Abstract
Purpose
Sleep disturbance, fatigue and depression are common complaints in patients with cancer, and often contribute to worse quality of life (QoL). Circadian activity rhythms (CARs) are often disrupted in cancer patients. These symptoms worsen during treatment, but less is known about their long-term trajectory.
Methods
Sixty-eight women with stage I-III breast cancer (BC) scheduled to receive ≥4 cycles of chemotherapy, and age-, ethnicity- and education-matched normal, cancer-free controls (NC) participated. Sleep was measured with actigraphy (nocturnal total sleep time [nocturnal TST] and daytime total nap time [NAPTIME]) and with the Pittsburgh Sleep Quality Index (PSQI); fatigue with the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF); depression with the Center of Epidemiological Studies-Depression (CES-D). CARs were derived from actigraphy. Several measures of QoL were administered. Data were collected at three time points: before (Baseline), end of cycle 4 (Cycle-4), and one year post-chemotherapy (1-Year).
Results
Compared to NC, BC had longer NAPTIME, worse sleep quality, more fatigue, more depressive symptoms, more disrupted CARs and worse QoL at Baseline (all p’s<0.05). At Cycle-4, BC showed worse sleep, increased fatigue, more depressive symptoms, and more disrupted CARs compared to their own Baseline levels and to NC (all p’s<0.05). By 1-year, BC’s fatigue, depressive symptoms and QoL returned to Baseline levels but were still worse than those of NC, while NAPTIME and CARs did not differ from NC’s.
Conclusion
Additional research is needed to determine if beginning treatment of these symptoms before the start of chemotherapy will minimize symptom severity over time.
Keywords: Sleep disturbance, fatigue, depression, circadian activity rhythms, quality of life, breast cancer
INTRODUCTION
Sleep disturbance, fatigue and depressive symptoms are common in and often negatively impact patients with breast cancer (BC) [1,2]. These symptoms often exist before the start of chemotherapy treatment [3–5], and significantly impact patients’ quality of life (QoL) [6–9]. However, less is known about the trajectory of these symptoms over time, particularly after the end of chemotherapy.
The most common sleep-related complaints of many cancer patients are symptoms of insomnia [10]. In one previous study, 52% of mixed types of cancer patients reported sleeping difficulties, 66% reported their insomnia began before their cancer diagnosis, and 58% reported that cancer aggravated their sleep problem [11]. The fact that cancer aggravated sleep suggests that the challenges faced by cancer patients may perpetuate insomnia, which in turn may exacerbate other symptoms associated with cancer [10]. While insomnia often becomes chronic in other populations, little is known about the long term trajectory of insomnia in cancer patients, particularly after treatment ends.
Cancer related fatigue (CRF) is another chronic and debilitating symptom in patients with cancer, characterized by extreme tiredness and inability to function due to lack of energy. Our research group has shown that in women with breast cancer, CRF worsens during cancer treatment, whether or not it was present before chemotherapy [3,12,13]. CRF negatively impacts QoL and productivity. At times CRF leads to poor compliance with chemotherapy regimens [14,15] and often is the reason for patients discontinuing treatment [16]. CRF has also been shown to predict other symptoms such as depression [17], which is found in 40–82% of cancer patients [18,19].
Circadian rhythms (i.e., 24-hour rhythms) include changes or alternations of hormone secretion, body temperature, and sleep-wake activity cycles. Disrupted circadian rhythms have been associated with mortality in dementia [20], older men [21], and older women [22], as well as in cancer patients [23]. Actigraphy has been used to measure circadian activity rhythms (CARs) in patients with cancer [1,3,23,24]. We have previously shown that the first administration of chemotherapy is associated with transient circadian disruption but that repeated administration of chemotherapy results in progressively worse and more enduring rhythm impairments [25].
Little is known about the long term trajectory of any of these symptoms or the relationship between these symptoms and QoL. Lower QoL and its negative effects on daily functioning have been reported in patients undergoing chemotherapy as well as in some breast cancer survivors after chemotherapy [26,27]. In addition, cancer patients and their health care providers have recently become more aware of these problems and are now more focused on maintaining QoL [28,29]. The studies that are available on cancer and its treatment-related symptoms are limited by either small samples, cross-sectional design, absence of baseline data, absence of long-term follow-up data, or no healthy controls. This study explored changes in sleep, CRF, depressive symptoms, CARs and QoL from pre-chemotherapy to the end of four cycles of chemotherapy and one year later. Results were compared to those in non-cancer matched women. We hypothesized that women with breast cancer would experience more symptoms, more disrupted CARs, and worse QoL at all time-points compared to controls, and that the symptoms would be worse at cycle 4 and 1-year than at baseline.
METHODS
Participants
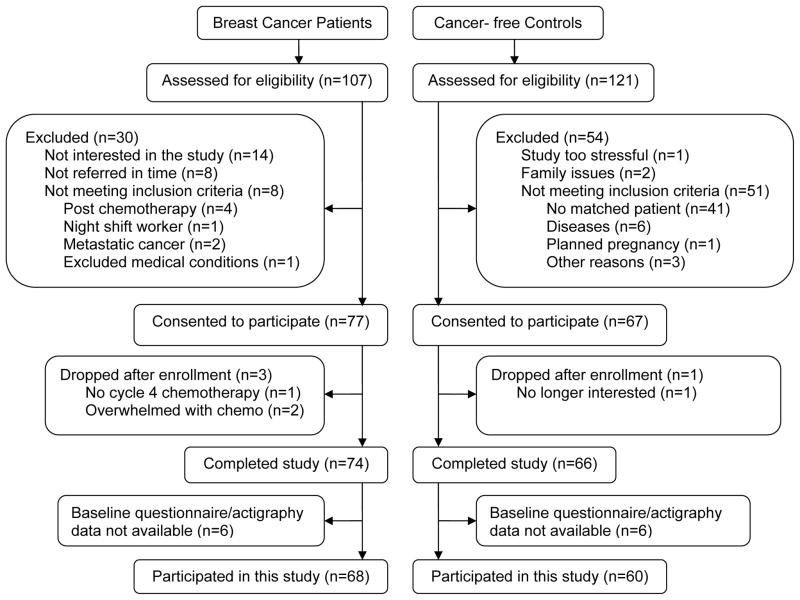
One-hundred and seven women with newly diagnosed stage I-III breast cancer, scheduled to receive at least four cycles of adjuvant or neoadjuvant chemotherapy were referred by oncologists in the San Diego area, mainly from the UCSD Moores Cancer Center. Each was screened for inclusion and 68 were enrolled in the study (see Fig. 1). Non-cancer control women were recruited as a comparison group. Each BC was asked to nominate a friend, or when a friend was not available, matched volunteers were recruited. Each “friend” was matched to the cancer patient by age (within five years), ethnicity, and education. A total of 121 cancer-free healthy women were screened as potential controls, and 60 (normal control; NC) were included in this study. “$150 was provided to each participant at the end of participation and was prorated for those who did not complete the protocol.
Fig. 1.
Screening and Enrollment Flowchart
Exclusion criteria included pregnancy, current bone marrow transplants and radiotherapy, metastatic breast cancer, confounding underlying medical illnesses, significant pre-existing anemia or other physical or psychological impairments. Exclusion criteria for the NC also included any history of cancer.
The study was approved by the UCSD Human Research Protections Committee and the UCSD Moores Cancer Center’s Protocol Review and Monitoring Committee.
Procedures
After informed consent was obtained, BC’s medical records were abstracted for medical history and current medication use. Objective sleep and CAR data and questionnaires on sleep, fatigue, depressive symptoms and QoL were collected at three time points: before the start of chemotherapy (Baseline), at the end of cycle 4 chemotherapy (Cycle-4), and one year after the start of chemotherapy (1-Year). The timing of the baseline data collection varied depending on patients’ start day of chemotherapy, but was always at least 3 days before chemotherapy. Data for NC were collected at the same time points. All participants completed a sleep log on bedtime and rising time for each night during which the actigraph was worn. The time to complete all questionnaires at each time point was about one hour. All data were collected either at the UCSD Moores Cancer Center or at the participants’ homes.
Measures
Sleep
Objective Sleep Measures
Sleep was measured with wrist activity. All women wore an actigraph for three consecutive days (72 hours). While the ideal recording time for an actigraph is generally one week, due to potential subject burden, the minimum of three days, as suggested by the American Academy of Sleep Medicine (AASM) practice parameters for actigraphy [30], was used in this study.
The Actillume-II (Ambulatory Monitoring Inc, Ardsley, New York) and the Actiwatch-Light (Philips Respironics Mini Mitter, Bend, OR) were used. Technical specification of the devises was previously provided [31], however, briefly, the Actillume-II actigraph is a small device approximately 1x3x6 cm in size, contains a piezoelectric linear accelerometer (sensitive to 0.003 g and above), a microprocessor, 32K RAM memory, and associated circuitry. The Actiwatch-Light is a watch-like device approximately 1x2.5x5 cm in size, uses a piezoelectric linear accelerometer (sensitivity <.01 g-force) with a sampling rate of 32Hz to measure and record wrist movement. Actigraphy data were downloaded onto a desktop computer and hand-edited with additional information from a sleep log completed by the participants. The Action-4 software for Actillume-II and Actiware-5 software for Actiwatch-Light were used to score sleep and wake. A one-minute epoch setting was used for both actigraphy devices. For the sleep/wake analysis, the SUMACT (summary activity) channel was used for Actillume, and the default (medium) activity sensitivity threshold was set for the Actiwatch-Light. In this study a nap was defined as >10 continuous minutes of inactivity during the out-of-bed time. Two parameters were used in this study, total nocturnal sleep time (nocturnal TST) and total nap time (NAPTIME).
Thirteen BC patients and seven controls wore an Actillume-II, and the rest of participants wore an Actiwatch-Light. To establish equivalency between the two devices, a validation study in eight volunteers was conducted with both devices worn concurrently on the same wrist for 72-hours. The Actillume-II-derived SUMACT count and the Actiwatch-Light-derived activity count data, as well as the software-scored sleep/wake data based on the two types of activity count were highly correlated (all r’s >0.85), and therefore deemed equivalent for the purpose of this study.
For each participant, actigraphy recordings were initiated on the same day at each time point. The day chosen was based on the day of the chemotherapy administration for patients.
Subjective Sleep Measures
Subjective measures of sleep quality were collected with the Pittsburgh Sleep Quality Index (PSQI) [32,33], which measures reported sleep patterns and sleep problems, including perceived sleep quality, sleep latency, sleep efficiency and napping behavior. The PSQI is a 19-item questionnaire that has been demonstrated to have high internal consistency (0.83), test-retest reliability (0.85) and diagnostic validity. A global sleep quality score is derived from the PSQI over the prior one-week period and was utilized in these analyses. Global sleep quality scores are continuous (range 0–21) with high scores reflecting poor sleep quality.
Fatigue
The Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) [34,35] was used to measure fatigue. The MFSI-SF has 30-items which are collapsed into five subscales: General, Physical, Emotional, Mental, and Vigor. Each subscale includes six items and each item is rated on a 5-point scale indicating how true the statement was during the last week (0=not at all, 4=extremely). The sum of General, Physical, Emotional, and Mental subscale scores minus the Vigor subscale score generates a total score. Range of possible score for each subscale is 0 to 24, and the range for total score is −24 to 96, with higher score indicating more severe fatigue, except for the Vigor subscale, where larger score indicates less fatigue.
Depressive symptoms
Center of Epidemiological Studies-Depression (CES-D) [36] was used to measure depressive symptoms. Answers to the 20-item items on the scale are based on the degree to which symptoms were experienced during the last week. This scale has been shown to have high reliability and validity in the assessment of depressive symptoms [36]. Since the CES-D reflects cognitive and affective symptoms rather than somatic symptoms of depression, it is highly recommended for use with patients with medical problems.
Circadian Activity Rhythms (CARs)
CARs were derived from the actigraphy activity level per epoch (minute) by fitting each subject’s activity data to a 5-parameter extended cosine model. This 5-parameter model is an anti-logistic transformation of the standard cosine curve, and it allows for estimation of parameters describing the shape of the 24-hour rest/activity rhythm [37]. A series of variables could be calculated from this model, including the R-squared. R-squared is the reduction in squared error which results from using a model to summarize data (and predict future values) compared to the mean [25]. In other words, more rhythmic and stronger rhythms have a larger R-squared value. The R-squared is therefore reported in the study as the measurement of circadian activity rhythmicity.
Quality of Life
QoL was assessed with both the Functional Outcomes of Sleep Questionnaire (FOSQ) [38] and the Medical Outcomes Study Short Form (SF-36) health survey [39].
The FOSQ is designed to measure functional status in situations that produce sleepiness. It is comprised of 30 items, which collapse into five sub-scales: Vigilance, Intimacy and Sexual Relationships, General Productivity, Activity Level, and Social Outcome. The scales are added together as a weighted total score, which ranges from 0–20. Lower scores indicate worse sleepiness-related QoL [38].
The SF-36 is a commonly used measurement of health related QoL, it is a generic 36-item health status instrument, with eight subscales measuring eight domains of health: physical functioning, role limitations because of physical problems, bodily pain, general health perceptions, vitality, social functioning, role limitations because of emotional problems, and mental health [39]. Each subscale is scored on a range from 0 to 100, with lower scores indicating worse QoL. The norm-based Physical Component Summary (PCS) and Mental Component Summary (MCS) scores are calculated from these eight subscales. The PCS and MCS both have a mean of 50 and a standard deviation of 10.
Medical co-morbidity
All participants were asked to reported if they had any of the following co-morbid conditions at each time point: cardiovascular disease, pulmonary disease, central nervous disorders, gastrointestinal disease, renal disease, endocrine disease, connective tissue disease, infections, dementia, arthritis, diabetes, ulcer, hiatal hernia, esophageal disease, neck/back/spine problems, epilepsy, headaches, high blood pressure, kidney problems, stroke, asthma, emphysema, edema, thyroid disease, or other disorders.
Data Analysis
Descriptive statistics (mean, standard deviation and standard error) were calculated for all outcomes at all three time points. T-tests and Chi-square or Fisher’s exact tests were used to examine the differences in demographic characteristics between the two groups, and between those who completed and who did not completed the Cycle-4 of 1-Year study in each group. Chi-square tests or Fisher’s exact tests were also used to examine possible group differences in medical co-morbidities and drop-out rates. Pearson correlation analysis was performed between outcome variables and age and body mass index (BMI). Cohen’s d effect size and 95% confidence interval (CI) was also calculated for nocturnal TST and NAPTIME at each time point (BC vs. NC).
A mixed model analysis [40] was used to examine the changes in each outcome variable from Baseline to the other two time points, with group and time included as fixed covariate effects. Baseline was the reference time point and NC was the reference group. A group-x-time interaction was also tested; significance of the group-x-time term would indicate the changes from Baseline between the two groups were significantly different. A random intercept was included in each mixed model to account for subject-specific effects. If a significant group, time or group-x-time interaction was found, further post-hoc tests were conducted using appropriate contrasts: between group differences at each time point, and/or within group changes between any two time points.
Age and BMI were significantly associated with outcome measures at one or more time points for both BC and NC, therefore these two factors were adjusted in the mixed model analyses.
All analyses were performed using version 9.3 of SAS (SAS Institute Inc. 2010). All statistical tests with p-values <0.05 are reported as statistically significant.
RESULTS
Demographics, participant characteristics, and medical co-morbidities
BC and NC demographics and BC disease characteristics are listed in Table 1. There were no significant differences between the two groups in age, BMI, race, education, marital status, household annual income or baseline menopause status (all p’s>0.2).
Table 1.
Demographic and disease characteristics
| Characteristics | BC (n=68) | NC (n=60) |
|---|---|---|
|
| ||
| Age (years) | ||
| Mean (SD) | 51.3 (9.1) | 52.4 (9.4) |
| Range | 31 – 76 | 29 – 81 |
|
| ||
| Body mass index (BMI, kg/m2) | ||
| Mean (SD) | 27.6 (7.4) | 25.9 (7.0) |
| Range | 19.3 – 61.9 | 19.1 – 56.7 |
|
| ||
| Race [n (%)] | ||
| Caucasian | 60 (88.2) | 51 (85.0) |
| Non-Caucasian | 8 (11.8) | 9 (15.0) |
|
| ||
| Education [n (%)] | ||
| Below completed college | 32 (47.1) | 21 (35.0) |
| Completed college and above | 36 (52.9) | 39 (65.0) |
|
| ||
| Marital status [n (%)] | ||
| Never married | 3 (4.4) | 7 (11.7) |
| Divorced/separated/widowed | 19 (27.9) | 12 (20.0) |
| Married | 46 (67.7) | 41 (68.3) |
|
| ||
| Household annual income [n (%)] | ||
| ≤ $100,000 | 25 (36.8) | 21 (35.0) |
| > $100,000 | 43 (63.2) | 39 (65.0) |
|
| ||
| Baseline menopausal status [n (%)] | ||
| pre-menopause | 28 (41.2) | 20 (33.9) |
| peri-menopause | 8 (11.8) | 8 (13.6) |
| post-menopause | 27 (39.7) | 22 (37.3) |
| hysterectomy | 5 (7.3) | 9 (15.3) |
| Not available | 0 | 1 |
|
| ||
| Cancer stage [n (%)] | - | |
| Stage I | 19 (28.4) | |
| Stage II | 27 (40.3) | |
| Stage III | 21 (31.3) | |
| Not available | 1 | |
|
| ||
| Surgery type | - | |
| Lumpectomy | 31 (45.6) | |
| Mastectomy | 31 (45.3) | |
| Double mastectomy | 3 (4.4) | |
| No surgery before Chemotherapy | 3 (4.4) | |
| Not available | 0 | |
|
| ||
| Days between surgery and start of chemotherapy | - | |
| Available (n=47) | ||
| Mean (SD) | 42.0 (16.7) | |
| Range | 14 – 106 | |
| Not available (n=21) | - | |
|
| ||
| Chemotherapy regimen [n (%)] | - | |
| AC | 13 (19.7) | |
| AC followed by docetaxel | 6 (9.1) | |
| AC followed by paclitaxel | 34 (51.5) | |
| Other | 13 (19.7) | |
| Not available | 2 | |
Note: BC= women with breast cancer, NC=cancer-free controls. No significant differences between the two groups in age, BMI, race, education, marital status, household annual income and baseline menopause status (all p’s>0.2). AC = Doxorubicin + Cyclophosphamide.
Of the enrolled 68 BC, 6 were dropped from the study before Cycle-4 due to family issues (divorce), medical problems or too much study burden, and 18 only completed the Cycle-4 but not the 1-Year follow-up due to completion of the study; of the 60 NC, 8 dropped from the study before Cycle-4 due to multiple reasons, and 17 only completed the Cycle-4 but not the 1-Year follow-up due to completion of the study. The drop-out rates in the two groups at Cycle-4 and 1-Year were not significantly different (both p’s>0.7). There were no significant differences between those who completed the Cycle-4 study or 1-Year study and those who did not in age, BMI in both groups, and in cancer stage and chemotherapy regimen in BC (all p’s>0.06).
Reported medical co-morbidities over time are listed in Table 2. At Baseline, there were no significant group differences for any of the reported diseases (all p’s>0.06). There were also no significant changes across the three time points in each group for any of the diseases (all p’s>0.06).
Table 2.
Medical co-morbidities over time [n (%)]
| Diseases | Baseline | Cycle-4 | 1-Year | |||
|---|---|---|---|---|---|---|
| BC (n=66) | NC (n=55) | BC (n=58) | NC (n=49) | BC (n=41) | NC (n=28) | |
| Cardiovascular diseases | 2 (3.0) | 0 (0.0) | 2 (3.5) | 0 (0.0) | 1 (2.4) | 0 (0.0) |
| Pulmonary diseases | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 1 (2.4) | 0 (0.0) |
| Central nervous diseases | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.4) | 0 (0.0) |
| Gastrointestinal diseases | 4 (6.1) | 0 (0.0) | 2 (3.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Renal disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Endocrine disease | 5 (7.6) | 0 (0.0) | 1 (1.7) | 2 (4.1) | 0 (0.0) | 1 (3.6) |
| Connective tissue diseases | 1 (1.5) | 0 (0.0) | 2 (3.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Infections | 3 (4.6) | 0 (0.0) | 2 (3.5) | 2 (4.1) | 2 (4.9) | 1 (3.6) |
| Dementia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Arthritis | 8 (12.2) | 4 (7.3) | 6 (10.3) | 5 (10.2) | 11 (26.8) | 7 (25.0) |
| Diabetes | 4 (6.1) | 0 (0.0) | 3 (5.2) | 0 (0.0) | 2 (4.9) | 1 (3.6) |
| Ulcer | 2 (3.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 1 (2.4) | 0 (0.0) |
| Hiatal hernia | 0 (0.0) | 1 (1.8) | 0 (0.0) | 1 (2.4) | 2 (4.9) | 0 (0.0) |
| Esophageal diseases | 1 (1.5) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neck/back/spine problems | 10 (15.2) | 9 (16.4) | 10 (17.2) | 11 (22.5) | 9 (21.9) | 6 (21.4) |
| Epilepsy | 0 (0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 1 (2.4) | 0 (0.0) |
| Headaches | 12 (18.2) | 6 (10.9) | 15 (25.9) | 5 (10.2) | 12 (29.3) | 6 (21.4) |
| High blood pressure | 10 (15.2) | 11 (20.0) | 8 (13.8) | 11 (22.5) | 8 (19.5) | 7 (25.0) |
| Kidney problems | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stroke | 0 (0.0) | 1 (1.8) | 1 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asthma | 6 (9.1) | 3 (5.5) | 6 (10.3) | 4 (8.2) | 3 (7.3) | 1 (3.6) |
| Emphysema | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.4) | 0 (0.0) |
| Edema | 2 (3.0) | 0 (0.0) | 3 (5.2) | 1 (2.4) | 2 (4.9) | 1 (3.6) |
| Thyroid disease | 12 (18.2) | 3 (5.5) | 10 (17.2) | 4 (8.2) | 8 (19.5) | 3 (10.7) |
| Other diseases | 8 (12.2) | 4 (7.3) | 13 (22.4) | 1 (2.4) | 6 (14.6) | 2 (7.1) |
Note: BC= breast cancer patients, NC= cancer-free controls. Data for 2 BC and 5 NC were not available at Baseline; 8 more BC and 6 more NC at Cycle-4, and 25 more BC and 27 more NC at 1-Year, were either dropped or stopped due to the end of study. There were no significant group differences at Baseline (all p’s>0.5); and no changes over time in both groups for all disease (all p’s>0.06).
Sleep
Objective measures
Nocturnal TST
There were no significant differences in nocturnal TST between BC and NC at any time points; nor were there any significant changes over time in either group (see Table 3).
Table 3.
Objectively measured sleep [mean±SD (n, median)] and effect size (95% CI)
| Group | Baseline | Cycle-4 | 1-Year | |
|---|---|---|---|---|
|
| ||||
| Nocturnal TST (hours) | BC | 7.08±1.09 (68, 7.21) | 7.23±1.21 (56, 7.22) | 7.01±0.74 (40, 7.00) |
| NC | 7.15±0.92 (60, 7.05) | 7.11±0.87 (51, 7.18) | 7.07±0.66 (31, 7.15) | |
| Effect size | −0.06 (−0.41, 0.29) | 0.12 (−0.26, 0.50) | −0.08 (−0.55, 0.39) | |
|
| ||||
| NAPTIME (hours)* | BC | 0.80±0.94 (68, 0.44)† | 1.02±1.02 (56, 0.79)†† | 0.49±0.47 (40, 0.37)‡ ‡‡ |
| NC | 0.47±0.50 (60, 0.27) | 0.46±0.42 (51, 0.38) | 0.36±0.44 (31, 0.18) | |
| Effect size | 0.43 (0.07, 0.78) | 0.71 (0.31, 1.10) | 0.28 (−0.19, 0.76) | |
BC= breast cancer patients, NC= cancer-free controls. TST=total sleep time, NAPTIME=total nap time. Age and BMI were adjusted in the mixed model.
NAPTIME: group effect p=0.0008, time effect for BC p=0.0003. Compared to NC at the same time point:
p=0.0095,
p<0.0001. Compared to Baseline in BC:
p=0.010; compare to Cycle-4 in BC:
p<0.0001. No significant time effect in NC for NAPTIME. No significant group effect, time effect in both BC and NC, or group-x-time interaction for nocturnal TST.
NAPTIME
Mixed model analysis showed that there was a significant group effect (p=0.0008); further post-hoc testing of between group differences at each time-point indicated that compared to NC, BC spent more time napping at Baseline (p=0.0095) and Cycle-4 (p<0.0001), but not at 1-Year (p=0.63). BC also had a larger effect size at Baseline (0.43, 95% CI=0.07, 0.78) and Cycle-4 (0.71, 95% CI=0.31, 1.10) than NC. There was also a significant time effect for BC (p=0.0003); post-hoc analysis showed that BC spent significantly less time napping at Year-1 compare to Baseline (p=0.010) and to Cycle-4 (p<0.0001); the increases from Baseline to Cycle-4 was not significant (p=0.071). There were no significant within-group changes in NAPTIME for NC (p’s>0.5) nor any group-x-time interactions at any time points (all p>0.1) (see Table 3).
Subjective measures
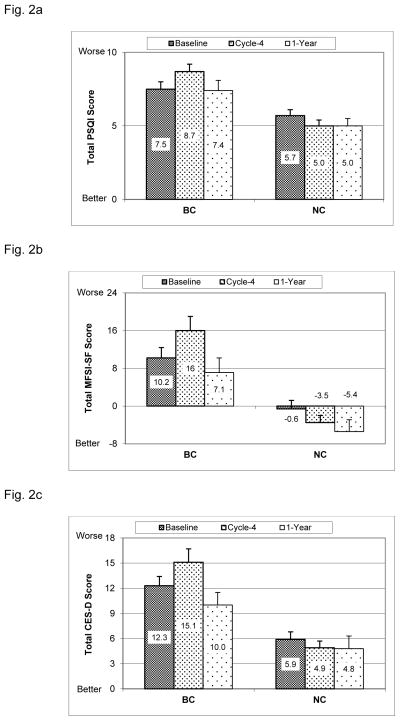
Total PSQI scores are shown in Fig. 2a. Mixed model analysis showed that there was a significant group effect (p<0.0001); further post-hoc analysis showed that compared to NC, BC reported significantly worse sleep quality (higher PSQI scores) at Baseline (p=0.011), Cycle 4 (p<0.0001) and 1-Year (p=0.020). There was a significant time effect for BC (p=0.0003), post-hoc analysis showed that BC reported significantly higher total PSQI scores at Cycle-4 compared to Baseline (p=0.0030), with scores significantly decreasing from Cycle-4 back to Baseline levels at 1-Year (p=0.0018). There were no significant within-group changes in NC (p’s>0.1). There was however a significant group-x-time interaction (p=0.0030), with the increases in BC patients from Baseline to Cycle-4 being significantly higher than in NC (p=0.0015).
Fig. 2. Sleep, Fatigue and Depressive symptoms.
(mean+SE). BC= breast cancer patients, NC= cancer-free controls. Baseline = before the start of chemotherapy, Cycle-4 = at the end of cycle 4 chemotherapy, 1-Year = 1 year after the start of chemotherapy. Age and BMI were adjusted in the mixed model.
Fig. 2a. PSQI (Pittsburgh Sleep Quality Index, higher score indicates poorer sleep quality): Between groups (group effect p<0.0001): Baseline p=0.011; Cycle-4 p<0.0001; Year-1 p=0.020. Within BC group (time effect p=0.0003): changes form Baseline to Cycle-4 p=0.030; from Cycle-4 to Year-1 p=0.0018. No significant within-group changes for NC. Group-x-time interaction p=0.0030.
Fig. 2b: MFSI-SF (Multidimensional Fatigue Symptom Inventory-Short Form, higher score indicates more fatigue): Between groups (group effect p<0.0001): Baseline p=0.0019; Cycle-4 p<0.0001; Year-1 p=0.0091. Within BC group (time effect p<0.0001): changes from Baseline to Cycle-4 p=0.0031; from Cycle-4 to 1-Year p<0.0001. No significant within-group changes for NC. Group-x-time interaction p=0.0097.
Fig. 2c: CES-D (Center of Epidemiological Studies-Depression, higher score indicates more depressive symptoms): Between groups (group effect p<0.0001): Baseline p=0.0003; Cycle-4 p<0.0001; Year-1 p=0.039. Within BC group (time effect p<0.0001): changes from Baseline to Cycle-4 p=0.0087; from Cycle-4 to 1-Year p<0.0001; from Baseline to 1-Year p=0.037. No significant within-group changes for NC. Group-x-time interaction p=0.0093.
Fatigue
Fatigue data are shown in Fig. 2b. Mixed model analysis indicated that there was a significant group effect (p<0.0001); post-hoc analysis indicated that compared to NC, BC had significantly higher total MSFI-SF scores at Baseline (p=0.0019), Cycle-4 (p<0.0001) and 1-Year (p=0.0091). There was a significant time effect for BC (P<0.0001), with BC reporting more fatigue at Cycle 4 than at Baseline (p=0.0031), but significantly decreasing at 1-Year from Cycle-4 with levels returning to Baseline levels (p<0.0001). There were no significant within-group changes in NC (p’s>0.1). There was a significant group-x-time interaction (p=0.0097), with the increases in reports of fatigue from Baseline to Cycle-4 in BC being significantly higher than in NC (p=0.0057).
Depressive Symptoms
Depressive symptoms are shown in Fig. 2c. Mixed model analysis showed that there was a significant group effect (p<0.0001); post-hoc analysis showed that compared to NC, BC had significantly more depressive symptoms (higher depression scores) at all three time points (Baseline p=0.0003, Cycle-4 p<0.0001, 1-Year p=0.039). There was a significant time effect for BC (p<0.0001); BC reported more depression at Cycle-4 than at Baseline (p=0.0087), but the depression scores decreased significantly from Cycle-4 to 1-Year (p<0.0001) and from Baseline to 1-Year (p=0.037). There were no significant within-group changes in depression scores for NC (p’s>0.5). There was a significant group-x-time interaction (p=0.0093) with increases in depression scores from Baseline to Cycle-4 in BC being significantly higher than in NC (p=0.025).
CAR robustness
The R-squared parameter is shown in Fig. 3. Mixed model analysis showed that there was a significant group effect (p=0.0039); post-hoc analysis indicated that compared to NC, BC had significantly smaller R-squared at Baseline (p=0.047) and Cycle 4 (p=0.0001), but not at 1-Year (p=0.47). There was a significant time effect for BC (p=0.0014), the post-hoc analysis showed that BC had significantly lower R-squared at Cycle-4 than at Baseline (p=0.014), but significantly higher R-squared at 1-Year when compared to Cycle-4 (p=0.0004). There were no significant within-group changes in R-squared for NC (p’s>0.7). There was however a significant group-x-time interaction (p=0.042) with decreases in R-squared from Baseline to Cycle-4 in BC being higher than in NC (p=0.059).
Fig. 3. Robustness of circadian activity rhythm.
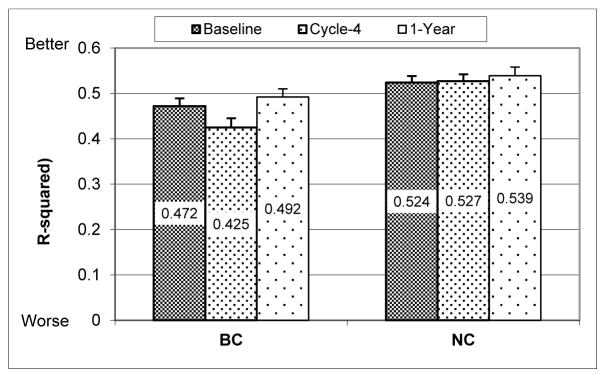
(mean+SE, bigger R-squared value indicates more robust rhythm). BC= breast cancer patients, NC= cancer-free controls. Baseline = before the start of chemotherapy, Cycle-4 = at the end of cycle 4 chemotherapy, 1-Year = 1 year after the start of chemotherapy. Age and BMI were adjusted in the mixed model. Between groups (group effect p=0.0039): Baseline p=0.047; Cycle-4 p=0.0001; 1-Year p=0.47. Within BC group (time effect p=0.0014): changes from Baseline to Cycle-4 p=0.014; from Cycle-4 to 1-Year p=0.0004. No significant within-group changes for NC. Group-x-time interaction p=0.042.
Quality of life
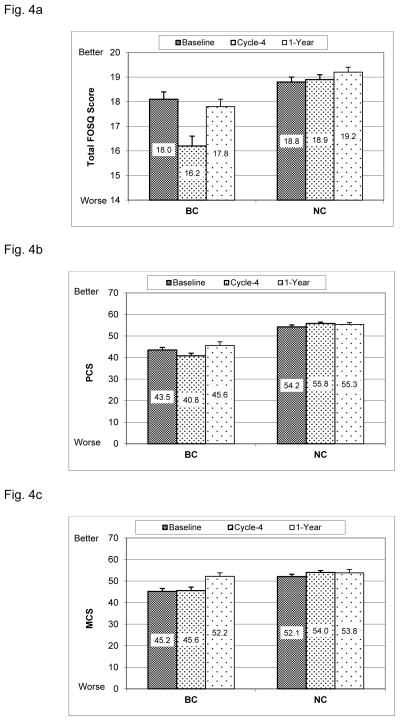
FOSQ (Fig. 4a)
Fig. 4. Quality of life.
(mean+SE, higher scores indicate better QoL). BC= breast cancer patients, NC= cancer-free controls. Baseline = before the start of chemotherapy, Cycle-4 = at the end of cycle 4 chemotherapy, 1-Year = 1 year after the start of chemotherapy. Age and BMI were adjusted in the mixed model.
Fig. 4a: FOSQ (Functional Outcomes of Sleep Questionnaire): Between groups (group effect p<0.0001): Baseline p=0.039; Cycle-4 p<0.0001; Year-1 p=0.029. Within BC group (time effect p<0.0001): changes from Baseline to Cycle-4 p<0.0001; from Cycle-4 to 1-Year p<0.0001. No significant within-group changes for NC. Group-x-time interaction p<0.0001.
Fig. 4b: PCS (SF-36 Physical Component Summary): Between groups (group effect p<0.0001): Baseline p<0.0001; Cycle-4 p<0.0001; Year-1 p<0.0001. Within BC group (time effect p<0.0001): changes from Baseline to Cycle-4 p=0.013; from Cycle-4 to 1-Year p<0.0001; from Baseline to 1-Year p=0.021. No significant within-group changes for NC. Group-x-time interaction p=0.0060.
Fig. 4c: MCS (SF-36 Metal Component Summary): Between groups (group effect p=0.0026): Baseline p=0.0010; Cycle-4 p<0.0001; 1-Year p=0.79. Within BC group (time effect p<0.0001): changes from Baseline to 1-Year p<0.0001; from Cycle-4 to 1-Year p<0.0001. No significant within-group changes for NC. Group-x-time interaction p=0.014.
Mixed model analysis showed that there was a significant group effect (p<0.0001), with BC reporting lower FOSQ scores than NC at Baseline (p=0.039), Cycle-4 (p<0.0001) and 1-Year (p=0.029). There was a significant time effect for BC (p<0.0001), with FOSQ scores in BC decreasing significantly at Cycle-4 compared to Baseline (p<0.0001), but at 1-Year recovering back to Baseline levels from Cycle-4 (p<0.0001). There were no significant changes in FOSQ scores for NC (p’s>0.8). There was a significant group-x-time interaction (p<0.0001), showing that decreases from Baseline to Cycle-4 in BC was significantly larger than in NC (p<0.0001).
SF-36 Physical Component Summary (PCS) score (Fig. 4b)
Mixed model analysis showed that there was a significant group effect (p<0.0001), with BC reporting lower PCS scores than NC at Baseline (p<0.0001), Cycle-4 (p<0.0001) and 1-Year (p<0.0001). There was a significant time effect for BC (p<0.0001), with PCS scores in BC decreasing significantly at Cycle-4 compared to Baseline (p=0.013), but recovering at 1-Year from Baseline (p=0.021) and from Cycle-4 (p<0.0001). There were no significant within-group changes in PCS scores for NC (p’s>0.1). There was however a significant group-x-time interaction (p=0.0022), showing that decreases from Baseline to Cycle-4 in BC was significantly larger than in NC (p=0.0060).
SF-36 Mental Component Summary (MCS) score (Fig. 4c)
Mixed model analysis showed that there was a significant group effect (p=0.0026), with BC reporting lower MCS scores than NC at Baseline (p=0.0010) and Cycle-4 (p<0.0001), but not at 1-Year (p=0.79). There was a significant time effect for BC (p<0.0001), BC’s MCS scores increased significantly at 1-Year when compared to Baseline (p<0.0001) and to Cycle-4 (p<0.0001). There were no significant within-group changes in MCS scores for NC (p’s>0.2). There was a significant group-x-time interaction (p=0.0054), the increases from Baseline to 1-Year in BC were significantly larger than in NC (p=0.014). (Insert Fig. 4 about here)
DISCUSSION
This longitudinal study showed that fatigue, sleep, depressive symptoms, quality of life and CARs were worse in patients than controls pre-chemotherapy, with all symptoms getting worse by the end of four cycles of chemotherapy. By one year, many symptoms returned to baseline levels, but were still worse than controls. The objective measures, total sleep time, total nap time and CAR, were no longer significantly different from controls at one year. Future studies need to explore whether there are physiological reasons, for example, chronic psychological distress or long term toxicity of chemotherapy, that might play a role in the differences of subjectively and objectively measured variables.
The findings of continued sleep disturbances, more fatigue and more depressive symptoms during chemotherapy are consistent with previous studies [1,2,41], but extend the findings longitudinally. A few other studies have examined the cancer-related symptoms longitudinally. A recent study by Trudel-Fitzgerald et al. [17] explored the temporal relationship between cancer-related symptoms of anxiety, depression, insomnia, fatigue and pain over 18-months and across six time points in a large sample of non-metastatic cancer patients, finding that the severity level of any one symptom significantly predicted the level of that same symptom at the subsequent time points. This finding supports previous work from our laboratory which showed that symptoms presenting pre-chemotherapy were all worse at the end of cycle 4 chemotherapy [13]. However, these previous studies, including our own, had no cancer-free comparison group.
Previous studies in cancer patients, including those from our group, reported CAR findings during chemotherapy, with most reporting more disruption in CARs during chemotherapy [1,25,31,42,43]. This current study not only confirmed that CAR is impaired during chemotherapy, but that at one year post chemotherapy, CAR recovered to levels similar to controls. Previous studies have also suggested that CRF is associated with disrupted CAR [31]. While this seems to be true during chemotherapy, other factors seem to be contributing to CRF at one year. Future studies will need to explore these associations.
In this study, QoL was measured with multiple tools. The FOSQ measured daytime sleepiness related outcomes, and the SF-36 measured health related functioning. Both FOSQ and the physical component scores of the SF-36 showed a pattern similar to the other symptoms, i.e., BC patients experienced worse QoL than controls at all three time points, with QoL being lowest during chemotherapy. On the other hand, the mental component score in BC patients showed a different pattern: compared to controls, although still lower before and during chemotherapy, there was total recovery at the one year time point. These results suggest that the changes of sleepiness-related QoL were more in line with the physical component of QoL, than with the mental component, particularly at one year. These data suggest that BC patients mentally adapted to the cancer and related treatment after one year, although their physical condition did not.
This study was the first to show the trajectory of multiple symptoms experienced before, during and up to one year after cancer treatment, and was able to compare data of BC patients to non-cancer controls. Nevertheless, this study has some limitations. The data were only collected in women with stage I-III BC, so the conclusions cannot be extended to patients with stage IV or metastatic breast cancer, with other types of cancer, or male patients with breast cancer. Patients were adjuvant and neo-adjuvant, but individual groups were too small to look for differences between them. The patients were all drawn from a single geographic region, and one that has quite narrow seasonal variations, a factor which might help reduce the study participants’ physical and, hence, mental distress and capacity to cope with a cancer diagnosis.
In summary, results of this controlled, longitudinal study showed that patients with breast cancer have symptoms that are worse than those of non-cancer controls even before the start of chemotherapy. This observation is not surprising given the emotional distress associated with a new diagnosis of breast cancer. By the end of four cycles of chemotherapy, compared to their own baseline levels and to levels reports by controls, BC had significantly worse symptoms. By one year after the start of chemotherapy, the patients’ symptoms returned to their own baseline levels, but were still worse than controls on subjective measures, but not on objective measures of sleep and circadian activity rhythms. Additional research is needed to determine if treatment of these symptoms started before chemotherapy will alleviate symptom severity over time.
Acknowledgments
This study was supported by the National Cancer Institute (CA112035 and P30 CA023100), the UCSD Clinical & Translational Research Institute (UL1RR031980) and the Department of Veterans Affairs San Diego Center of Excellence for Stress and Mental Health (CESAMH). The authors would like to thank all women who participated in this study either as patients or controls.
Footnotes
Conflict of Interest: The study was funded by NIH. The corresponding author has full control of all primary data and agrees to allow the Journal to review the data if requested.
References
- 1.Fernandes R, Stone P, Andrews P, Morgan R, Sharma S. Comparison between fatigue, sleep disturbance, and circadian rhythm in cancer inpatients and healthy volunteers: evaluation of diagnostic criteria for cancer-related fatigue. J Pain Symptom Manage. 2006;32:245–254. doi: 10.1016/j.jpainsymman.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37:715–736. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Liu L, Marler M, Parker BA, Jones V, Robins Sadler G, Dimsdale JE, Cohen-Zion M, Fiorentino L. Fatigue, sleep and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger AM, Farr LA, Kuhn BR, Fischer P, Agrawal S. Values of Sleep/Wake, Activity/Rest, Circadian Rhythms, and Fatigue Prior to Adjuvant Breast Cancer Chemotherapy. J Pain Symptom Manage. 2007;33:398–409. doi: 10.1016/j.jpainsymman.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miaskowski C, Lee K, Dunn L, Dodd M, Aouizerat BE, West C, Paul SM, Cooper B, Wara W, Swift P. Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nurs. 2011;34:255–268. doi: 10.1097/NCC.0b013e3181f65d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 7.Byar KL, Berger AM, Bakken SL, Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol Nurs Forum. 2006;33:E18–26. doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- 8.Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs. 2010;14:101–110. doi: 10.1016/j.ejon.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandadi S, Frasure HE, Broderick MJ, Waggoner SE, Miller JA, von Gruenigen VE. The effect of sleep disturbance on quality of life in women with ovarian cancer. Gynecol Oncol. 2011;123:351–355. doi: 10.1016/j.ygyno.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Fiorentino L, Ancoli-Israel S. Insomnia and its Treatment in Women with Breast Cancer. Sleep Med Rev. 2006;10:419–429. doi: 10.1016/j.smrv.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583–590. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Marler M, Parker BA, Jones V, Johnson S, Cohen-Zion M, Fiorentino L, Sadler GR, Ancoli-Israel S. The relationship between fatigue and light exposure during chemotherapy. Support Care Cancer. 2005;13:1010–1017. doi: 10.1007/s00520-005-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Fiorentino L, Natarajan L, Parker BA, Mills PJ, Sadler GR, Dimsdale JE, Rissling M, He F, Ancoli-Israel S. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psycho-oncology. 2009;18:187–194. doi: 10.1002/pon.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 15.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118:2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 16.Morrow GR, Andrews PLR, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Support Care Cancer. 2002;10:389–398. doi: 10.1007/s005200100293. [DOI] [PubMed] [Google Scholar]
- 17.Trudel-Fitzgerald C, Savard J, Ivers H. Which symptoms come first? Exploration of temporal relationships between cancer-related symptoms over an 18-month period. Ann Behav Med. 2013;45:329–337. doi: 10.1007/s12160-012-9459-1. [DOI] [PubMed] [Google Scholar]
- 18.So WK, Marsh G, Ling WM, Leung FY, Lo JC, Yeung M, Li GK. The symptom cluster of fatigue, pain, anxiety, and depression and the effect on the quality of life of women receiving treatment for breast cancer: a multicenter study. Oncol Nurs Forum. 2009;36:E205–214. doi: 10.1188/09.ONF.E205-E214. [DOI] [PubMed] [Google Scholar]
- 19.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 20.Gehrman PR, Marler M, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. The timing of activity rhythms in patients with dementia is related to survival. Journal of Gerontology: Medical Sciences. 2004;59A:1050–1055. doi: 10.1093/gerona/59.10.m1050. [DOI] [PubMed] [Google Scholar]
- 21.Paudel M, Taylor BC, Ancoli-Israel S, Blackwell T, Stone KL, Tranah G, Redline SS, Cummings SR, Ensrud KE for the Osteoporotic Fractures in Men(MrOS) Study Group. Rest/Activity Rhythms and mortality rates in older men: The MrOs Sleep Study. Chronobiol Int. 2010;27:363–377. doi: 10.3109/07420520903419157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tranah GJ, Blackwell TL, Ancoli-Israel S, Paudel M, Ensrud K, Cauley JA, Hillier TA, Cummings SR, Stone KL for the SOF Research Group. Circadian activity rhythms and mortality: the Study of Osteoporotic Fractures. J Am Geriatr Soc. 2010;58:282–291. doi: 10.1111/j.1532-5415.2009.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, Touitou Y, Levi F. Marked 24-h rest/activity rhythms are associated with better quality of life, better response and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000;6:3038–3045. [PubMed] [Google Scholar]
- 24.Berger AM, Hertzog M, Geary CR, Fischer P, Farr L. Circadian rhythms, symptoms, physical functioning, and body mass index in breast cancer survivors. J Cancer Surviv. 2012;6:305–314. doi: 10.1007/s11764-012-0218-x. [DOI] [PubMed] [Google Scholar]
- 25.Savard J, Liu L, Natajaran L, Rissling M, Neikrug AB, He F, Dimsdale JE, Mills PJ, Parker BA, Sadler GR, Ancoli-Israel S. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep. 2009;32:1155–1160. doi: 10.1093/sleep/32.9.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 27.Broeckel J, Jacobsen PB, Horton J, Balducci L, Lyman GH. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. Journal of Cinical Oncology. 1998;16:1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]
- 28.Pockaj BA, Degnim AC, Boughey JC, Gray RJ, McLaughlin SA, Dueck AC, Perez EA, Halyard MY, Frost MH, Cheville AL. Quality of life after breast cancer surgery: What have we learned and where should we go next. J Surg Oncol. 2009;99:447–455. doi: 10.1002/jso.21151. [DOI] [PubMed] [Google Scholar]
- 29.Trentham-Dietz A, Sprague BL, Klein R, Klein BE, Cruickshanks KJ, Fryback DG, Hampton JM. Health-related quality of life before and after a breast cancer diagnosis. Breast Cancer Res Treat. 2008;109:379–387. doi: 10.1007/s10549-007-9653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ancoli-Israel S, Cole R, Alessi CA, Chambers M, Moorcroft WH, Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Rissling MB, Neikrug AB, Fiorentino L, Natarajan L, Faierman M, Sadler GR, Dimsdale JE, Mills PJ, Parker BA, Ancoli-Israel S. Fatigue and circadian activity rhythms in breast cancer patients before and after chemotherapy: a controlled study. Fatigue: Biomedicine, Health & Behavior. 2013;1:12–26. doi: 10.1080/21641846.2012.741782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CRI, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14(4):331–338. [PubMed] [Google Scholar]
- 33.Buysse DJ, Reynolds CFI, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 34.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 35.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 37.Marler MR, Martin JL, Gehrman PR, Ancoli-Israel S. The Sigmoidally-transformed Cosine Curve: A Mathematical Model for Circadian Rhythms with Symmetric Non-sinusoidal Shapes. Stat Med. 2006;25:3893–3904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- 38.Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, Smith PL, Schwartz AR, Redline S, Pack AI, Dinges DF. An instrument to measure functional status outcome for disorders of excessive sleepiness. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- 39.Ware JE, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 2002. [Google Scholar]
- 40.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. New York, NY: Oxford University Press; 1994. [Google Scholar]
- 41.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 42.Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, Andrews PLR. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Supportive Care in Cancer. 2002;10:329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 43.Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2009 doi: 10.1007/s00520-009-0636-0. [DOI] [PubMed] [Google Scholar]