram P-wave and the trough of a waveform (t) as follows: cfPWV = D/t (m s−1).
Carotid intima-media thickness
Carotid intima-media thickness (cIMT) is a well-validated surrogate measure of atherosclerosis of the right and left carotid arteries. Participants rested supine, with their necks in a neutral position and rotated 45° from the midline. Two-dimensional (2D) ultrasound images of the carotid artery were obtained using a 12-MHz linear array transducer, and data were digitally recorded on an external computer for offline analysis. An automated edge detection software (Medical Imaging Applications, Coralville, IA, USA) was utilized to measure the carotid artery diameter (intima–intima) and the cIMT of both sides ~1 cm distal to the carotid bulb. All cIMT outcome measures were determined by averaging data from a minimum of 10 cardiac cycles at end-diastole. Both near and far wall cIMTs between the right and left carotid arteries were obtained and averaged. Intra-observer coefficients of variability for near and far wall cIMTs were 2.9 and 1.8%, respectively.
Blood chemistry assays
Samples were assayed for a determination of the total cholesterol, high-density lipoprotein and triglycerides using the Olympus AU400 Chemistry Analyzer (Center Valley, PA, USA). Low-density lipoprotein (LDL) was calculated using the Friedewald equation.22 Oxidized LDL (oxLDL) (Mercodia Laboratories, Upsala, Sweden) and high-sensitivity CRP (Alpco, Salem, NH, USA) concentrations were determined using enzyme-linked immunosorbent assay. Interleukin (IL)-8, vascular endothelial growth factor (VEGF), matrix metallopeptidase-9, myeloperoxidase, total plasminogen activator inhibitor-1, soluble E-Selectin (sE-selectin), soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1 and monocyte chemotactic protein-1 were determined using Millipore Multiplex assay (Billerica, MA, USA). All analytes were measured in duplicates.
Statistical analyses
Non-parametric analyses were chosen for statistical inference due to the presence of non-normally distributed data (using both graphical and numerical methods, that is, the Shapiro–Wilk test), unequal samples sizes and heteroscedasticity. Power calculations were based on Heffernan et al.,23 to detect an ~2% change in central pressure within a group with a large effect size. The overall effects of the intervention were tested for evaluable participants. Between-group significance was calculated using Wilcoxon rank-sum test on post-test–pre-test change scores. Within-group significance was calculated using Wilcoxon signed-rank test; confidence intervals were derived using bias-corrected bootstrap methods (105 permutations). Post hoc Spearman correlation analyses were used to determine the relationships between changes in cSBP and changes in inflammatory and atherogenic biomarkers. Statistical analyses were performed using Stata Statistical Software 11.2 (StataCorp LP., College Station, TX, USA). Given that the nature of this study is that it is a pilot, the analysis initially focused on the effects of central pressure, whereas all other analyses were exploratory; across-group changes were detected alongside within-group changes and significant P-values (<0.05) were reported. All data are reported as median (interquartile range).
RESULTS
Evaluable intervention participants were young adult males (21 years old (20–23 years old)), who were both sedentary and overweight/obese (body mass index (BMI): 31.4 kg m−2 (29.7–34.7 kg m−2) and waist circumference: 103 cm (99–111 cm)). In total, participants attended 99.7% of their training sessions; 27 out of 28 RT participants performed 36 of the 36 training sessions, whereas one participant performed 35 of the 36 training sessions.
Anthropometrics, body composition and strength
The lean body mass increased and the total body fat percentage decreased in the RT group compared with the control group (P = 0.0002 and P = 0.03, respectively) (Table 1). BMI increased in the RT group (P = 0.03). 1RM chest press, 1RM leg press, 1RM back row and the composite strength score all increased significantly in the RT group compared with the control group (all P<0.001).
Table 1.
Anthropometrics, body composition and strength
| Outcomes |
Median (25th–75th percentile)
|
Median change (95% confidence interval)
|
P-value | ||
|---|---|---|---|---|---|
| Pre-test | Post-test | Within-group changes | Between-group changes | ||
| Age (years) | — | --b | |||
| Control | 22.0 (20.8–22.8) | — | --w | ||
| RT | 21.5 (20.0–23.0) | — | --w | ||
| Height (m) | — | -- | |||
| Control | 1.74 (1.70–1.77) | — | -- | ||
| RT | 1.77 (1.73–1.81) | — | -- | ||
| Weight (kg) | 1.7 (−0.56–3.7) | 0.07 | |||
| Control | 98.5 (91.6–106.2) | 98.0 (90.9–105.9) | 0.02 (−1.4–0.57) | 0.58 | |
| RT | 96.6 (90.0–103.5) | 97.1 (91.2–105.4) | 1.8 (0.00–3.0) | 0.06 | |
| BMI (kgm−2) | 0.58 (−0.22–1.4) | 0.06 | |||
| Control | 33.6 (31.2–34.7) | 33.2 (31.3–34.6) | −0.19 (−0.57–0.19) | 0.16 | |
| RT | 30.9 (29.7–32.7) | 31.2 (30.3–32.7) | 0.39 (−0.18–0.96) | 0.03 | |
| WC (cm) | −0.27 (−3.4–2.9) | 0.77 | |||
| Control | 106.5 (96.1–110.8) | 106.7 (93.6–112.0) | −0.28 (−2.0–1.9) | 0.83 | |
| RT | 103.3 (99.4–111.3) | 101.4 (96.8–108.7) | −0.55 (−2.4–0.85) | 0.24 | |
| Total fat (%) | −1.4 (−3.4–0.50) | 0.03 | |||
| Control | 26.2 (24.3–31.9) | 27.9 (22.7–31.3) | −0.48 (−1.6–1.0) | 0.67 | |
| RT | 28.6 (26.3–31.2) | 26.2 (24.2–29.6) | −1.9 (−2.9 to−0.52) | <0.001 | |
| Lean mass (kg) | 3.1 (1.2–4.1) | 0.0002 | |||
| Control | 69.3 (68.0–71.9) | 70.2 (68.6–71.4) | −0.45 (−0.70–1.4) | 0.58 | |
| RT | 69.5 (64.7–72.8) | 70.9 (66.5–76.2) | 2.7 (2.0–3.4) | <0.0001 | |
| 1RM chest (kg) | 15.9 (10.2–21.6) | <0.001 | |||
| Control | 70.3 (65.8–96.4) | 70.3 (65.8–99.8) | 0.00 (0.00–13.6) | 0.45 | |
| RT | 70.3 (56.7–80.5) | 86.2 (70.3–94.1) | 15.9 (13.6–19.3) | <0.0001 | |
| 1RM leg (kg) | 59.0 (15.9–95.3) | <0.001 | |||
| Control | 272.2 (272.2–347.0) | 281.2 (260.8–349.3) | 9.1 (−9.1–54.4) | 0.45 | |
| RT | 251.7 (237.0–293.7) | 331.1 (311.9–365.1) | 68.0 (56.7–90.7) | <0.0001 | |
| 1RM row (kg) | 18.1 (4.5–29.5) | 0.015 | |||
| Control | 81.7 (70.3–95.3) | 88.5 (82.8–89.6) | −2.3 (−4.5–17.0) | 0.67 | |
| RT | 79.4 (72.0–90.7) | 94.1 (90.2–104.9) | 15.9 (13.6–20.4) | <0.0001 | |
| Strength score (kg) | 77.1 (28.4–124.7) | <0.001 | |||
| Control | 455.9 (399.2–511.4) | 437.7 (411.6–526.2) | 22.7 (−11.3–77.1) | 0.40 | |
| RT | 409.4 (373.7–470.6) | 517.1 (479.1–560.8) | 99.8 (88.5–129.3) | <0.0001 | |
Abbreviations: BMI: body mass index; 1RM: 1-repetition maximum; strength score: sum of all three strength measures, WC: waist circumference. Significance was calculated using Wilcoxon signed-rank test. P-values with a superscript b indicate between-group analysis, and w indicates within-group analysis. Median values are presented with 25th and 75th percentile in parentheses, and median change scores are presented with the accompanied 95% confidence intervals.
Significant P values (P<0.05) are bolded.
Central and peripheral blood pressure and indices of arterial stiffness
Figure 1 depicts the change in central and peripheral blood pressures for the RT and control groups. Both cSBP (P = 0.01) and cDBP (P = 0.02) decreased with training (Table 2). These changes were noted in concert with decreases in bSBP (P = 0.03), branchial diastolic pressure (P = 0.01), P1 (P = 0.01), P2 (P = 0.04) and resting heart rate (P = 0.01). No significant changes occurred in the control group for central or brachial blood pressures. Conversely, RT resulted in no change in cfPWV (P = 0.43) or AIx (P = 0.34), although an increase in AIx was seen in the control group (P = 0.04). Furthermore, there were no changes in near or far wall cIMTs (all P>0.1).
Figure 1.
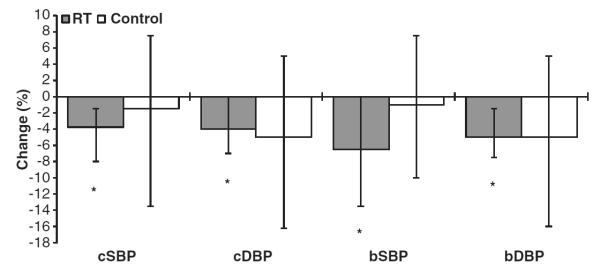
Central and brachial blood pressures. Bar graphs present median and 95% CI. *P<0.05 for within-group significance using Wilcoxon signed-rank test.
Table 2.
Central and peripheral blood pressure and indices of arterial stiffness
| Outcomes |
Median (25th–75th percentile)
|
Median change (95% confidence interval)
|
P-value | ||
|---|---|---|---|---|---|
| Pre-test | Post-test | Within-group changes | Between-group changes | ||
| cfPWV (m s−1) | −0.65 (−1.5–0.15) | 0.14b | |||
| Control | 6.9 (6.5–7.4) | 7.2 (7.1–7.5) | 0.45 (0.02–1.2) | 0.09w | |
| RT | 6.7 (6.2–7.4) | 6.6 (5.9–7.5) | −0.20 (−0.65–0.40) | 0.43w | |
| AIx(hr75) | −2.8 (−11.0–4.0) | 0.25 | |||
| Control | −10.3 (−13.6–−7.8) | −5.0 (−9.1–0.00) | 6.0 (2.5–11.5) | 0.04 | |
| RT | −7.3 (−12.1–−0.50) | −2.8 (−10.9–4.0) | 3.3 (−2.0–7.0) | 0.34 | |
| SEVR | 18.3 (−7.0–51.0) | 0.07 | |||
| Control | 166.0 (149.3–193.9) | 153.3 (149.6–157.1) | −11.3 (−38.5–6.3) | 0.21 | |
| RT | 157.3 (142.0–183.6) | 167.8 (147.5–189.0) | 7.0 (−8.0–16.0) | 0.33 | |
| P1 (mmHg) | −6.25 (−13.0–5.0) | 0.59 | |||
| Control | 112.0 (100.0–116.9) | 104.8 (103.5–109.3) | 0.75 (−2.5–7.0) | 0.29 | |
| RT | 110.0 (105.8–114.8) | 106.0 (103.0–107.0) | −5.5 (−9.3–5.3) | 0.01 | |
| P2 (mmHg) | −3.0 (−15.0–11.5) | 0.57 | |||
| Control | 111.0 (101.0–114.5) | 105.3 (103.4–108.9) | −1.0 (−12.5–9.0) | 0.40 | |
| RT | 109.3 (104.6 115.4) | 106.3 (102.4–108.9) | −4.0 (−8.75–0.00) | 0.04 | |
| PPA | 0.01 (−0.06–0.06) | 0.27 | |||
| Control | 1.70 (1.65–1.74) | 1.67 (1.60–1.68) | −0.05 (−0.11–−0.04) | 0.09 | |
| RT | 1.66 (1.57–1.71) | 1.62 (1.56–1.71) | −0.04 (−0.05–0.00) | 0.07 | |
| cSBP (mmHg) | −2.25 (−12.0–11.5) | 0.52 | |||
| Control | 112.5 (102.5–116.9) | 106.5 (104.3–110.1) | −1.5 (−13.5–7.5) | 0.40 | |
| RT | 111.5 (106.4–117.5) | 106.5 (104.6–109.0) | −3.8 (−8.0–−1.5) | 0.01 | |
| cDBP (mmHg) | 1.0 (−8.5–18.0) | 0.79 | |||
| Control | 85.5 (71.5–89.3) | 75 (70.9–77.8) | −5.0 (−16.3–5.0) | 0.16 | |
| RT | 80.8 (75.9–85.6) | 76.3 (72.0–79.5) | −4.0 (−7.0–0.0) | 0.02 | |
| bSBP (mmHg) | −5.5 (−17.0–6.0) | 0.39 | |||
| Control | 131.0 (121.0–135.8) | 127.5 (122.0–130.0) | −1.0 (−10.0–7.5) | 0.40 | |
| RT | 132.0 (123.8–137.0) | 123.5 (119.8–129.8) | −6.5 (−13.5–0.0) | 0.03 | |
| bDBP (mmHg) | 0.00 (−10.0–17.0) | 0.86 | |||
| Control | 84.5 (70.5–88.3) | 74.0 (69.8–76.8) | −5.0 (−16.0–5.0) | 0.16 | |
| RT | 81.0 (75.0–84.0) | 75.5 (71.5–78.5) | −5.0 (−7.5–−1.5) | 0.01 | |
| Heart Rate (b.p.m.) | −3.5 (−17.5–5.0) | 0.15 | |||
| Control | 74.5 (60.5–80.8) | 72.0 (67.8–79.3) | −0.50 (−5.5–14.0) | 0.89 | |
| RT | 74.0 (63.3–80.0) | 64.5 (60.0–72.0) | −4.0 (−8.0–1.5) | 0.01 | |
| cIMT far wall (mm) | −0.005 (−0.09–0.05) | 0.39 | |||
| Control | 0.48 (0.47–0.53) | 0.51 (0.50–0.55) | −0.01 (−0.06–0.06) | 0.61 | |
| RT | 0.52 (0.50–0.55) | 0.51 (0.49–0.54) | −0.01 (−0.05–0.01) | 0.49 | |
| cIMT near wall (mm) | −0.03 (−0.16–0.02) | 0.88 | |||
| Control | 0.52 (0.49–0.55) | 0.55 (0.51–0.58) | 0.05 (−0.06–0.07) | 0.44 | |
| sRT | 0.52 (0.50–0.55) | 0.54 (0.50–0.58) | 0.02 (−0.01–0.05) | 0.15 | |
Abbreviations: Aix, aortic augmentation index; bDBP, brachial diastolic blood pressure; bSBP, brachial systolic blood pressure; cDBP, central diastolic blood pressure; cfPWV, carotid–femoral pulse wave velocity; cIMT, carotid intima-media thickness; cSBP, central systolic blood pressure; P1, is the forward wave amplitude caused by stroke volume ejection at the aorta; P2, is the reflected wave amplitude; PPA, pulse pressure amplification; SEVR, subendocardial viability ratio. Significance was calculated using Wilcoxon signed-rank test. P-values with a superscript b indicate between-group analysis, and w indicates within-group analysis. Median values are presented with 25th and 75th percentile in parentheses, and median change scores are presented with the accompanied 95% confidence intervals. Significant P values (P<0.05) are bolded.
Lipids and inflammatory and atherogenic markers
Regarding blood lipids, total cholesterol, high-density lipoprotein, LDL and triglycerides did not change in either group with the intervention (all P>0.5) (Table 3). As for atherogenic and inflammatory markers, VEGF significantly increased after training (P = 0.03). CRP, metallopeptidase-9, soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1, myeloperoxidase, IL-8, total plasminogen activator inhibitor-1, sE-selectin, monocyte chemotactic protein-1, and oxLDL did not change with RT (all P≥0.1) (Table 4). An increase in oxLDL (P = 0.03) and a decrease in monocyte chemotactic protein-1 (P = 0.046) were noted in the control group.
Table 3.
Lipids
| Outcomes |
Median (25th–75th percentile)
|
Median change (95% confidence interval)
|
P-value | ||
|---|---|---|---|---|---|
| Pre-test | Post-test | Within-group changes | Between-group changes | ||
| Cholesterol (mg dl−1) | −12.5 (−23.5–5.0) | 0.047 b | |||
| Control | 163.0 (150.5–183.8) | 166.0 (153.0–199.3) | 13.5 (2.5–18.0) | 0.07w | |
| RT | 163.0 (140.0–176.0) | 164.0 (134.0–174.0) | 1.0 (−8.0–7.0) | 0.53w | |
| HDL (mg dl−1) | −1.0 (−6.0–4.0) | 0.62 | |||
| Control | 39.0 (37.0–40.0) | 41.0 (37.0–45.0) | 1.0 (1.0–6.0) | 0.34 | |
| RT | 41.0 (39.0–45.0) | 40.0 (39.0–46.0) | 0.0 (−1.5–4.0) | 0.53 | |
| LDL (mg dl−1) | −9.5 (−27.0–4.0) | 0.15 | |||
| Control | 102.0 (77.3–124.5) | 104.5 (91.3–123.8) | 7.5 (0.5–25.0) | 0.07 | |
| RT | 85.0 (66.0–108.0) | 84.0 (72.0–101.0) | −2.0 (−12.0–10.0) | 0.66 | |
| TG (mg dl−1) | 9.5 (−59.5–72.5) | 0.98 | |||
| Control | 124.0 (117.8–138.5) | 107.5 (99.5–115.5) | −16.5 (−55.5–94.0) | 0.60 | |
| RT | 118.0 (85.5–164.5) | 120.0 (75.0–154.0) | −7.0 (−26.0–25.0) | 0.51 | |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride. Significance was calculated using the Wilcoxon signed-rank test. Pvalues with a superscript b indicate between-group analysis, and w indicates within-group analysis. Median values are presented with 25th and 75th percentile in parentheses, and median change scores are presented with the accompanied 95% confidence intervals. Significant P values (P<0.05) are bolded.
Table 4.
Inflammatory and Atherogenic Markers
| Outcomes |
Median (25th–75th percentile)
|
Median change ( 95% confidence interval)
|
P-value | ||
|---|---|---|---|---|---|
| Pre-test | Post-test | Within-group changes | Between-group changes | ||
| oxLDL (Ul−1) | −5.5 (−11.9–0.89) | 0.08b | |||
| Control | 53.2 (52.9–61.2) | 56.6 (56.5–71.7) | 3.4 (1.9–8.1) | 0.03 w | |
| RT | 51.2 (41.0–70.8) | 48.5 (43.2–60.0) | −2.1 (−8.4–1.8) | 0.11w | |
| CRP (mg l−1) | −0.45 (−2.4–2.4) | 0.29 | |||
| Control | 1.4 (0.47–4.7) | 1.5 (0.75–3.6) | 0.33 (−1.5–2.8) | 0.60 | |
| RT | 1.4 (0.47–3.1) | 1.3 (0.54–2.0) | −0.12 (−0.50–0.02) | 0.15 | |
| VEGF (pgml−1) | 47.0 (−311.5–210.8) | 0.62 | |||
| Control | 241.0 (193.0–436.0) | 374.0 (297.5–641.5) | −6.0 (−34.3–711.0) | 0.89 | |
| RT | 460.0 (246.5–644.5) | 520.0 (233.8–939.5) | 41.0 (10–159.5) | 0.03 | |
| MMP-9 (ng ml−1) | −46.1 (−142.0–35.5) | 0.37 | |||
| Control | 249.0 (198.8–261.0) | 266.5 (221.0–289.5) | 20.1 (−15.5–112.0) | 0.25 | |
| RT | 227.0 (169.0–271.0) | 214.0 (185.0–262.0) | −26.0 (−45.0–48.0) | 0.83 | |
| PAI-1 total (ng ml−1) | 23.0 (−0.90–50.4) | 0.12 | |||
| Control | 125.5 (102.7–157.5) | 114.5 (90.8–142.0) | −8.0 (−25.5–16.8) | 0.35 | |
| RT | 115.5 (79.6–175.5) | 148.0 (101.5–210.3) | 15.0 (4.0–26.9) | 0.06 | |
| sE-Selectin (ng ml−1) | −2.0 (−16.6–2.7) | 0.16 | |||
| Control | 34.1 (26.3–46.1) | 47.4 (29.4–48.2) | −0.45 (−2.4–12.5) | 0.92 | |
| RT | 33.9 (26.5–46.2) | 31.4 (23.3–43.1) | −2.4 (−4.8–1.2) | 0.20 | |
| sICAM-1 (ng ml−1) | 0.10 (−21.8–37.4) | 1.00 | |||
| Control | 146.4 (85.5–194.8) | 122.3 (92.1–192.5) | 0.90 (−22.1–27.6) | 0.75 | |
| RT | 139.0 (102.8–184.0) | 146.5 (108.5–173.0) | 1.0 (−7.4–5.5) | 0.95 | |
| sVCAM-1 (ng ml−1) | −25.0 (−152.0–171.0) | 0.92 | |||
| Control | 730.0 (664.5–834.5) | 744.0 (596.0–883.0) | 22.0 (−111.0–153.0) | 0.75 | |
| RT | 825.5 (768.3–962.5) | 900.5 (798.0–991.8) | −3.0 (−40.0–76.5) | 0.88 | |
| MPO (ng ml−1) | −46.1 (−261.6–47.8) | 0.21 | |||
| Control | 160.5 (140.3–267.8) | 251.5 (119.8–405.0) | 25.5 (−37.0–231.0) | 0.17 | |
| RT | 154.0 (82.1–237.8) | 118.0 (93.1–250.0) | −20.6 (−112.0–42.5) | 0.59 | |
| MCP-1 (pg ml−1) | 126.8 (7.5–201.8) | 0.11 | |||
| Control | 407.3 (373.8–490.3) | 343.3 (306.8–409.0) | −97.8 (−124.0–58.5) | 0.046 | |
| RT | 422.5 (303.5–559.3) | 421.3 (352.8–582.3) | 29.0 (−50.0–89.5) | 0.56 | |
| IL-8 (pg ml−1) | 3.4 (−189.4–12.7) | 0.35 | |||
| Control | 14.2 (11.8–28.6) | 22.6 (13.5–159.5) | −1.2 (−292.5–189.3) | 0.60 | |
| RT | 22.0 (11.3–41.5) | 19.4 (11.4–63.3) | 2.2 (−0.44–12.5) | 0.07 | |
Abbreviations: CRP, C-reactive protein; MCP-1, monocyte chemotactic protein-1; MMP-9, matrix metallopeptidase-9; MPO, myeloperoxidase; IL-8, interleukin-8; PAI-1 total, plasminogen activator inhibitor-1 total; oxLDL, oxidized LDL; sE-selectin, soluble E-selectin; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor. Significance was calculated using the Wilcoxon signed-rank test. P-values with a superscript b indicate between-group analysis, and w indicates within-group analysis. Median values are presented with 25th and 75th percentile in parentheses, and median change scores are presented with the accompanied 95% confidence intervals. Significant P values (P<0.05) are bolded.
Correlation between cSBP and atherosclerotic risk markers
As cSBP is strongly related to atherosclerotic disease and cardiovascular events11 and was significantly decreased after training, we correlated changes in cSBP with changes in markers of atherosclerotic risk (Table 5). In the RT group, cSBP was positively correlated with oxLDL (r = 0.42, P = 0.03) and sE-selectin (r = 0.41, P = 0.04).
Table 5.
Spearman correlation coefficients for changes in cSBP and changes in atherogenic markers
| Biomarkers | R | P-value |
|---|---|---|
| oxLDL | 0.42 | 0.03 |
| CRP | 0.21 | 0.31 |
| VEGF | −0.1 | 0.61 |
| MMP-9 | −0.17 | 0.41 |
| PAI-1 total | −0.09 | 0.65 |
| sE-Selectin | 0.41 | 0.04 |
| sICAM-1 | 0.08 | 0.68 |
| sVCAM-1 | −0.13 | 0.53 |
| MPO | −0.06 | 0.78 |
| MCP-1 | −0.22 | 0.27 |
| IL-8 | 0.28 | 0.16 |
Abbreviations: CRP, C-reactive protein; IL-8, interleukin-8; MMP-9, matrix metallopeptidase-9; oxLDL, oxidized LDL; PAI-1 total, plasminogen activator inhibitor-1 total; sE-selectin, soluble E-selectin; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; MPO, myeloperoxidase; MCP-1, monocyte chemotactic protein-1; VEGF, vascular endothelial growth factor. Data are presented as Spearman correlation coefficient (r) and level of significance (P value). Significant P values (P<0.05) are bolded.
DISCUSSION
The principal findings of this study are that high-intensity RT, with progressive increases in both training intensity and volume, improved central and brachial blood pressures without weight loss and had no effect on indices of arterial stiffness in overweight/obese young men. These findings were accompanied by significant increases in VEGF, muscle strength and lean body mass and a decrease in body fat percentage. To our knowledge, this is the first study to determine the effects of an RT intervention on central blood pressure and arterial stiffness in overweight/obese young men, although Heffernan et al.23 did include some overweight participants in their prior study.
Central and peripheral blood pressure
Recently, it has become apparent that noninvasive determination of central blood pressure may more accurately reflect loading conditions of the ventricles and coronary arteries, more strongly relate to cardiovascular function and end-organ damage, and therefore, may more accurately estimate future CVD risk, compared with peripheral blood pressure.11,12 In addition, RT has been demonstrated to be associated with reduced risk of coronary heart disease, although the mechanism(s) for the beneficial effects are currently unclear.24 In the present study, we demonstrated that one potential mechanism by which RT may decrease CVD risk is through a decrease in central blood pressure. It is important to note that the decreases in central pressure occurred in the absence of any change in the indices of arterial stiffness and wave reflection, including cfPWV and AIx, respectively. Additionally, the decrease in brachial blood pressure, also noted, was similar to that approximated using a meta-analysis,25 and these decreases were not seen in the control group. The improvement in central pressures is in agreement with Heffernan et al.23 These authors demonstrated reductions in central blood pressures after short-term RT in both African American and Caucasian men, which occurred without a change in brachial blood pressure and was independent of carotid stiffness and aortic pulse wave velocity, although an ethnic difference was noted for brachial stiffness, with an increase observed in African American young men. Furthermore, studies by Taaffe et al.26 and Figueroa et al.27 have suggested a reduction in central pressures with RT in older adults and postmenopausal women, respectively. The improvement in central pressure in the present study may be attributed to a decrease in peripheral vascular resistance, as suggested by an accompanied decrease in P2 (defined as the reflected pressure wave that augments the incident wave amplitude). Indeed, Anton et al.28 showed that 13 weeks of RT resulted in a significant decrease in femoral vascular resistance. This suspected decrease in vascular resistance may be a result of an improvement in microvascular perfusion and/or endothelial function. Previous studies indicate that RT for a duration of 12 weeks to 1 year can improve endothelial function (via brachial artery flow-mediated dilation) in populations at risk of impaired vascular reactivity—obese adults,29 overweight women30 and postmenopausal women.31 Unsurprisingly, RT does not affect flow-mediated dilation in healthy subjects with minimal or no endothelial impairment at baseline.10,32 Additionally, VEGF not only has a role in stimulating angiogenesis, but also acts as a vascular protective factor.33 The elevated levels of VEGF suggest a physiologic adaptation to exercise, which may indicate increased angiogenesis and preservation of the endothelial function.
Arterial stiffness
Similar to our findings, several studies indicate no change in arterial stiffness after RT.10,23,32,34 In addition, Fahs et al.35 noted that muscle strength is inversely associated with aortic stiffness. However, it has been suggested by others that RT can increase stiffness.6–8 Differences in arterial stiffness in response to RT may be due to several factors, including differences in training regimens, measurement protocols, study population and/or post-exercise timing of measurement. It was previously proposed that high-intensity RT was the main factor contributing to an increase in stiffness.10,36 In response, Casey et al.10 devised an RT intervention with progressive increases in intensity without volume increases, which did not result in changes in cfPWV or AIx. Our study, which also resulted in no change in cfPWV and AIx, incorporated progressive increases in both intensity and volume and utilized a higher training volume per session (as many as 24 sets) compared with training programs that resulted in increased stiffness (~18 sets). 6,7 It is possible that the short rest periods between sets in our RT intervention may have contributed to the physiologic responses to RT. Indeed, Kawano et al.7 noted that a combination of RT and endurance exercises appeared to minimize arterial stiffening compared with RT alone. Collectively, it appears that differences in training volume and intensity may not be the primary reasons for the reported discrepancies. Furthermore, it is unclear whether the reductions in arterial compliance seen in some RT interventions truly indicate a pathologic process, or are simply a physiologic adaptation that occurs with this type of exercise. Moreover, it is appropriate to mention that resistance-trained men performing vigorous RT for more than 10 years exhibit reduced arterial compliance but no difference in cIMT or endothelial function, compared with age-matched sedentary controls.37 Thus, although reduced arterial compliance has been associated with endothelial dysfunction,38 there is no evidence that links continuous RT (for any length of time) with impaired endothelial function.
Inflammation and atherogenic markers
Inflammatory cytokines and other atherogenic markers are linked with vascular dysfunction and atherosclerotic disease.13 In agreement with previous findings,15,17 we noted no significant improvements in blood lipids. In addition, the lack of change in CRP is also not surprising, as exercise appears to only decrease CRP in subjects with CRP levels >3.0 mg l−1.18,39 We also assessed cell adhesion molecules, soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1 and sE-Selectin, whose expression on the surface of a variety of cells, including endothelial cells, is believed to mediate the immune response to inflamed and injured vessels. We noted no change in these molecules, in agreement with Olson et al.,17 the only other RT intervention to investigate the potential effects of RT on cell adhesion molecules. In early-risk populations, RT seems to have little effect on measured inflammatory markers.
In addition, inflammation has been associated with indices of vascular stiffness. For example, CRP has been positively correlated with cfPWV,40–42 AIx40,43 and brachial pulse pressure.44 Furthermore, Vlachopoulos et al.45 demonstrated an etiologic association between acute systemic inflammation and increased pulse wave velocity. Indeed, we observed moderate positive correlations between changes in cSBP and both sE-Selectin and oxLDL. Improvements in cSBP might contribute to reductions in sE-Selectin and oxLDL and vascular injury, or vice versa.
A limitation of this investigation was that it was designed as an exploratory study to examine central blood pressure and arterial stiffness indices and powered to detect within-group changes.8,23 Also, we observed that a few participants in the control group did not strictly adhere to the directive to not participate in exercise training, which may limit interpretation of between-group comparisons. However, robust statistical analysis was applied to minimize spurious findings. A 3:1 randomization scheme was chosen to ensure that this study had adequate power to detect an effect size for within-group analysis on central blood pressure and indices of arterial stiffness based on similar studies. An additional limitation is the gender-specific population studied.
In summary, this study demonstrated that RT, with progressive increases in both training intensity and volume, reduces both central and brachial blood pressures without weight loss and independently of effects on arterial stiffness in overweight/obese young men. These findings suggest that high-intensity RT may decrease CVD risk by improving central blood pressure without compromising arterial stiffness in overweight/obese young adults. Future studies should examine whether the effects of RT on central blood pressures are related to true changes in risk for future CVD events.
What is known about this study
Previous studies suggest that RT decreases the risk of CVD; however, the mechanisms by which RT decreases CVD risk are unclear.
Central blood pressure is a better predictor of CVD risk than brachial blood pressure.
The effects of RT on central blood pressures are generally unknown.
There are conflicting evidence and conclusions regarding the effects of RT on arterial stiffness, and changes induced by RT may be dependent upon the subject population.
What this study adds
This study suggests that RT decreases central blood pressures in obese young men.
The decreases in central blood pressures occur independently of changes in the indices of arterial stiffness, such as AIx and cfPWV, weight loss or changes in biomarkers of CVD risk.
This study suggests that the decreases in central blood pressures, noted with RT, may be a contributing mechanism to the cardioprotective effects of RT.
ACKNOWLEDGEMENTS
We would like to thank Mary Lee, Brian Le, Ergit Paparisto, Stacy Young, Kristin Anderson, Yashesh Shah and the entire Exercise and Metabolic Disease Research (EMDR) team for their commitment to this study. We thank the dedicated nurses and staff of the UCLA GCRC, Gonda (Goldschmied) Diabetes Center, Diabetes and Endocrinology Research Center (DERC) and Elisa Terry and colleagues at the John Wooden Recreation Center. We also thank the UCLA Academic Technology Services Statistical Consulting Group for their statistical support. Furthermore, we thank all participants for their time and effort.
This work was supported by the American Heart Association (BGIA no 0765139Y to CKR.), the National Heart, Lung and Blood Institute (P50 HL105188 to CKR.) and the National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR000124. RAH. is supported in part by the American Heart Association (10SDG305006).
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
Author contributions: CKR, DMC and RAH contributed to the conception and design of the research; DMC led the training intervention; SLK, CSO, MK and CL performed experiments; DMC analyzed data; DMC and SLK interpreted results of experiments; DMC SLK and CKR drafted and revised the manuscript; DMC, CKR, SLK, RAH, CL, MK and CSO edited the manuscript; DMC, CKR, SLK, RAH, CL, MK and CSO approved the final version of the manuscript.
REFERENCES
- 1.Roberts CK, Barnard RJ. Effects of exercise and diet on chronic disease. J Appl Physiol. 2005;98(1):3–30. doi: 10.1152/japplphysiol.00852.2004. [DOI] [PubMed] [Google Scholar]
- 2.Telama R, Yang X, Viikari J, Valimaki I, Wanne O, Raitakari O. Physical activity from childhood to adulthood: a 21-year tracking study. Am J Prev Med. 2005;28(3):267–273. doi: 10.1016/j.amepre.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Telama R, Hirvensalo M, Mattsson N, Viikari JS, Raitakari OT. The long-itudinal effects of physical activity history on metabolic syndrome. Med Sci Sports Exerc. 2008;40(8):1424–1431. doi: 10.1249/MSS.0b013e318172ced4. [DOI] [PubMed] [Google Scholar]
- 4.Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation. 2006;113(22):2642–2650. doi: 10.1161/CIRCULATIONAHA.105.584060. [DOI] [PubMed] [Google Scholar]
- 5.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 6.Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, et al. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation. 2004;110(18):2858–2863. doi: 10.1161/01.CIR.0000146380.08401.99. [DOI] [PubMed] [Google Scholar]
- 7.Kawano H, Tanaka H, Miyachi M. Resistance training and arterial compliance: keeping the benefits while minimizing the stiffening. J Hypertens. 2006;24(9):1753–1759. doi: 10.1097/01.hjh.0000242399.60838.14. [DOI] [PubMed] [Google Scholar]
- 8.Cortez-Cooper MY, DeVan AE, Anton MM, Farrar RP, Beckwith KA, Todd JS, et al. Effects of high intensity resistance training on arterial stiffness and wave reflection in women. Am J Hypertens. 2005;18(7):930–934. doi: 10.1016/j.amjhyper.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Rakobowchuk M, McGowan CL, de Groot PC, Bruinsma D, Hartman JW, Phillips SM, et al. Effect of whole body resistance training on arterial compliance in young men. Exp Physiol. 2005;90(4):645–651. doi: 10.1113/expphysiol.2004.029504. [DOI] [PubMed] [Google Scholar]
- 10.Casey DP, Beck DT, Braith RW. Progressive resistance training without volume increases does not alter arterial stiffness and aortic wave reflection. Exp Biol Med. 2007;232(9):1228–1235. doi: 10.3181/0703-RM-65. [DOI] [PubMed] [Google Scholar]
- 11.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50(1):197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 12.Safar ME, Jankowski P. Central blood pressure and hypertension: role in cardiovascular risk assessment. Clin Sci (Lond) 2009;116(4):273–282. doi: 10.1042/CS20080072. [DOI] [PubMed] [Google Scholar]
- 13.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6(6):399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 14.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension. 2003;42(4):468–473. doi: 10.1161/01.HYP.0000090360.78539.CD. [DOI] [PubMed] [Google Scholar]
- 15.Klimcakova E, Polak J, Moro C, Hejnova J, Majercik M, Viguerie N, et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab. 2006;91(12):5107–5112. doi: 10.1210/jc.2006-0382. [DOI] [PubMed] [Google Scholar]
- 16.Donges CE, Duffield R, Drinkwater EJ. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med Sci Sports Exerc. 2010;42(2):304–313. doi: 10.1249/MSS.0b013e3181b117ca. [DOI] [PubMed] [Google Scholar]
- 17.Olson TP, Dengel DR, Leon AS, Schmitz KH. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int J Obes (Lond) 2007;31(6):996–1003. doi: 10.1038/sj.ijo.0803534. [DOI] [PubMed] [Google Scholar]
- 18.Heffernan KS, Jae SY, Vieira VJ, Iwamoto GA, Wilund KR, Woods JA, et al. C-reactive protein and cardiac vagal activity following resistance exercise training in young African-American and white men. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R1098–R1105. doi: 10.1152/ajpregu.90936.2008. [DOI] [PubMed] [Google Scholar]
- 19.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38(4):932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95(7):1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(Pt 1):263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultra-centrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 23.Heffernan KS, Fahs CA, Iwamoto GA, Jae SY, Wilund KR, Woods JA, et al. Resistance exercise training reduces central blood pressure and improves microvascular function in African American and white men. Atherosclerosis. 2009;207(1):220–226. doi: 10.1016/j.atherosclerosis.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 24.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288(16):1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 25.Cornelissen VA, Fagard RH. Effect of resistance training on resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2005;23(2):251–259. doi: 10.1097/00004872-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Taaffe DR, Galvao DA, Sharman JE, Coombes JS. Reduced central blood pressure in older adults following progressive resistance training. J Hum Hypertens. 2007;21(1):96–98. doi: 10.1038/sj.jhh.1002115. [DOI] [PubMed] [Google Scholar]
- 27.Figueroa A, Arjmandi BH, Wong A, Sanchez-Gonzalez MA, Simonavice E, Daggy B. Effects of hypocaloric diet, low-intensity resistance exercise with slow movement, or both on aortic hemodynamics and muscle mass in obese postmenopausal women. Menopause. 2013;20(9) doi: 10.1097/GME.0b013e3182831ee4. (e-pub ahead of print 18 March 2013) [DOI] [PubMed] [Google Scholar]
- 28.Anton MM, Cortez-Cooper MY, DeVan AE, Neidre DB, Cook JN, Tanaka H. Resistance training increases basal limb blood flow and vascular conductance in aging humans. J Appl Physiol. 2006;101(5):1351–1355. doi: 10.1152/japplphysiol.00497.2006. [DOI] [PubMed] [Google Scholar]
- 29.Schjerve IE, Tyldum GA, Tonna AE, Stolen T, Loennechen JP, Hansen HEM, et al. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin Sci. 2008;115(9):283–293. doi: 10.1042/CS20070332. [DOI] [PubMed] [Google Scholar]
- 30.Olson TP, Dengel DR, Leon AS, Schmitz KH. Moderate resistance training and vascular health in overweight women. Med Sci Sports Exerc. 2006;38(9):1558–1564. doi: 10.1249/01.mss.0000227540.58916.0e. [DOI] [PubMed] [Google Scholar]
- 31.Casey D, Pierce G, Howe K, Mering M, Braith R. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive post-menopausal women. Eur J Appl Physiol. 2007;100(4):403–408. doi: 10.1007/s00421-007-0447-2. [DOI] [PubMed] [Google Scholar]
- 32.Rakobowchuk M, McGowan CL, de Groot PC, Hartman JW, Phillips SM, MacDonald MJ. Endothelial function of young healthy males following whole body resistance training. J Appl Physiol. 2005;98(6):2185–2190. doi: 10.1152/japplphysiol.01290.2004. [DOI] [PubMed] [Google Scholar]
- 33.Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol. 2001;280(6):C1375–C1386. doi: 10.1152/ajpcell.2001.280.6.C1375. [DOI] [PubMed] [Google Scholar]
- 34.Yoshizawa M, MAEDA S, Miyaki A, Misono M, Saito Y, Tanabe K, et al. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: A randomized controlled trial in women aged 32-59. Br J Sports Med. 2008;43(8):615–618. doi: 10.1136/bjsm.2008.052126. [DOI] [PubMed] [Google Scholar]
- 35.Fahs CA, Heffernan KS, Ranadive S, Jae SY, Fernhall B. Muscular strength is inversely associated with aortic stiffness in young men. Med Sci Sports Exerc. 2010;42(9):1619–1624. doi: 10.1249/MSS.0b013e3181d8d834. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto T, Masuhara M, Ikuta K. Effect of low-intensity resistance training on arterial function. Eur J Appl Physiol. 2011;111(5):743–748. doi: 10.1007/s00421-010-1702-5. [DOI] [PubMed] [Google Scholar]
- 37.Kawano H, Tanimoto M, Yamamoto K, Sanada K, Gando Y, Tabata I, et al. Resistance training in men is associated with increased arterial stiffness and blood pressure but does not adversely affect endothelial function as measured by arterial reactivity to the cold pressor test. Exp Physiol. 2008;93(2):296–302. doi: 10.1113/expphysiol.2007.039867. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura M, Sugawara S, Arakawa N, Nagano M, Shizuka T, Shimoda Y, et al. Reduced vascular compliance is associated with impaired endothelium-dependent dilatation in the brachial artery of patients with congestive heart failure. J Card Fail. 2004;10(1):36–42. doi: 10.1016/s1071-9164(03)00585-2. [DOI] [PubMed] [Google Scholar]
- 39.Lakka TA, Lakka HM, Rankinen T, Leon AS, Rao DC, Skinner JS, et al. Effect of exercise training on plasma levels of C-reactive protein in healthy adults: the HERITAGE Family Study. Eur Heart J. 2005;26(19):2018–2025. doi: 10.1093/eurheartj/ehi394. [DOI] [PubMed] [Google Scholar]
- 40.Kullo IJ, Seward JB, Bailey KR, Bielak LF, Grossardt BR, Sheedy Ii PF, et al. C-Reactive protein is related to arterial wave reflection and stiffness in asymptomatic subjects from the community. Am Jl Hypertens. 2005;18(8):1123–1129. doi: 10.1016/j.amjhyper.2005.03.730. [DOI] [PubMed] [Google Scholar]
- 41.Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24(5):969–974. doi: 10.1161/01.ATV.zhq0504.0173. [DOI] [PubMed] [Google Scholar]
- 42.Nagano M, Nakamura M, Sato K, Tanaka F, Segawa T, Hiramori K. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180(1):189–195. doi: 10.1016/j.atherosclerosis.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Kampus P, Kals J, Ristimae T, Fischer K, Zilmer M, Teesalu R. High-sensitivity C-reactive protein affects central haemodynamics and augmentation index in apparently healthy persons. J Hypertens. 2004;22(6):1133–1139. doi: 10.1097/00004872-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Abramson JL, Weintraub WS, Vaccarino V. Association between pulse pressure and C-reactive protein among apparently healthy US adults. Hypertension. 2002;39(2):197–202. doi: 10.1161/hy0202.104270. [DOI] [PubMed] [Google Scholar]
- 45.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112(14):2193–2200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]


