Abstract
Development of a vaccine against pulmonary tuberculosis (TB) may require immunization strategies that induce a high frequency of antigen-specific CD4 and CD8 T cells in the lung. The nonhuman primate (NHP) model is essential for testing such approaches because it has predictive value for how vaccines elicit responses in humans. Here, we used an aerosol (AE) vaccination strategy to administer AERAS-402, a replication-defective recombinant adenovirus (rAd) type 35 expressing Mycobacterium tuberculosis (M.tb) antigens Ag85A, Ag85B, and TB10.4, in bacille Calmette-Guerin (BCG)-primed or unprimed rhesus macaques. Immunization with BCG generated low purified protein derivative (PPD)-specific CD4 T cell responses in blood and bronchoalveolar lavage (BAL). In contrast, aerosolized AERAS-402 alone or following BCG induced potent and stable Ag85A/b-specific CD4 and CD8 effector T cells in BAL that largely produced IFN-γ, as well as TNF and IL-2. Such responses induced by BCG, AERAS-402, or both failed to confer overall protection following challenge with 275 CFU of M.tb Erdman, although vaccine-induced responses associated with reduced pathology were observed in some animals. Anamnestic T cell responses to Ag85A/b were not detected in blood of immunized animals after challenge. Overall, our data suggest that a high M.tb challenge dose may be a critical factor in limiting vaccine efficacy in this model. However, the ability of AE rAd immunization to generate potent cellular immunity in the lung by AE rAd immunization suggests that using different or more immunogens, alternative rAd serotypes with enhanced immunogenicity, and a physiological challenge dose may achieve protection against M.tb.
Introduction
With one third of the world’s population infected, 8.6 million new cases of active tuberculosis (TB) and 1.3 million deaths per year, Mycobacterium tuberculosis (M.tb) remains one of the most deadly pathogens in human history (1). Developing a vaccine that provides high-level protection against pulmonary TB, as for other infectious diseases requiring cellular immunity for protection (e.g., malaria, Leishmania major), remains elusive (2). Optimally, vaccine-elicited immunity should eliminate the bacteria, or at least limit the progression to active TB disease during primary or latent infection (3). Bacille Calmette-Guerin (BCG), the only licensed vaccine against TB, is given at birth in endemic areas and protects infants from disseminated TB; however, BCG-elicited immunity wanes and fails to uniformly prevent pulmonary disease in adolescents and adults (4, 5). Thus, vaccine approaches that enhance the long-term efficacy of BCG to protect against pulmonary TB are urgently needed.
Heterologous prime-boost immunization is an important vaccine approach in which a viral or protein/adjuvant vaccine is given following BCG priming to enhance immunity and protection through several mechanisms (6, 7). First, heterologous boosting can increase the magnitude and duration of BCG-elicited immunity by using antigens in the boosting vaccine that are expressed in BCG; second, it can broaden the immune response by including virulence-associated antigens expressed by M.tb but not BCG; third, boosting with viral vectors can elicit CD8 T cell responses not efficiently induced by BCG; and fourth, altering the route of vaccine delivery for the boost can enhance immunity at the site of infection (lung).
A unique aspect of TB pathogenesis is a delayed onset of T cell responses in the lymph nodes (LN) draining the lung, allowing establishment of infection and progression to active disease (8–11). The arrival of effector T cells in the lung can take up to 20 days after primary TB infection—late compared to respiratory viral infections such as influenza (12, 13). Mice immunized with M.tb (and cured with drug treatment) or BCG respond slightly earlier to reinfection, resulting in enhanced bacterial containment in the lung but not sterilizing immunity (13–16). Populating the lung with increasing numbers of TB-specific transgenic Th1 cells, however, protects mice from challenge in a dose-dependent fashion (17). Thus, a likely limitation of BCG and other vaccine strategies is an insufficient number of effector cells in the lung at the time of infection. In this regard, mucosal immunization to induce pulmonary cellular immune responses offers an advantage over parenteral immunization for protecting against respiratory and mucosal pathogens including TB (18–21). Indeed, it has been long appreciated that mice, guinea pigs and macaques are protected to a greater extent following intranasal (i.n.) or aerosol (AE) delivery, compared to parenteral delivery, of BCG (22–27). However, in humans BCG is administered by the intradermal (i.d.) route; therefore we chose a replication-defective recombinant adenovirus (rAd) vaccine formulation and AE delivery strategy as a boost following i.d. BCG that would elicit potent Th1 and CD8 T cell responses in the lung.
rAds have been widely used as vaccine delivery vectors and are particularly suited for respiratory mucosal vaccination due to their natural tropism for the respiratory tract, their safety profile, and their capacity to promote potent immunogenicity of the antigen insert (21, 28–31). In the mouse model of TB, mucosal (i.n.) immunization with an rAd serotype 5 expressing an immunogenic mycobacterial antigen, Ag85A (AdAg85A), resulted in a greater accumulation and retention of memory CD4 and CD8 T cells within the airway lumen compared to intramuscular (i.m.) immunization that predominantly elicited responses in the peripheral tissues (32). Furthermore, such T cell responses within the airway lumen correlated better with protection against TB challenge in mice than peripheral responses against the same antigen, presumably because such cells are poised at the appropriate anatomical location to protect against respiratory infection (33–35). In the setting of BCG-priming, i.n. boosting of mice or guinea pigs with AdAg85A or Modified Vaccinia Virus Ankara expressing the same antigen (MVA85A) enhanced the level of BCG-mediated protection to a greater extent than parenteral immunization (27, 36–38). In addition, both MVA85A and a rAd serotype 35 (rAd35) vector expressing TB antigens (AERAS-402) elicited T cell responses in the lungs of macaques following AE administration (21, 39). Although there is some immunogenicity and protection in mice or guinea pigs with these vectors, protection of nonhuman primates (NHPs) against TB following mucosal administration of such viral vectors has not been assessed.
In this study, rhesus macaques were used to assess immunogenicity and protection against M.tb challenge following BCG and/or AE immunization with AERAS-402, a rAd35 vector expressing the M.tb antigens Ag85A, Ag85B, and TB10.4 (40). The primary goal of the study was to establish whether a high frequency of TB-specific T cells in the lung at the time of challenge confers protection against M.tb challenge. NHPs are a particularly relevant animal model for evaluating vaccine-elicited immune correlates of protection against human TB based on the immunological similarities between the species, and also because they are susceptible to pulmonary TB infection with strains of M.tb that are pathogenic in humans (41, 42). AERAS-402 was chosen because it protects mice from lung challenge with M.tb following i.m. or i.n. immunization (43) and elicits TB-specific, multifunctional CD4 and CD8 T cell responses when given i.m. to BCG-vaccinated macaques and humans (44–46). Overall, the data presented here provide insights into how to elicit robust pulmonary T cell responses and guide future studies for optimizing the NHP model for vaccine development against M.tb infection.
Materials and Methods
Animals
Thirty-two healthy, specific pathogen-free, male Indian origin rhesus monkeys (Macaca mulatta) with a mean age and weight of 2.7 years (2.2–5.2) and 4.4 kg (2.5–7.7) were pre-screened for prior mycobacterial exposure by PRIMAGAM® (Prionics AG, Schlieren-Zurich, Switzerland) and for Ad35 neutralizing titers (NVITAL, Gaithersburg, MD). Animals were housed in animal biosafety level 2 (immunization phase) and level 3 (challenge phase) facilities at the Tulane National Primate Research Center (TNPRC) and were monitored throughout the study for: physical health, food consumption, body weight, temperature, complete blood count, respiratory mechanics, thoracic radiographs, serum chemistry (including but not limited to C-reactive protein, albumin, globulin) and PRIMAGAM®. Peripheral blood samples were collected throughout the study (vaccination and challenge phases) while bronchoalveolar lavage (BAL) samples were collected only during the vaccination phase. Predefined humane endpoints were applied to alleviate discomfort, as described below. All animals were cared for in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and the Guide for the Care and Use of Laboratory Animals prepared by the National Research Council. The TNPRC is AAALAC-accredited, licensed by the US Department of Agriculture, and has an assurance filed with the NIH Office of Laboratory Animal Care. The Tulane Institutional Animal Care and Use Committee and the Tulane Institutional Biosafety Committee approved all studies.
Immunizations
32 animals were divided into 4 vaccine groups (see Results and Fig. 1). Randomization was done based on three criteria: (1) Animals with negative Ad35 neutralization titers were preferentially assigned to AERAS-402 immunization groups (G3 and G4). (2) Following pre-immune screening, “background” ICS responses (i.e. stimulation without antigen) were graded as low, medium, or high for each animal; equal numbers of each level were assigned to each group. (3) Final randomization was by weight. Resulting groups did not significantly differ in average age. Animals were immunized i.d. at wk 0 with physiological saline (saline) or 5 × 105 BCG Danish strain 1331 (Statens Serum Institute; provided by CBER/FDA, Bethesda, MD), prepared and administered according to the manufacturer’s instructions for human use. AE immunization with 3 × 1010 viral particles (vp) of Ad35-null (empty rAd35 vector) or AERAS-402 (Crucell Holland BV, Leiden, The Netherlands), a replication-defective (E1/E3-deleted) rAd35 vector encoding M.tb antigens Ag85A, Ag85B, and TB10.4 (40), occurred 14 wks later. For AE immunization, the e-Flow® Nebulizer System (PARI Pharma GmbH, Munich, Germany) was used to deliver 4.4 μm diameter aerosol droplets deep into the lung, as described previously (21). All immunizations were performed in a biosafety cabinet with the animals under anesthesia (10 mg/kg ketamine HCl and 8 mg/kg tiletamine HCl/zolazepam HCl).
FIGURE 1. Experimental design.
32 rhesus macaques were vaccinated (V) as follows: Group 1, saline control (Ctrl); Group 2, BCG prime followed by an empty Ad35 (Ad35-null); Group 3, BCG prime followed by an AERAS-402; Group 4, saline (Ctrl) followed by AERAS-402. For priming, 5 × 105 CFU of BCG or saline was administered ID at wk 0. For boosting, 3 × 1010 viral particles (vp) of Ad35-null or AERAS-402 were administered AE at wk 14. All animals were challenged (C) with 275 CFU of M.tb (Erdman) at wk 26 and followed for an additional 24–26 wks or until clinical endpoint criteria were met. Immune analyses in PBMC (◆) or BAL (❖) were performed at the indicated weeks.
M. tuberculosis challenge and necropsy procedures
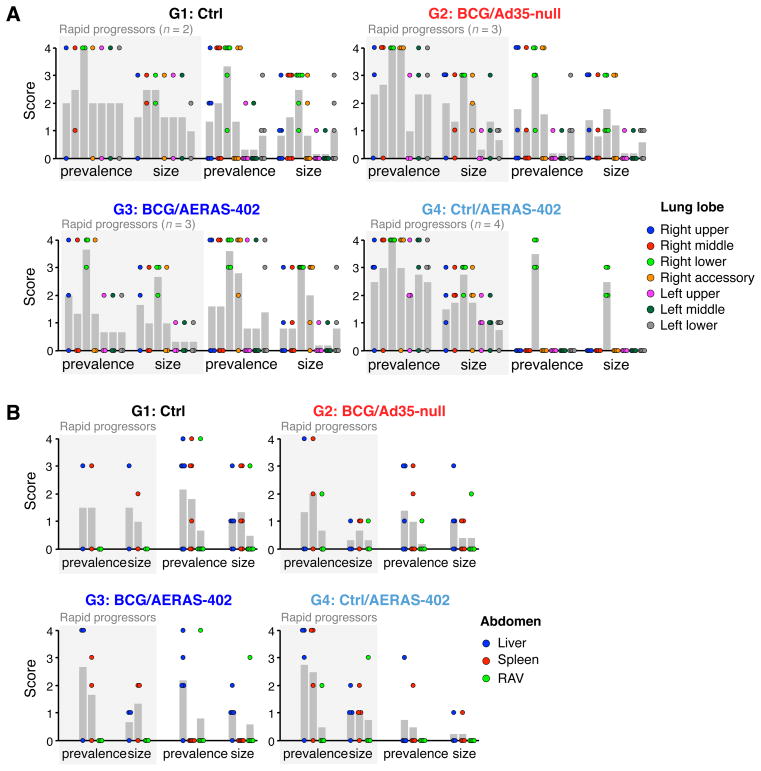
M.tb Erdman lot K01 (Mycos Inc., provided by CBER/FDA) was diluted in saline and sonicated briefly. Animals were anesthetized and bacteria were delivered to the right caudal lung lobe using a bronchoscope in a volume of 2 ml. 4 animals from each group were infected on two consecutive days using the same diluted stock of M.tb (stored at 4°C overnight). Actual number of bacteria delivered was 283 ± 83 on day 1 and 263 ± 22 on day 2. The following predetermined humane endpoint criteria were applied for euthanasia prior to the scheduled (fixed) endpoint: loss of 25% body weight, major organ failure or medical conditions unresponsive to treatment, severe respiratory distress or complete anorexia for 4 days. At the humane or scheduled endpoint (24–26 wks post-challenge), necropsy was performed that included gross pathologic scoring as well as histologic analysis and bacterial quantitation using a non-biased stereologic sampling method (47). Gross pathology of each lung lobe, thoracic lymph nodes (LN) and abdominal viscera were scored using a semiquantitative grading system such that granulomas were assigned a numeric point value (0–4) based on prevalence (0, none visible; 1, 1–3 visible; 2, 4–10 visible; 3, >10 visible; 4, miliary pattern) and size (0, none present; 1, <1–2 mm; 2, 3–4 mm; 3, >4 mm). Approximately 15–20 samples per lung were evaluated for histology and bacterial burdens. Histologic analysis was done using SPOT Imaging Software (Diagnostics Instruments, Sterling Heights, MI) on a Leica DM-LB photomicroscope (Leica Microsystems, Buffalo Grove, IL) at 67.5x magnification. The number of lesion points (granulomatous inflammation, necrosis, hemorrhage, and edema) within the total points counted was used to calculate the lesion percent in each lung lobe. Bacterial burdens were quantified at necropsy; tissues were homogenized and decontaminated in Mycoprep (Becton Dickinson, Franklin Lakes, NJ), diluted in Mycoprep SALINE, plated in triplicate on Middlebrook 7H11 agar containing fungicide, incubated at 37°C, and colony-forming units (CFUs) counted 3 and 5 wks later.
T cell assays and flow cytometry
Immune assays on BAL were conducted on freshly harvested samples. BAL was obtained by collecting 3 consecutive 20 ml washes with room temperature saline using a feeding tube. Samples were resuspended in warm (37°C) R10 medium (RPMI with 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% heat-inactivated fetal bovine serum) containing 2 U/ml DNase (Roche Applied Science, Indianapolis, IN) and filtered through a 70 μM cell strainer. BAL cells were rested for 4 h before overnight (16 h) stimulation with antigen. Peripheral blood mononuclear cells (PBMC) were batch-analyzed from cryopreserved samples at the completion of the study. PBMC were isolated from heparin anti-coagulated whole blood with Lymphocyte Separation Medium (MP Biomedicals, Solon, OH) using standard methods. For immune analysis, PBMC were thawed in a 37°C water bath, washed twice in warm R10 containing 50 U/ml Benzonase (EMD Millipore, Billerica, MA), and rested at 37°C overnight before a 6 h stimulation with antigen. For in vitro T cell cytokine assays, cells were cultured with overlapping peptide pools (15-mer peptides overlapping by 11 amino acids) for Ag85A/b (peptides spanning the entire Ag85A sequence and regions of the Ag85B sequence that are not present in Ag85A) or ESAT-6 (obtained from Aeras) at 2 μg/ml or tuberculin purified protein derivative S-2 (PPD, provided by CBER/FDA) at 20 μg/ml, in the presence of anti-CD28 (PE-Cy5 conjugate) and anti-CD49d co-stimulation. Brefeldin A (BD GolgiPlug, BD Biosciences, San Jose, CA) was added 2h after antigens at a final concentration of 10 μg/ml. For flow cytometric analysis (Supp. Fig. 1), cells were first incubated with a LIVE/DEAD® Fixable Aqua Dead Cell Stain (Life Technologies, Grand Island, NY) and then immunostained for cell surface markers (CD4 QDot 605 and CD8 QDot 655; CD45RA ECD (Beckman Coulter, Indianapolis, IN), CCR7 Alexa Fluor 680, and CD95 biotin followed by streptavidin Qdot 705 for PBMC panel). Intracellular staining on fixed and permeabilized (BD Cytofix/Cytoperm; BD Biosciences) samples was performed using the following antibodies: CD3 APC-Cy7, IFNγ FITC, TNF PE-Cy7, IL-2 APC and granzyme B PE-Cy5.5 or Alexa Fluor 700). Except where noted, antibodies were purchased from BD Biosciences. As shown in Supp. Fig. 1, cytokine frequencies of viable CD3+ memory T cell populations were assessed in PBMC after gating out naïve CD4+CD95−CD28+ or CD8+CD45RA+CCR7+ cells; such memory gating was not necessary for BAL where nearly all T cells are memory phenotype (48). Flow cytometric data were acquired using a modified BD LSR II and analyzed using FlowJo® (Tree Star Inc., Ashland, OR) software; data processing and presentation were performed using Pestle (M. Roederer, NIH) and SPICE software (49). All antigen-specific cytokine frequencies are reported after background-subtraction of the frequency of the identically gated population of cells from the same sample incubated without antigen. Background responses in BAL were typically greater than those observed in PBMC.
Statistics
For cytokine frequencies, statistical significance was determined using a two-tailed Wilcoxon Rank Sum test using SPICE (v. 4.3) software (49); for pie charts, significance was determined by two-tailed Students t test. Survival curves were analyzed using a log-rank (Mantel-Cox) test in Prism (GraphPad Software, La Jolla, CA). Clinical parameters, bacterial burdens, and immune correlates were evaluated by Student’s t test or Spearman’s rank correlation, using either SPICE or JMP (SAS Institute, Cary, NC). For all comparisons, p values ≤ 0.05 are noted.
Results
Experimental design: animals, immunizations and challenge
32 Indian origin rhesus macaques were randomized into 4 study groups based on Ad35 serology, baseline BAL T cell responses, and weight (Materials and Methods and Fig. 1). G1 (Control; Ctrl) received only saline at wk 0; G2 (BCG/Ad35-null) and G3 (BCG/AERAS-402) were immunized i.d. with BCG at wk 0 (prime) followed by AE immunization with Ad35-null (control for AERAS-402) or AERAS-402 at wk 14 (boost), respectively; G4 (Ctrl/AERAS-402) received AERAS-402 (AE) at wk 14 without prior BCG-priming. All animals were challenged 12 wks after Ad35-null or AERAS-402 (wk 26) with approximately 275 CFU of M.tb Erdman, instilled by bronchoscope into the right lower lung. Euthanasia and necropsy for each animal was scheduled 24–26 wks after challenge, unless humane endpoint criteria were met at an earlier time point. Cellular immune responses in PBMC and BAL as well as various clinical parameters were monitored throughout the pre- and/or post-challenge phases of the study.
Aerosol vaccination with AERAS-402 elicits transient cellular immune responses in PBMC
The primary objective of this study was to determine whether a vaccine delivered by the AE route would elicit robust and protective TB-specific T cell cytokine responses in the lung, the initial site of TB infection. We also investigated how the aerosolized AERAS-402 vaccine would affect the magnitude and quality of antigen-specific T cell responses in peripheral blood when administered alone or following a primary i.d. immunization with BCG.
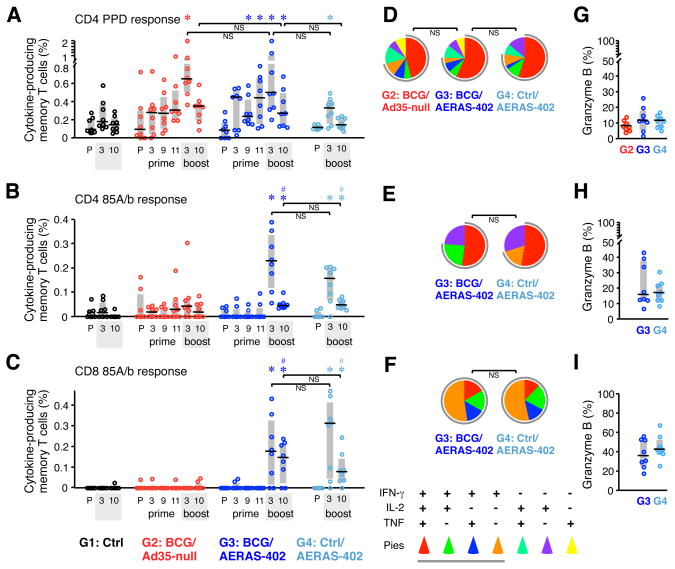
The magnitude of antigen-specific memory T cell responses was assessed as the total frequency of IFN-γ-, IL-2-, or TNF-producing cells (Supp. Fig. 1); these cytokines were chosen based on their importance in controlling TB infection (IFN-γ, TNF) and the ability to augment T cell expansion (IL-2) (9, 50–52). In PBMC, the frequency of PPD-specific memory CD4 T cells gradually increased after BCG priming (G2 and G3) with the highest responses (~0.6%) observed 17 wks after BCG (Fig. 2A). Three weeks after AE immunization with AERAS-402 alone (G4), low but significant PPD-specific CD4 T cell responses were elicited; such responses are likely attributed to the presence of Ag85A and Ag85B in PPD (53). However, all PPD responses in the blood decreased by 10 wks post-boost, the last measurement before challenge. For AERAS-402-induced immune responses, we focused on the Ag85A/b antigens since our previous studies had shown that T cell responses to TB10.4 were much lower than Ag85A/b following AE AERAS-402 immunization in both the PBMC (submitted for publication) and BAL (Supp. Fig. 2A and (21)), consistent with prior reports in mice (40, 43) or BCG-primed humans vaccinated i.m. with AERAS-402 (44, 45). Although BCG expresses Ag85A and Ag85B, Ag85A/b-specific T cells were low to undetectable in PBMC following BCG vaccination (Fig. 2B, C). In contrast, Ag85A/b-specific CD4 and CD8 T cells in vaccine groups that received AERAS-402 were readily detectable at 3 wks post-boost. By 10 wks post-boost these responses waned to similar frequencies, irrespective of BCG priming, but remained significantly higher than Ctrl animals (G1) prior to challenge. Thus, AERAS-402 administered into the lung elicits a transient increase in Ag85A/b-specific CD4 and CD8 T cell responses in the blood that were not significantly augmented or sustained by prior BCG immunization.
FIGURE 2. Pre-challenge cellular immune responses in PBMC.
(A–C) The frequency of memory CD4 (A, B) and CD8 (C) T cells in PBMC producing IFN-γ, IL-2, or TNF in response to PPD (A) or Ag85A/b peptides (B, C) was measured by flow cytometry at a pre-immune (P) time point, 3, 9, and 11 wks after the BCG prime (G2 and G3), and 3 and 10 wks after the Ad35-null or AERAS-402 boost (G2–G4). Interquartile range (shaded box) and median (black line) of individual responses are indicated. (D–F) The quality of the T cell responses in panels A–C, respectively, 10 wks post-boost. Pie charts show the fraction of total cytokine-response comprising any combination of IFN-γ, IL-2, or TNF (gray pie arcs show the proportion of IFN-γ-producing cells). Pies (average of n = 8) are shown for groups with measurable responses. (G–I) The percent of cytokine-producing T cells in panels A–C, respectively, producing granzyme B 10 wks post-boost. *p ≤ 0.05 compared to pre-immune within same group; #p ≤ 0.05 compared to Ctrl at 10 wks post-boost; NS: not significant.
The quality (cytokine expression profile) of the vaccine-elicited T cell response has implications for disease outcome in TB and other infections that require T cells for protection (36, 54–56). Thus, we assessed the quality of CD4 and CD8 T cell responses in PBMC prior to challenge (Fig. 2D–F and Supp. Fig. 2B, left). Approximately 50% of the memory CD4 T cell cytokine response in each vaccine group produced IFN-γ, IL-2, and TNF simultaneously, whereas CD8 T cell responses were far less multifunctional with >50% of responses producing only IFN-γ. A transient decrease in the proportion of multifunctional PPD-specific memory CD4 T cells was noted 3 wks after the Ad35-null or AERAS-402 boost (G2 and G3; Supp. Fig 2B, left) suggesting that non-specific innate factors induced by the Ad35 vector (57–59) may alter the quality of the response. To explore the potential cytolytic capacity of TB-specific responses, the proportion of cytokine-positive T cells expressing granzyme B was assessed at the last time point before challenge (Fig. 2G–I). Granzyme B expression was higher in cytokine-producing CD8 T cells compared to CD4 T cells, but did not differ between vaccine groups.
Aerosol vaccination with AERAS-402 elicits robust and sustained cellular immune responses in BAL
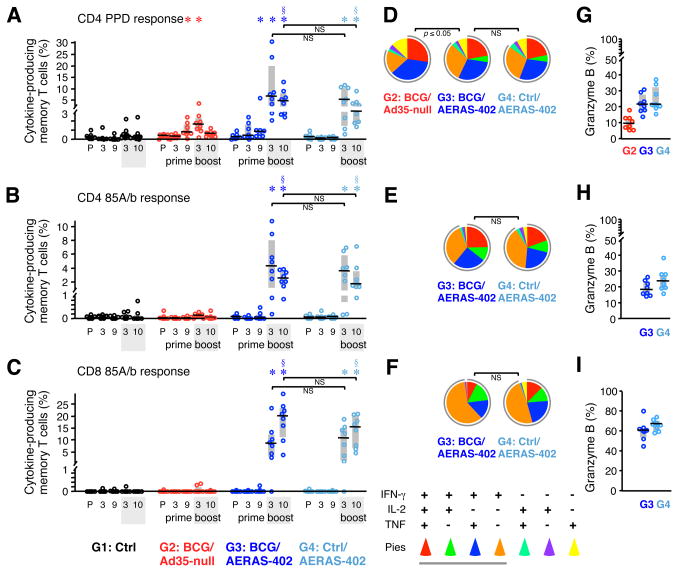
T cell responses in BAL were assessed before immunization, 3 and 9 wks after priming with BCG, and 3 and 10 wks after AE boosting with AERAS-402 or Ad35-null (Fig. 3). In contrast to the relatively low and transient systemic PBMC responses after AE AERAS-402, CD4 and CD8 T cell responses were high and sustained in BAL. Median frequencies of antigen-specific CD4 T cells 3 wks after AERAS-402 immunization ranged between 4 and 7% to PPD or Ag85A/b (Fig. 3A, B) and such responses were maintained with a modest decrease at 10 wks post-boost. By contrast, low frequencies of PPD-specific CD4 responses were detected in BAL 17 wks after BCG—3 wks after Ad35-null boost (G2, Fig. 3A). There was no additional increase in PPD or Ag85A/b CD4 T cell responses after the AE AERAS-402 boost of BCG-primed animals (G3) compared to that in animals that received AERAS-402 without BCG-priming (G4). Last, potent Ag85A/b-specific memory CD8 T cell responses (~8.5–10.5% median) were elicited by AE AERAS-402 at 3 wks post-boost and such responses were sustained or even increased (~15–20% median) between 3 and 10 wks post-boost (Fig. 3C). Prior BCG-priming did not affect Ag85A/b-specific CD8 T cells generated by AE AERAS-402 administration. Thus, at the final assessment prior to challenge, robust TB-specific CD4 and CD8 T cell cytokine responses were detected only in BAL of animals that received AE AERAS-402 (G3 and G4).
FIGURE 3. Pre-challenge cellular immune responses in BAL.
(A–C) The frequency of memory CD4 (A, B) and CD8 (C) T cells in BAL producing IFN-γ, IL-2, or TNF in response to PPD (A) or Ag85A/b peptides (B, C) was measured at a pre-immune (P) time point, 3 and 9 wks after the BCG prime, and 3 and 10 wks after the Ad35-null or AERAS-402 boost. Interquartile range (shaded box) and median (black line) are indicated (n = 8 per group). (D–F) The quality of T cell responses in panels A–C, respectively, 10 wks post-boost, as described in Fig. 2. (G–I) The percent of cytokine-producing T cells in panels A–C, respectively, producing granzyme B 10 wks post-boost. *p ≤ 0.05 compared to pre-immune within same group; §p ≤ 0.05 compared to Ctrl and BCG/Ad35-null at time of challenge; NS: not significant.
We also examined the quality of the T cell responses in BAL prior to challenge and found no significant effect of BCG-priming on the cytokine profile elicited by AERAS-402 (Fig. 3D–F). Nearly all antigen-specific CD4 T cells in BAL produced IFN-γ and, compared with PBMC, were comprised of fewer IFN-γ, IL-2, and TNF triple-positive cells and a higher proportion of IFN-γ and TNF double-positive and IFN-γ single-positive cells. This is consistent with effector sites such as the lung having a differentiated effector T cell cytokine profile and phenotype (48). The proportion of memory CD4 T cells producing only IFN-γ in BAL increased following AERAS-402 boosting in BCG-primed animals (Supp. Fig. 2B, right). Similarly, rAd vaccines have previously been shown to alter the quality of primed CD4 T cell responses (60). As noted above in the PBMC, CD8 T cell responses in the BAL following AERAS-402 were comprised of >50% IFN- γ single-positive cells (Fig. 3F). Expression of granzyme B (Fig. 3G–I) by cytokine-producing CD8 T cells was higher in BAL compared with PBMC but did not differ between AERAS-402 groups. In contrast, granzyme B expression in PPD-specific CD4 T cells was lower in BCG/Ad35-null animals than in animals that received AERAS-402.
Survival, bacterial burdens and clinical response after M.tb challenge
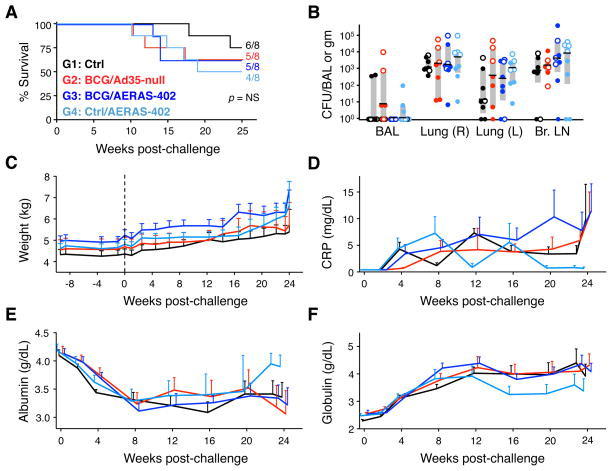
To determine whether the vaccine-elicited responses conferred protection against challenge, 275 CFU of M.tb Erdman were instilled by bronchoscope into the right lower lung at wk 26 (12 wks after AERAS-402 or Ad35-null AE administration) and multiple clinical parameters were followed (41). Between 4 and 8 wks post-challenge, all animals exhibited similar radiographic signs of disease (parenchymal abnormalities) at the site of infection (lower right lung lobe; data not shown). Additionally, beginning 10 wks after challenge and continuing through the fixed study endpoint (wk 50–52), multiple animals in each group met humane endpoint criteria and required euthanasia. Such animals (2 animals in G1, 3 animals in G2, 3 animals in G3, and 4 animals in G4) are henceforth referred to as “rapid progressors” to distinguish them from animals that survived until the fixed endpoint (survivors). There was no significant difference in the rate of survival between vaccinated groups and unvaccinated control animals after challenge (Fig. 4A).
FIGURE 4. Survival, bacterial burdens, and clinical data.
(A) Survival curves following M.tb challenge. Shown are the fraction and percentage of animals that survived until the fixed study endpoint for each vaccine group (B) M.tb CFU per total BAL volume harvested or per gram of tissue for the right (R) and left (L) lung lobes and bronchial (Br.) lymph nodes for each animal at time of necropsy. Interquartile range (shaded box) and median (black bar) are indicated for each group. Open symbols indicate rapid progressors. (C) Average weight (with SEM) for each vaccine group at the indicated week relative to challenge (dashed line). Individual animal weights are shown in Supp. Fig. 3. Average serum levels of C-reactive protein (CRP; D), albumin (E) and globulin (F) for each group with SEM at the indicated wks post-challenge. Values were calculated using available data from 4–8 animals per group per time point.
Overall, considering all animals (rapid progressors and survivors), there was no reduction in bacterial CFUs cultured at necropsy from BAL, right lung (infection site), left lung or bronchial lymph nodes in any of the vaccine groups (Fig. 4B). Furthermore, there were no differences in the severity of lymph node pathology (enlargement, granulomas, or necrosis) between vaccinated and unvaccinated macaques (data not shown). Thus, despite high frequencies of antigen-specific, cytokine-producing CD4 and CD8 T cells in BAL at the time of challenge, animals were not protected against M.tb with respect to survival rate or bacterial burdens. Furthermore, BCG vaccination (BCG/Ad35-null) also failed to elicit any protection compared to the Ctrl group.
Progressive weight loss after M.tb challenge can occur during TB infection; however, here an overall increase in average weight was observed in each group, regardless of challenge, as the study progressed (Fig. 4C and Supp. Fig. 3). With respect to other disease-related clinical parameters, the non-specific inflammatory marker, C-reactive protein (CRP) is often elevated in the serum of animals with active tuberculosis; similarly, decreased serum albumin and increased serum globulin can be indicative of disease. In this study, CRP (Fig. 4D), albumin (Fig. 4E) and globulin (Fig. 4F) levels in the serum did not differ significantly between vaccinated groups and control animals. Thus, no differences in clinical outcome could be discerned between groups if rapid progressors and survivors were considered together.
Gross pathology, histology and correlation analyses of rapid progressors and survivors
All animals underwent full necropsy procedures regardless of whether they were sacrificed early due to disease (rapid progressors) or survived until the scheduled study endpoint (survivors). Granuloma prevalence and size in each lung lobe (Fig. 5A) and the abdominal viscera (Fig. 5B) were scored grossly, using a scale of 0 to 4. Rapid progressors displayed similar granuloma severity throughout the lung lobes across the different vaccine groups (Fig. 5, shaded area). By contrast, among the surviving animals, both granuloma prevalence and size scores were reduced in the Ctrl/AERAS-402-vaccinated animals (G4) compared to other vaccine groups; in these animals, only the right lower lobe (inoculation site) contained grossly visible granulomas, suggesting confinement of M.tb infection (G4, unshaded area). Concordant with lower granuloma scores observed in the Group 4 survivors was a decrease in additional pulmonary findings (pleural adhesions, pleural thickening, and cavitation; data not shown). Furthermore, histopathologic analysis of lung sections confirmed that, in the surviving animals that received Ctrl/AERAS-402, lesions were largely restricted to the right lower lobe (Supp. Fig. 4A). These findings were notable because the same animals displayed higher serum albumin and lower serum globulin throughout the post-challenge phase, compared to survivors in other groups (Supp. Fig. 4B). Taken together, these data suggested that AE AERAS-402 vaccination might be associated with mildly improved outcome. However, the confinement of granulomas to the right lower lobe in the Group 4 surviving animals did not translate into decreased in bacterial burdens in the lung, as measured in this study. While animals that survived until the fixed endpoint (closed symbols; Fig. 4B) had generally lower CFU than rapid progressors (open symbols) within the same group, there was no difference in bacterial burdens between groups when only surviving animals were considered (data not shown). Finally, abdominal granulomas (an indication of extra-thoracic spread) were generally less abundant than in the lung but with no apparent difference between vaccine groups (Fig. 5B).
FIGURE 5. Gross pathology observations.
Granuloma severity in the lung (A) and abdominal viscera (B). The prevalence and size of granulomas were scored on a scale ranging from 0 to 4 (with increasing severity) in each lung lobe (A; right lower lobe is site of challenge) as well as in the liver, spleen, and remaining abdominal viscera (RAV; B) in each animal at the time of necropsy. Each vaccine group is shown on a separate graph with data segregated by whether the animal was a rapid progressor (shaded area) or survivor (unshaded area). Gray bars indicate the mean score.
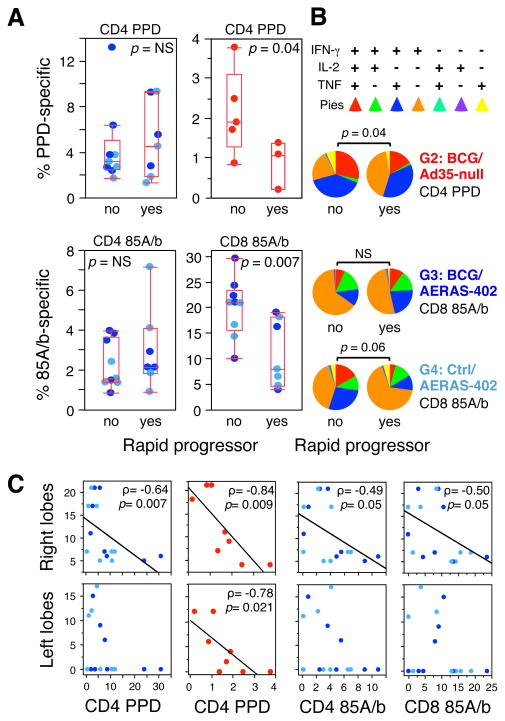
Finally, we investigated potential immune correlates between the cellular immune response before challenge and survival after challenge. Prior to challenge, PPD- and Ag85A/b-specific CD4 T cell cytokine responses in BAL were similar among all animals immunized with AERAS-402 (Fig. 6A, left). However, Ag85A/b-specific CD8 T cell responses in BAL were higher in surviving animals compared to rapid progressors (Fig. 6A, lower right), whereas no correlation was observed in PBMC (data not shown). Despite the comparatively low peak magnitude of CD4 PPD responses in the BAL following BCG/Ad35-null vaccination (Fig. 3A), such responses were significantly higher in survivors compared to rapid progressors within the same vaccine group (Fig. 6A, upper right). Furthermore, there was a difference (p = 0. 04) in the cytokine expression pattern of the pre-challenge PPD-specific CD4 T responses in survivors and rapid progressors vaccinated with BCG/Ad35-null (Fig. 6B). Similarly, greater than half of the antigen-specific CD8 T cells in the surviving Ctrl/AERAS-402-immunized animals (that confined granulomas to the site of inoculation) produced IFN-γ in addition to IL-2, TNF or both, compared with rapid progressors (Fig. 6B). Combined, these data suggest that both the magnitude and quality of the vaccine-induced lung T-cell response present at the time of challenge may limit disease progression.
FIGURE 6. Correlation of pre-challenge immune responses with protection against M.tb.
(A) CD4 and CD8 T cell cytokine responses (IFN-γ, IL-2 or TNF) against PPD or Ag85A/b peptides in the BAL are compared between survivors (no) and rapid progressors (yes) in AERAS-402- (light/dark blue symbols; 10 wks post-boost) or BCG/Ad35-null- (red symbols; peak response) immunized groups. (B) Comparison of the T cell cytokine expression profiles in survivors and rapid progressors for peak PPD-specific CD4 responses (G2) and Ag85A/b-specific CD8 responses (G3, G4). Significant p values (t-test) are indicated; NS: not significant. (C) Spearman correlation between BAL T cell responses in AERAS-402- or BCG/Ad35-null- immunized animals 3 wks post-boost and lung granuloma severity at necropsy. Granuloma prevalence and size scores were summed for either the right or left lung lobes. Spearman’s rho (ρ) and p values are indicated for significant correlations; linear regression fit is shown for illustration.
To determine if vaccination impacted granuloma formation in the lung, we studied the relationship between vaccine-induced BAL T-cell responses and granuloma scores. The magnitude of the PPD-specific CD4 T-cell response 3 wks post-boost inversely correlated with granuloma severity in the right lung lobe (inoculation site) among all AERAS-402-vaccinated animals, as well as in the right and left lobes of animals that received BCG/Ad35-null (Fig. 6C). Ag85A/b-specific CD4 and CD8 T-cell responses in animals that received AERAS-402 were also associated with reduced granuloma scores in the right lobe. Analysis of the AERAS-402-elicited T-cell responses 10 wks post-boost did not reveal significant correlations (data not shown). These data implicate local CD4 and CD8 T cell responses in constraining granuloma size and prevalence in the lungs.
Vaccine-elicited cellular immune responses are not boosted by M.tb infection in PBMC
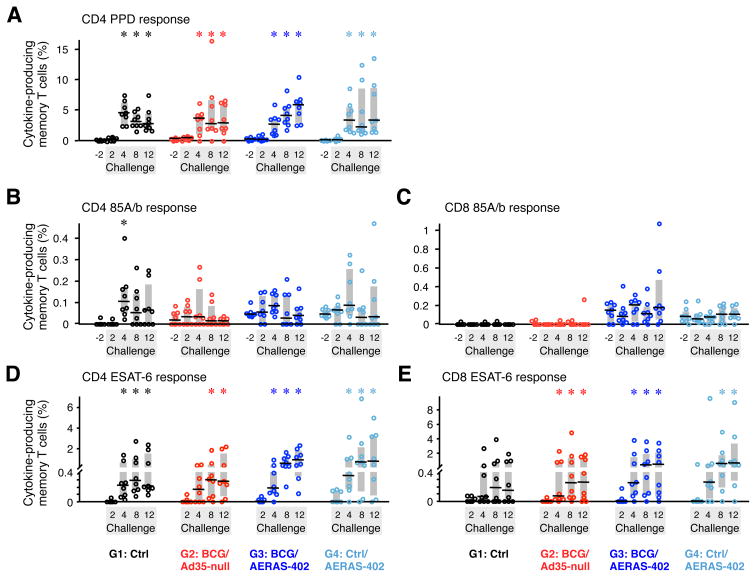
A critical aspect that will impact protective efficacy is whether the antigen-specific vaccine elicited responses are rapidly expanded after the infectious challenge. Therefore, we assessed the kinetics of the anamnestic T cell responses in PBMC to the vaccine antigens following M.tb infection (Fig. 7). By 4 wks post-infection, all animals in vaccine and control groups had high and comparable frequencies (~4%) of PPD-specific CD4 T cells that exceeded pre-challenge levels (Fig. 7A). In striking contrast, CD4 T cell responses to Ag85A/b in PBMC were remarkably lower than responses to PPD (<0.1%). Low de novo CD4 T cell responses to Ag85A/b were generated in Ctrl (G1) animals by 4 wks post-challenge (Fig. 7B). Of note, BCG- or AERAS-402-vaccinated animals showed neither an accelerated response nor enhanced magnitude of Ag85A/b-specific CD4 T cells compared to unvaccinated Ctrl animals at any time point post-challenge. Thus, the frequency of Ag85A/b-specific CD4 T cells in vaccinated animals was not increased from pre-challenge levels. In contrast, Ag85A/b-specific CD8 T cells were neither generated de novo nor boosted in the blood after challenge (Fig. 7C). Taken together, these data indicate that the low-level pre-existing Ag85A/b-specific CD4 and CD8 T cells generated by AERAS-402 immunization in the blood were not appreciably boosted by M.tb infection.
FIGURE 7. Post-challenge cellular immune responses in PBMC.
The frequency of memory CD4 (A, B, D) and CD8 (C, E) T cells in PBMC producing IFN-γ, IL-2, or TNF in response to PPD (A), Ag85A/b peptides (B, C), or ESAT-6 peptides (D, E) was measured 2 wks before (excluding ESAT-6) and 2, 4, 8, and 12 wks after M.tb challenge. Interquartile range (shaded box) and median (black line) for 8 animals per group are indicated. *p ≤ 0.05 compared to pre-challenge (A–C) or 2 wk post-challenge (D, E) within same group.
Last, we measured post-challenge CD4 and CD8 T cell responses to the M.tb-specific antigen, ESAT-6 (Early Secreted Antigenic Target of 6 kDa), which is expressed neither by BCG nor AERAS-402. Between 2 and 4 wks after challenge, nearly all control and vaccinated groups generated significant CD4 T cell responses against ESAT-6 (Fig. 7D). At later time points, the frequency of ESAT-6-specific CD4 T cells in vaccine groups that received AERAS-402 (G3 and G4) trended higher but were not significantly different than the Ctrl group (Fig. 7D). Similarly, ESAT-6-specific CD8 T cells were generated in each group after infection; however, only vaccinated groups reached significance with a trend toward AERAS-402-immunized animals having greater median frequencies at 8 and 12 wks post-challenge (Fig. 7E). No significant correlations were found between ESAT-6-specific T cell responses after challenge and outcome (data not shown).
Discussion
In this study we used a vaccination strategy that induces strong cellular immune responses directly in the lung to assess the hypothesis that a high frequency of antigen-specific T cells at the site of infection would protect against TB. We show that aerosolized delivery of rAd35 expressing Ag85A, Ag85B and TB10.4 (AERAS-402) induces potent Ag85A/b-specific, cytokine-producing, effector CD4 and CD8 T cell responses in BAL. Nevertheless, these responses did not confer enhanced survival or decrease bacterial burdens among all animals following challenge with 275 CFU of M.tb Erdman in this fixed endpoint protocol. Overall, based on the results, we have identified several areas for improving possible protective efficacy using the NHP model. These include: (1) antigen selection, (2) type of viral vector used for AE delivery, (3) species of macaque, and (4) dose of M.tb used for challenge.
BCG protects infants from disseminated TB but has varying efficacy (0–80%) against pulmonary infection (4), which is the principal target of current vaccine efforts (7). To model a TB vaccine for humans, who are given BCG at birth, we immunized rhesus macaques with BCG i.d. before AE administration of AERAS-402. We hypothesized that the AE route would most directly and effectively induce T cell immunity in the lung and thereby improve protection against pulmonary TB. A key variable in optimizing adaptive immunity to heterologous prime-boost immunization is the time interval between the prime and boost. In this study, a longitudinal analysis of TB-specific T cell responses in PBMC following BCG immunization showed that the CD4 responses to PPD continued to increase between wk 11 (~0.3%) and wk 17 (~0.6%; Fig. 2). Thus, in contrast to the peak of the response following an adjuvanted protein or viral vector vaccine that occurs between 10–21 days after immunization, T cell responses after BCG appear to peak much later. A similar prolonged kinetic of BCG-induced immunity has been documented in young adults where PPD-specific T cell responses gradually increase up to one year after immunization (61). These data suggest that for optimizing T cell responses to a BCG prime, a systemic boost using the i.m., i.d., or s.c route should be administered after a prolonged interval (~20 weeks or later) to avoid boosting during the peak of the primary effector response. Indeed, longer intervals between a prime and boost with heterologous vaccines have been shown to optimize both antibody and T cell responses in NHPs and humans with a variety of vaccine platforms (62, 63).
Intradermal BCG immunization elicited PPD-specific CD4 T cells in BAL that waned from ~1.5% at peak (wk 17) to ~0.5% before challenge (Fig. 3A). These data raise questions as to whether the magnitude of the T cell response in BAL after BCG immunization is sufficient to mediate protection against TB in the lung. The low BAL responses may explain variability in the protection afforded by BCG in humans. Therefore, to enhance T cell responses at the site of infection, BCG-primed or naïve animals were given AERAS-402 by the AE route. Prior studies by others and us have shown that lung delivery of rAd5 using TB or SIV antigens induces robust T cell responses in the lung that confer protection in mice and macaques, respectively (19, 32, 35, 64). Consistent with these data, animals boosted with AERAS-402 AE generated robust and sustained CD4 and CD8 T cells responses to Ag85A/b in BAL. Importantly, BCG-priming did not augment such responses (Fig. 3), consistent with BCG inducing low to undetectable levels of Ag85A/b-specific T cells in the lung (as shown here), or in PBMC (39, 45, 65). Furthermore, AE delivery of AERAS-402 had no significant effect on the BCG-primed systemic response (Fig. 2A). Taken together, these data show that the different prime and boost vaccination routes used for BCG and AERAS-402 direct immune responses to the periphery or lung mucosa, respectively, with limited boosting. Nevertheless, generating responses in both the lung and the periphery should be optimal for protection—by having a high frequency of effectors present in the lung at the time of infection, as well as peripheral TB-specific cells that can migrate into the lung to enhance and sustain protection. Indeed, previous experiments in mice have shown that i.n. delivery of Ad5-85A maintains a high frequency of lung-resident effector cells that protect early after TB infection while systemic BCG immunization generates a reservoir of peripheral memory cells that can be recruited into lung by inflammation at later phases of infection (64, 66). In this regard, boosting by both AE and i.m., after i.d. BCG, would generate robust short-term effector responses in the lung as well as increase the magnitude of BCG-primed T cell responses in the periphery. Accordingly, prior studies demonstrated enhanced systemic responses following BCG-priming in humans when AERAS-402 or Ad5-85A boosts were given i.m. (45, 67).
Proteins of the Ag85 mycolyl transferase complex have been considered promising immune targets and have been widely used as vaccine immunogens to improve or extend the efficacy of BCG because they are abundantly secreted and largely conserved among Mycobacteria spp (68, 69). The esat-6 gene family member, TB10.4, is strongly recognized by BCG-vaccinated and TB patients (70) and was included in the AERAS-402 construct to enhance the breadth of the T cell responses. However, we and others have demonstrated that AERAS-402 vaccination elicits comparatively lower responses to TB10.4 (Supp. Fig. 2A and (40, 43–45), due possibly to immunodominance of Ag85A/b epitopes or the greater size of the Ag85A and Ag85B proteins. Thus, we focused our immune analysis on Ag85A/b responses. Ag85 proteins are immunogenic in humans (44, 45, 67, 71–75) and elicit protection either alone or when combined with other antigens in small animal models when administered in various vaccine formulations including protein plus adjuvant (54, 76–81), recombinant BCG (82–84), and recombinant viral vectors (27, 32, 36, 38, 43, 64, 66, 79, 85). However, in this study, despite the robust T cell responses to Ag85A/b in the lung prior to challenge, there was no appreciable protection. Furthermore, the lack of a robust anamnestic response to Ag85A/b in AERAS-402-vaccinated animals in the blood after M.tb challenge is notable (Fig. 7). Taken together, these data raise the concern as to whether Ag85A/B alone is a sufficiently effective target during early TB infection in vivo. Prior studies have evaluated prime-boost immunization with BCG followed by i.d. MVA85A in rhesus macaques. In one study, BCG/MVA85A did not significantly improve protection (bacterial burdens or pathology) compared to BCG alone following an intratracheal challenge with 1000 CFU M.tb Erdman (65). A second study in which animals were challenged AE with a low-dose (40–60 CFU) of M.tb. Erdman reported that neither BCG nor BCG/MVA85A immunization enhanced survival despite each group displaying an equal reduction in pulmonary disease burden (lesion volume/lung volume) compared to unvaccinated controls (86). In both studies, there were no significant anamnestic T cell responses to Ag85A after M.tb challenge. Finally, any protective effect of BCG/MVA85A immunization in NHPs as measured by various parameters (58) did not translate into protection in a recent phase 2b trial in infants (87). While we cannot exclude the possibility that anamnestic responses to Ag85A/b occurred in BAL, which we did not assess in order to avoid disrupting the lung after infection, experiments in mice have carefully demonstrated that Ag85B-specific cells in the lung are suboptimally activated after TB infection due to limited expression of Ag85B that decreases further as the infection progresses (88). Decreased expression of Ag85B relative to other TB antigens, such as ESAT-6, has been observed in macaques and mice after challenge (76, 89) as the adaptive immune response begins to limit bacterial replication (90). Taken together, these data suggest that using different or additional mycobacterial immunogens in vaccines to generate T cells that will respond to highly expressed M.tb antigens post-challenge may be more protective.
While no recall response to Ag85A/b was observed in the blood of vaccinated animals, unvaccinated animals generated CD4, but not CD8, T cells to Ag85A/b in response to infection. In contrast, several animals in each vaccine group generated de novo CD8 T cell responses to the M.tb-specific antigen ESAT-6 post-challenge (Fig. 7). These data illustrate a fundamental difference in antigen presentation of M.tb proteins that may have important consequences for protection by vaccines. A protective vaccine against TB might require immune responses to different or more antigens, perhaps most importantly those that are rapidly recognized by the immune system following infection, as well as those which engage the CD8 T cell compartment through cross-presentation.
Another important consideration for the lack of protection we observed in this study is the type of rAd vector used for AE delivery. AERAS-402 uses rAd35, which was developed as an alternative adenoviral vector to rAd5 because of its low global seroprevalance (that can impact systemic immunogenicity). However, rAd35 is the least immunogenic of all rAd vectors for priming T cell responses when given by the i.m. or s.c. route in mouse, NHP and human studies (28, 29, 91). Thus, while we observed potent effector T cell responses in the lung after AE AERAS-402 immunization, it remains possible that other rAd vectors would induce quantitatively and qualitatively different responses that may confer protection (29). Indeed, the protective efficacy of rAd immunization with TB antigens in mice has been achieved in multiple studies using rAd5 (31, 64, 79).
A notable finding was the lack of significant protection in animals immunized with BCG/Ad35-null compared to unvaccinated animals. The level of BCG-induced protection against TB in NHP is historically variable and may depend on multiple factors including: species or origin of macaque; challenge dose, strain, and route; and BCG vaccination route (22, 41, 65, 92, 93). Here as in other NHP studies, we were restricted by the number of animals we could infect (4 groups of 8 animals). As the major experimental group in the study was BCG/AERAS-402, the most appropriate control to confirm antigen-specificity of a protective effect conferred by AERAS-402 was to use an empty rAd vector boost following BCG. Thus, we did not have a group that received only BCG; therefore, we cannot exclude the possibility that the Ad35-null boost may have interfered with BCG-elicited protection. Although BCG-primed CD4 T cell responses continued to increase following the Ad35-null boost (Fig. 2A, 3A), it remains possible that innate factors elicited by Ad35-null boost may have induced a qualitative change. Indeed, rAd35 elicits high levels of innate cytokines in mice, NHP and humans compared with other rAd serotypes (57–59). Notwithstanding the Ad35-null boost, BCG-induced CD4 PPD-responses in the BAL were found to correlate with better survival (compared to rapid progressors within the same group) and less severe lung pathology (Fig. 6). These data indicate that BCG immunization did have some effect on disease outcome.
The NHP model can be used to predict vaccine immunogenicity of viral vector and protein/adjuvant formulations in humans and to model protective efficacy for infections such as SIV/HIV, TB, and malaria. Consistent with prior vaccine studies in macaques, we delivered a high number of M.tb (275 CFU) by bronchoscope to ensure infection of all animals. However, as shown here, with this challenge dose, rhesus macaques are highly susceptible to TB with a rapid course of infection (22, 92–94). These findings suggest that a far lower dose (<10 CFU) given by bronchoscope, AE (95), or by natural exposure from another infected animal, may be less overwhelming and thereby improve vaccine efficacy. Indeed, prior studies demonstrate that rhesus macaques will uniformly develop active TB infection following intrabronchial or AE challenge with doses of M.tb Erdman as low 10–50 CFU (86, 93, 96). It should be noted that the challenge dose required to induce active TB might differ in a less-susceptible NHP species, such as cynomolgus macaques (92, 93, 97, 98), or using a less-virulent M.tb lab strain, such as H37Rv (96). Notwithstanding the rigorous challenge used in this study, we identified immune responses in AERAS-402-vaccinated animals that may have been associated with mildly better outcome (Fig. 6) as well as a trend toward reduced lung pathology in surviving animals that received AE AERAS-402 without a BCG prime (Fig. 5A and Supp. Fig. 4). Despite these findings, M.tb CFUs were not diminished in any vaccinated group, regardless of whether all animals or only surviving animals were considered. Future studies using real-time, advanced imaging by PET/CT may provide an additional non-invasive method for assessing protection that will extend what is obtained by pathology and CFU quantitation (99). Identifying reliable clinical and immunological parameters that correlate with protection is critical to assessing vaccine efficacy in NHP as well as in humans.
In conclusion, our study shows that AE vaccination with AERAS-402 elicits robust and sustained T cell responses in the lung. Based on the absence of post-challenge anamnestic responses to Ag85A/b, the lack of cross-presentation of Ag85A and Ag85B following natural infection, and the lack of protection in vaccinated animals or humans, we conclude that these antigens alone are likely not sufficient to mediate overall protection, at least with this rAd vector serotype. Therefore, as with other infections (e.g., malaria, L. major) that require whole attenuated organisms for protective cellular immunity, vaccines against TB that incorporate many, rather than few or single antigens might have a greater chance of efficacy. Furthermore, rAd5 vectors or newly characterized chimpanzee-derived Ads with potent immunogenicity may enhance the magnitude and quality of the responses compared to rAd35 used here. Last, future NHP studies should use a much lower M.tb challenge dose to evaluate vaccine-mediated protection. Thus, although this study had limited efficacy, it provides critical insights into optimizing the NHP model for future TB vaccine development.
Supplementary Material
Acknowledgments
We thank Faith Schiro (TNPRC, Covington, LA) for sample collection and processing. We acknowledge Christine Anderson, Steven Derrick, and Amy Yang at CBER (FDA, Bethesda, MD) for assistance with BCG and M.tb stocks. We thank JP Todd and Alida Ault for assistance with AE immunizations and animal protocols.
This work was funded in part by Aeras and supported by the intramural research program of the Vaccine Research Center, NIAID, NIH as well as NIH grant P51RR000164/P51OD011104 to the TNPRC.
Abbreviations
- AE
aerosol
- AERAS-402
rAd type 35 expressing M.tb antigens Ag85A, Ag85B, and TB10.4
- BAL
bronchoalveolar lavage
- BCG
bacille Calmette-Guerin
- CRP
C-reactive protein
- Ctrl
control
- ESAT-6
Early Secreted Antigenic Target of 6 kDa
- i.d
intradermal
- i.m
intramuscular
- i.n
intranasal
- LN
lymph node
- M.tb
Mycobacterium tuberculosis
- MVA85A
Modified Vaccinia Virus Ankara expressing Ag85A
- NHP
nonhuman primate
- P
pre-immune
- PBMC
peripheral blood mononuclear cells
- PPD
purified protein derivative
- rAd
recombinant adenoviral vector
- s.c
subcutaneous
- TB
tuberculosis
- vp
viral particles
Footnotes
Disclosures
JCS is an employee of Crucell. The other authors have no financial conflicts of interest.
References
- 1.World Health Organization. Global Tuberculosis Report 2013. Geneva, Switzerland: 2013. [Google Scholar]
- 2.Seder RA, Hill AV. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406:793–798. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann SH. Future vaccination strategies against tuberculosis: thinking outside the box. Immunity. 2010;33:567–577. doi: 10.1016/j.immuni.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96:29–35. [PubMed] [Google Scholar]
- 5.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 6.Dalmia N, Ramsay AJ. Prime-boost approaches to tuberculosis vaccine development. Expert review of vaccines. 2012;11:1221–1233. doi: 10.1586/erv.12.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS pathogens. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infection and immunity. 2002;70:4501–4509. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper AM. Cell-mediated immune responses in tuberculosis. Annual review of immunology. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, Fountain JJ, Martino CA, Roberts AD, Cooper AM, Winslow GM, Woodland DL. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10961–10966. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. The Journal of experimental medicine. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. Journal of immunology (Baltimore, Md : 1950) 1990;144:3980–3986. [PubMed] [Google Scholar]
- 13.Jung YJ, Ryan L, LaCourse R, North RJ. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. The Journal of experimental medicine. 2005;201:1915–1924. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serbina NV, Flynn JL. CD8(+) T cells participate in the memory immune response to Mycobacterium tuberculosis. Infection and immunity. 2001;69:4320–4328. doi: 10.1128/IAI.69.7.4320-4328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor LM, Harvie MC, Rich FJ, Quinn KM, Brinkmann V, Le Gros G, Kirman JR. A key role for lung-resident memory lymphocytes in protective immune responses after BCG vaccination. European journal of immunology. 2010;40:2482–2492. doi: 10.1002/eji.200940279. [DOI] [PubMed] [Google Scholar]
- 16.Horvath CN, Shaler CR, Jeyanathan M, Zganiacz A, Xing Z. Mechanisms of delayed anti-tuberculosis protection in the lung of parenteral BCG-vaccinated hosts: a critical role of airway luminal T cells. Mucosal immunology. 2012;5:420–431. doi: 10.1038/mi.2012.19. [DOI] [PubMed] [Google Scholar]
- 17.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. The Journal of experimental medicine. 2008;205:2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horvath CN, Xing Z. Immunization strategies against pulmonary tuberculosis: considerations of T cell geography. Advances in experimental medicine and biology. 2013;783:267–278. doi: 10.1007/978-1-4614-6111-1_14. [DOI] [PubMed] [Google Scholar]
- 19.Bolton DL, Song K, Wilson RL, Kozlowski PA, Tomaras GD, Keele BF, Lovingood RV, Rao S, Roederer M. Comparison of systemic and mucosal vaccination: impact on intravenous and rectal SIV challenge. Mucosal immunology. 2012;5:41–52. doi: 10.1038/mi.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nature reviews Immunology. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 21.Song K, Bolton DL, Wei CJ, Wilson RL, Camp JV, Bao S, Mattapallil JJ, Herzenberg LA, Herzenberg LA, Andrews CA, Sadoff JC, Goudsmit J, Pau MG, Seder RA, Kozlowski PA, Nabel GJ, Roederer M, Rao SS. Genetic immunization in the lung induces potent local and systemic immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22213–22218. doi: 10.1073/pnas.1015536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barclay WR, Busey WM, Dalgard DW, Good RC, Janicki BW, Kasik JE, Ribi E, Ulrich CE, Wolinsky E. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. The American review of respiratory disease. 1973;107:351–358. doi: 10.1164/arrd.1973.107.3.351. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Wang J, Zganiacz A, Xing Z. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infection and immunity. 2004;72:238–246. doi: 10.1128/IAI.72.1.238-246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Contreras L, Wong YL, Muttil P, Padilla D, Sadoff J, Derousse J, Germishuizen WA, Goonesekera S, Elbert K, Bloom BR, Miller R, Fourie PB, Hickey A, Edwards D. Immunization by a bacterial aerosol. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4656–4660. doi: 10.1073/pnas.0800043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gheorghiu M. BCG-induced mucosal immune responses. International journal of immunopharmacology. 1994;16:435–444. doi: 10.1016/0192-0561(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 26.Giri PK, Verma I, Khuller GK. Protective efficacy of intranasal vaccination with Mycobacterium bovis BCG against airway Mycobacterium tuberculosis challenge in mice. The Journal of infection. 2006;53:350–356. doi: 10.1016/j.jinf.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. Journal of immunology (Baltimore, Md : 1950) 2003;171:1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 28.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O’Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. Journal of virology. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinn KM, Da Costa A, Yamamoto A, Berry D, Lindsay RW, Darrah PA, Wang L, Cheng C, Kong WP, Gall JG, Nicosia A, Folgori A, Colloca S, Cortese R, Gostick E, Price DA, Gomez CE, Esteban M, Wyatt LS, Moss B, Morgan C, Roederer M, Bailer RT, Nabel GJ, Koup RA, Seder RA. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. Journal of immunology (Baltimore, Md : 1950) 2013;190:2720–2735. doi: 10.4049/jimmunol.1202861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AV. Viral vectors as vaccine platforms: deployment in sight. Current opinion in immunology. 2011;23:377–382. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Xing Z, Lichty BD. Use of recombinant virus-vectored tuberculosis vaccines for respiratory mucosal immunization. Tuberculosis. 2006;86:211–217. doi: 10.1016/j.tube.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, Hitt M, Xing Z. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. Journal of immunology (Baltimore, Md : 1950) 2004;173:6357–6365. doi: 10.4049/jimmunol.173.10.6357. [DOI] [PubMed] [Google Scholar]
- 33.Jeyanathan M, Heriazon A, Xing Z. Airway luminal T cells: a newcomer on the stage of TB vaccination strategies. Trends in immunology. 2010;31:247–252. doi: 10.1016/j.it.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Santosuosso M, McCormick S, Roediger E, Zhang X, Zganiacz A, Lichty BD, Xing Z. Mucosal luminal manipulation of T cell geography switches on protective efficacy by otherwise ineffective parenteral genetic immunization. Journal of immunology (Baltimore, Md : 1950) 2007;178:2387–2395. doi: 10.4049/jimmunol.178.4.2387. [DOI] [PubMed] [Google Scholar]
- 35.Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. Journal of immunology (Baltimore, Md : 1950) 2005;174:7986–7994. doi: 10.4049/jimmunol.174.12.7986. [DOI] [PubMed] [Google Scholar]
- 36.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. Journal of immunology (Baltimore, Md : 1950) 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santosuosso M, McCormick S, Zhang X, Zganiacz A, Xing Z. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infection and immunity. 2006;74:4634–4643. doi: 10.1128/IAI.00517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing Z, McFarland CT, Sallenave JM, Izzo A, Wang J, McMurray DN. Intranasal mucosal boosting with an adenovirus-vectored vaccine markedly enhances the protection of BCG-primed guinea pigs against pulmonary tuberculosis. PloS one. 2009;4:e5856. doi: 10.1371/journal.pone.0005856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White AD, Sibley L, Dennis MJ, Gooch K, Betts G, Edwards N, Reyes-Sandoval A, Carroll MW, Williams A, Marsh PD, McShane H, Sharpe SA. Evaluation of the safety and immunogenicity of a candidate tuberculosis vaccine, MVA85A, delivered by aerosol to the lungs of macaques. Clinical and vaccine immunology : CVI. 2013;20:663–672. doi: 10.1128/CVI.00690-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Havenga M, Vogels R, Zuijdgeest D, Radosevic K, Mueller S, Sieuwerts M, Weichold F, Damen I, Kaspers J, Lemckert A, van Meerendonk M, van der Vlugt R, Holterman L, Hone D, Skeiky Y, Mintardjo R, Gillissen G, Barouch D, Sadoff J, Goudsmit J. Novel replication-incompetent adenoviral B-group vectors: high vector stability and yield in PER.C6 cells. The Journal of general virology. 2006;87:2135–2143. doi: 10.1099/vir.0.81956-0. [DOI] [PubMed] [Google Scholar]
- 41.Kaushal D, Mehra S, Didier PJ, Lackner AA. The non-human primate model of tuberculosis. Journal of medical primatology. 2012;41:191–201. doi: 10.1111/j.1600-0684.2012.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMurray DN. A nonhuman primate model for preclinical testing of new tuberculosis vaccines. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;30(Suppl 3):S210–212. doi: 10.1086/313885. [DOI] [PubMed] [Google Scholar]
- 43.Radosevic K, Wieland CW, Rodriguez A, Weverling GJ, Mintardjo R, Gillissen G, Vogels R, Skeiky YA, Hone DM, Sadoff JC, van der Poll T, Havenga M, Goudsmit J. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infection and immunity. 2007;75:4105–4115. doi: 10.1128/IAI.00004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, Makhethe L, Erasmus M, de Kock M, van der Merwe L, Hawkridge A, Veldsman A, Hatherill M, Schirru G, Pau MG, Hendriks J, Weverling GJ, Goudsmit J, Sizemore D, McClain JB, Goetz M, Gearhart J, Mahomed H, Hussey GD, Sadoff JC, Hanekom WA. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. American journal of respiratory and critical care medicine. 2010;181:1407–1417. doi: 10.1164/rccm.200910-1484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoft DF, Blazevic A, Stanley J, Landry B, Sizemore D, Kpamegan E, Gearhart J, Scott A, Kik S, Pau MG, Goudsmit J, McClain JB, Sadoff J. A recombinant adenovirus expressing immunodominant TB antigens can significantly enhance BCG-induced human immunity. Vaccine. 2012;30:2098–2108. doi: 10.1016/j.vaccine.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 46.Magalhaes I, Sizemore DR, Ahmed RK, Mueller S, Wehlin L, Scanga C, Weichold F, Schirru G, Pau MG, Goudsmit J, Kuhlmann-Berenzon S, Spangberg M, Andersson J, Gaines H, Thorstensson R, Skeiky YA, Sadoff J, Maeurer M. rBCG induces strong antigen-specific T cell responses in rhesus macaques in a prime-boost setting with an adenovirus 35 tuberculosis vaccine vector. PloS one. 2008;3:e3790. doi: 10.1371/journal.pone.0003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luciw PA, Oslund KL, Yang XW, Adamson L, Ravindran R, Canfield DR, Tarara R, Hirst L, Christensen M, Lerche NW, Offenstein H, Lewinsohn D, Ventimiglia F, Brignolo L, Wisner ER, Hyde DM. Stereological analysis of bacterial load and lung lesions in nonhuman primates (rhesus macaques) experimentally infected with Mycobacterium tuberculosis. American journal of physiology Lung cellular and molecular physiology. 2011;301:L731–738. doi: 10.1152/ajplung.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Liu J, Carville A, Mansfield KG, Lynch D, Barouch DH. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. Journal of virology. 2011;85:11007–11015. doi: 10.1128/JVI.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. The Journal of experimental medicine. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 52.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. The Journal of experimental medicine. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho YS, Dobos KM, Prenni J, Yang H, Hess A, Rosenkrands I, Andersen P, Ryoo SW, Bai GH, Brennan MJ, Izzo A, Bielefeldt-Ohmann H, Belisle JT. Deciphering the proteome of the in vivo diagnostic reagent “purified protein derivative” from Mycobacterium tuberculosis. Proteomics. 2012;12:979–991. doi: 10.1002/pmic.201100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aagaard C, Hoang TT, Izzo A, Billeskov R, Troudt J, Arnett K, Keyser A, Elvang T, Andersen P, Dietrich J. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PloS one. 2009;4:e5930. doi: 10.1371/journal.pone.0005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature medicine. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 56.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature reviews Immunology. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 57.Teigler JE, Iampietro MJ, Barouch DH. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. Journal of virology. 2012;86:9590–9598. doi: 10.1128/JVI.00740-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson MJ, Petrovas C, Yamamoto T, Lindsay RW, Lore K, Gall JG, Gostick E, Lefebvre F, Cameron MJ, Price DA, Haddad E, Sekaly RP, Seder RA, Koup RA. Type I IFN induced by adenovirus serotypes 28 and 35 has multiple effects on T cell immunogenicity. Journal of immunology (Baltimore, Md : 1950) 2012;188:6109–6118. doi: 10.4049/jimmunol.1103717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lore K, Adams WC, Havenga MJ, Precopio ML, Holterman L, Goudsmit J, Koup RA. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. Journal of immunology (Baltimore, Md : 1950) 2007;179:1721–1729. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. The Journal of experimental medicine. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ravn P, Boesen H, Pedersen BK, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guerin. Journal of immunology (Baltimore, Md : 1950) 1997;158:1949–1955. [PubMed] [Google Scholar]
- 62.Jiang G, Shi M, Conteh S, Richie N, Banania G, Geneshan H, Valencia A, Singh P, Aguiar J, Limbach K, Kamrud KI, Rayner J, Smith J, Bruder JT, King CR, Tsuboi T, Takeo S, Endo Y, Doolan DL, Richie TL, Weiss WR. Sterile protection against Plasmodium knowlesi in rhesus monkeys from a malaria vaccine: comparison of heterologous prime boost strategies. PloS one. 2009;4:e6559. doi: 10.1371/journal.pone.0006559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ledgerwood JE, Wei CJ, Hu Z, Gordon IJ, Enama ME, Hendel CS, McTamney PM, Pearce MB, Yassine HM, Boyington JC, Bailer R, Tumpey TM, Koup RA, Mascola JR, Nabel GJ, Graham BS, Team VRCS. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. The Lancet infectious diseases. 2011;11:916–924. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ronan EO, Lee LN, Tchilian EZ, Beverley PC. Nasal associated lymphoid tissue (NALT) contributes little to protection against aerosol challenge with Mycobacterium tuberculosis after immunisation with a recombinant adenoviral vaccine. Vaccine. 2010;28:5179–5184. doi: 10.1016/j.vaccine.2010.05.075. [DOI] [PubMed] [Google Scholar]
- 65.Verreck FA, Vervenne RA, Kondova I, van Kralingen KW, Remarque EJ, Braskamp G, van der Werff NM, Kersbergen A, Ottenhoff TH, Heidt PJ, Gilbert SC, Gicquel B, Hill AV, Martin C, McShane H, Thomas AW. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PloS one. 2009;4:e5264. doi: 10.1371/journal.pone.0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tchilian EZ, Ronan EO, de Lara C, Lee LN, Franken KL, Vordermeier MH, Ottenhoff TH, Beverley PC. Simultaneous immunization against tuberculosis. PloS one. 2011;6:e27477. doi: 10.1371/journal.pone.0027477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smaill F, Jeyanathan M, Smieja M, Medina MF, Thanthrige-Don N, Zganiacz A, Yin C, Heriazon A, Damjanovic D, Puri L, Hamid J, Xie F, Foley R, Bramson J, Gauldie J, Xing Z. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Science translational medicine. 2013;5:205ra134. doi: 10.1126/scitranslmed.3006843. [DOI] [PubMed] [Google Scholar]
- 68.Harth G, Lee BY, Wang J, Clemens DL, Horwitz MA. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infection and immunity. 1996;64:3038–3047. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiological reviews. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skjot RL, Brock I, Arend SM, Munk ME, Theisen M, Ottenhoff TH, Andersen P. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infection and immunity. 2002;70:5446–5453. doi: 10.1128/IAI.70.10.5446-5453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert review of vaccines. 2011;10:645–658. doi: 10.1586/erv.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AV. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nature medicine. 2004;10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 73.Meyer J, Harris SA, Satti I, Poulton ID, Poyntz HC, Tanner R, Rowland R, Griffiths KL, Fletcher HA, McShane H. Comparing the safety and immunogenicity of a candidate TB vaccine MVA85A administered by intramuscular and intradermal delivery. Vaccine. 2013;31:1026–1033. doi: 10.1016/j.vaccine.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Isaacs F, Keyser A, Moyo S, Brittain N, Lawrie A, Gelderbloem S, Veldsman A, Hatherill M, Hawkridge A, Hill AV, Hussey GD, Mahomed H, McShane H, Hanekom WA. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. European journal of immunology. 2010;40:279–290. doi: 10.1002/eji.200939754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Mauff K, Hughes EJ, Moyo S, Brittain N, Lawrie A, Mulenga H, de Kock M, Gelderbloem S, Veldsman A, Hatherill M, Geldenhuys H, Hill AV, Hussey GD, Mahomed H, Hanekom WA, McShane H. Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. The Journal of infectious diseases. 2011;203:1832–1843. doi: 10.1093/infdis/jir195. [DOI] [PubMed] [Google Scholar]
- 76.Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nature medicine. 2011;17:189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 77.Agger EM, Rosenkrands I, Olsen AW, Hatch G, Williams A, Kritsch C, Lingnau K, von Gabain A, Andersen CS, Korsholm KS, Andersen P. Protective immunity to tuberculosis with Ag85B-ESAT-6 in a synthetic cationic adjuvant system IC31. Vaccine. 2006;24:5452–5460. doi: 10.1016/j.vaccine.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 78.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infection and immunity. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elvang T, Christensen JP, Billeskov R, Thi Kim Thanh Hoang T, Holst P, Thomsen AR, Andersen P, Dietrich J. CD4 and CD8 T cell responses to the M. tuberculosis Ag85B-TB10.4 promoted by adjuvanted subunit, adenovector or heterologous prime boost vaccination. PloS one. 2009;4:e5139. doi: 10.1371/journal.pone.0005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infection and immunity. 2004;72:6148–6150. doi: 10.1128/IAI.72.10.6148-6150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Dissel JT, Arend SM, Prins C, Bang P, Tingskov PN, Lingnau K, Nouta J, Klein MR, Rosenkrands I, Ottenhoff TH, Kromann I, Doherty TM, Andersen P. Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naive human volunteers. Vaccine. 2010;28:3571–3581. doi: 10.1016/j.vaccine.2010.02.094. [DOI] [PubMed] [Google Scholar]
- 82.Hoft DF, Blazevic A, Abate G, Hanekom WA, Kaplan G, Soler JH, Weichold F, Geiter L, Sadoff JC, Horwitz MA. A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. The Journal of infectious diseases. 2008;198:1491–1501. doi: 10.1086/592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13853–13858. doi: 10.1073/pnas.250480397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun R, Skeiky YA, Izzo A, Dheenadhayalan V, Imam Z, Penn E, Stagliano K, Haddock S, Mueller S, Fulkerson J, Scanga C, Grover A, Derrick SC, Morris S, Hone DM, Horwitz MA, Kaufmann SH, Sadoff JC. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine. 2009;27:4412–4423. doi: 10.1016/j.vaccine.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 85.Williams A, Goonetilleke NP, McShane H, Clark SO, Hatch G, Gilbert SC, Hill AV. Boosting with poxviruses enhances Mycobacterium bovis BCG efficacy against tuberculosis in guinea pigs. Infection and immunity. 2005;73:3814–3816. doi: 10.1128/IAI.73.6.3814-3816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, McIntyre A, Gooch K, Clark S, Beveridge NE, Nuth E, White A, Marriott A, Dowall S, Hill AV, Williams A, Marsh PD. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clinical and vaccine immunology : CVI. 2010;17:1170–1182. doi: 10.1128/CVI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, Team MATS. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS pathogens. 2011;7:e1002063. doi: 10.1371/journal.ppat.1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, Bigbee M, Milk L, Gideon HP, Rodgers M, Cochran C, Guinn KM, Sherman DR, Klein E, Janssen C, Flynn JL, Andersen P. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. The Journal of clinical investigation. 2012;122:303–314. doi: 10.1172/JCI46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi L, North R, Gennaro ML. Effect of growth state on transcription levels of genes encoding major secreted antigens of Mycobacterium tuberculosis in the mouse lung. Infection and immunity. 2004;72:2420–2424. doi: 10.1128/IAI.72.4.2420-2424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, Siani L, Naddeo M, Grazioli F, Esposito ML, Ambrosio M, Sparacino A, Bartiromo M, Meola A, Smith K, Kurioka A, O’Hara GA, Ewer KJ, Anagnostou N, Bliss C, Hill AV, Traboni C, Klenerman P, Cortese R, Nicosia A. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Science translational medicine. 2012;4:115ra112. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Langermans JA, Andersen P, van Soolingen D, Vervenne RA, Frost PA, van der Laan T, van Pinxteren LA, van den Hombergh J, Kroon S, Peekel I, Florquin S, Thomas AW. Divergent effect of bacillus Calmette-Guerin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: implications for primate models in tuberculosis vaccine research. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11497–11502. doi: 10.1073/pnas.201404898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharpe SA, Eschelbach E, Basaraba RJ, Gleeson F, Hall GA, McIntyre A, Williams A, Kraft SL, Clark S, Gooch K, Hatch G, Orme IM, Marsh PD, Dennis MJ. Determination of lesion volume by MRI and stereology in a macaque model of tuberculosis. Tuberculosis. 2009;89:405–416. doi: 10.1016/j.tube.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 94.Good RC. Biology of the mycobacterioses. Simian tuberculosis: immunologic aspects. Annals of the New York Academy of Sciences. 1968;154:200–213. doi: 10.1111/j.1749-6632.1968.tb16710.x. [DOI] [PubMed] [Google Scholar]
- 95.Rayner EL, Pearson GR, Hall GA, Basaraba RJ, Gleeson F, McIntyre A, Clark S, Williams A, Dennis MJ, Sharpe SA. Early lesions following aerosol infection of rhesus macaques (Macaca mulatta) with Mycobacterium tuberculosis strain H37RV. Journal of comparative pathology. 2013;149:475–485. doi: 10.1016/j.jcpa.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 96.Gormus BJ, Blanchard JL, Alvarez XH, Didier PJ. Evidence for a rhesus monkey model of asymptomatic tuberculosis. Journal of medical primatology. 2004;33:134–145. doi: 10.1111/j.1600-0684.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- 97.Capuano SV, 3rd, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, Bissel S, Fuhrman C, Klein E, Flynn JL. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infection and immunity. 2003;71:5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walsh GP, Tan EV, dela Cruz EC, Abalos RM, Villahermosa LG, Young LJ, Cellona RV, Nazareno JB, Horwitz MA. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nature medicine. 1996;2:430–436. doi: 10.1038/nm0496-430. [DOI] [PubMed] [Google Scholar]
- 99.Lin PL, Ford CB, Coleman MT, Myers AJ, Gawande R, Ioerger T, Sacchettini J, Fortune SM, Flynn JL. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nature medicine. 2014;20:75–79. doi: 10.1038/nm.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.