Abstract
Acquired von Willebrand Syndrome (AVWS) is a rare bleeding disorder associated with hematoproliferative disorders, autoimmune conditions, neoplasia, and cardiovascular disorders that often presents a diagnostic challenge. Monoclonal gammopathy of undetermined significance (MGUS) is one of the most common causes of AVWS that typically presents later in life with mucocutaneous or postsurgical bleeding and multimers consistent with type I or II von Willebrand Disease (VWD). Here we present the case of a patient with a 32 year history of type III VWD that was ultimately found to be AVWS related to an IgG MGUS. In this case report, we highlight the diagnostic challenges of AVWS to ensure proper identification and potentially lifesaving treatment of this rare disorder.
Keywords: Howard C, Lin TL, Cunningham M, Lipe B
Introduction
First reported in 1968, Acquired von Willebrand Syndrome (AVWS) is a rare disorder of hemostasis that mimics congenital VWD and can result in life-threatening bleeding complications [1]. VWD results from a hereditary defect and is often accompanied by a family history of bleeding problems. AVWS is more commonly seen in elderly patients without a prior history of bleeding and frequently occurs as a result of an underlying medical condition, such as monoclonal hematoproliferative disorders (most commonly an IgG monoclonal gammopathy), malignancy, autoimmune disorders or cardiac disorders [2].
Bleeding in both VWD and AVWS is a result of abnormalities of the von Willebrand Factor (VWF), a large multimeric glycoprotein that is essential to primary hemostasis through the regulation of platelet adhesion and aggregation at the site of vascular injury. VWF also increases the half-life of factor VIII in circulation through stabilization as a carrier protein [3]. Hereditary VWD is separated into three main types and caused by abnormalities of VWF quantity (type 1), quality (type 2), or almost a complete absence of VWF (type 3) [4-6].
In contrast, AVWS is characterized by the increased clearance of qualitatively normal VWF . This increased clearance results in a relative deficiency of both VWF and factor VIII [2] and may occur through several potential mechanisms: autoantibodies to VWF that either inhibit functional sites or increase clearance from circulation; nonspecific antibodies that form circulating immune complexes and favor VWF clearance by Fc-bearing cells; proteolytic degradation and adsorption onto malignant clone cells; or increased shear stress as in cardiac valvular disorder [7, 8]. The diagnosis of AVWS is often difficult given the heterogeneity in presentation of the disease and laboratory results and lack of a specific diagnostic test [9]. In this report, we highlight the diagnostic challenges and importance of proper diagnosis in a patient with AVWS who was misdiagnosed with type III von Willebrand disease for 30 years.
Case Report
An 82 year old male was diagnosed with presumed type III VWD at the age of 50 after prolonged bleeding with phlebotomy, an abnormal bleeding time and prolonged activated partial thrombin time (aPTT). He had no prior personal or family history of bleeding problems. Over the next 30 years, he underwent several orthopedic procedures including knee and hip replacements for which he was treated preoperatively with factor VIII/ von Willebrand factor concentrate on at least two separate occasions. Post-operatively, he was noted to have had increased bleeding and minimal correction of factor VIII levels despite the administration of factor VIII/von Willebrand factor concentrate.
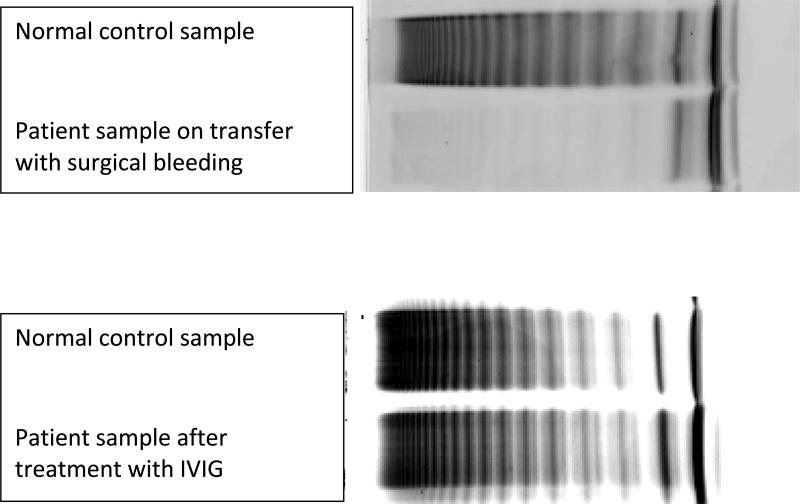
In 2013 he presented with a spontaneous lower extremity intramuscular hematoma and underwent hematoma evacuation and fasciotomy. He was treated at home with a 100% correction dose of Factor VIII/ VWF concentrate at 48 units/kg twice daily, but presented to the hospital when he continued to bleed from the surgical site requiring at least five units of blood. Initial laboratory evaluation revealed a prolonged aPTT of 146.2 seconds (normal 25-37 seconds) with normal prothrombin time and fibrinogen, low factor VIII activity (21%), low von Willebrand Factor Antigen (vWF: Ag) (26%), and low von Willebrand Factor activity (vWF:RCof) (<13%). Von Willebrand multimer analysis confirmed type III VWD with barely detectable multimers (Figure 1). After attempted replacement to 100% activity with VWF containing concentrate, peak activity and factor levels remained low: factor VIII activity 22-33%, vWF:Ag 22-50%, and vWF:RCof undetectable (Table 1). The lack of response to infused VWF concentrates was concerning for a VWF inhibitor. A von Willebrand factor propeptide antigen later returned at 151 IU/DL (normal 62-183), consistent with increased destruction of VWF as seen in AVWS.
Figure 1.
Patient's VWF multimer gel. On transfer, he had barely detectable multimers that corrected with IVIG admnisitration.
Table 1.
von Willebrand Profile before and after treatment with IVIG
| aPTT | FVIII (%) | VWF:Ag (%) | VWF:RCoF(%) | |
|---|---|---|---|---|
| During hip replacement in 2004 | 146.2 | 17 | 26 | <13 |
| On presentation after surgical bleed in 2013 | 48.1 | 21 | 26 | <13 |
| Post VWF/FVIII concentrates | 42.4 | 23 | 10 | <12 |
| Post-IVIG | 34.1 | 100 | 151 | 117 |
| 3 weeks post IVIG | 48.2 | 21 | 28 | 22 |
Given the concern for an inhibitor, a serum protein electrophoresis was checked and demonstrated the presence of an IgG paraprotein of 0.29 g/dL. Evaluation with a bone marrow biopsy and skeletal survey confirmed a diagnosis of monoclonal gammopathy of undetermined significance (MGUS). Given the IgG MGUS and clinically significant VWF inhibiting autoantibodies, IV immunoglobulin (IVIG) was administered at a dose of 1 gm/kg IV daily for two consecutive days. Within 48 hours of IVIG administration, complete correction of his coagulation profile was noted along with cessation of bleeding. Repeat multimer testing showed no evidence of VWD. A 1:1 mixing study of the ristocetin cofactor assay revealed no inhibitor, but the patient was felt clinically to have AVWS given the response to IVIG. The patient's AVWS recurred within three weeks of IVIG therapy and was again treated with IVIG with good response. His AVWS recurred 3 weeks after the second IVIG dosing and has not been retreated with IVIG as his bleeding has resolved.
Discussion
Abnormal bleeding symptoms in an elderly patient without a personal or family history of bleeding should prompt an investigation for AVWS, especially in the setting of laboratory abnormalities suggestive of VWD. Laboratory evaluation in AVWS will usually be consistent with a diagnosis of VWD and will show a prolonged activated partial thrombin time and PFA-100 (platelet function analyzer) with normal prothrombin time and platelet count [8]. Specific evaluation of von Willebrand factor will usually reveal a decrease in the von Willebrand antigen, von Willebrand activity, and Factor VIII activity, though increases can be seen. There is commonly a reduction in the antigen to activity level and the collagen binding to antigen ratio [8]. Multimer analysis usually reveals a loss of high molecular weight multimers, consistent with type I or type II VWD [2, 8]. To specifically diagnose AVWS, mixing studies can demonstrate the presence of an autoantibody in 15% of patients [8]. In IgG MGUS, an autoantibody may not be detected because the antibody is directed against non-functional epitopes or the inhibitor is saturated in complexes with VWF, preventing detection. Other potential inhibitor testing includes: ristocetin-induced agglutination co-factor, enzyme-linked immunosorbent assay, the demonstration of a neutralizing anti-vWF inhibitor with collagen-binding activity, and the analysis of the von Willebrand factor propeptide, but these tests are not standardized and can lead to false positive or negative results [1, 2, 9].
As laboratory evaluation alone is often insufficient to distinguish VWD from AVWS, clinical suspicion and recognition of risk factors is critical. A careful patient history and examination should be undertaken to look for evidence of AVWS-associated conditions. A routine complete blood count and serum protein electrophoresis can help to exclude an underlying hematologic condition or monoclonal gammopathy. Ultimately, a diagnosis should be made when a patient fails to respond as expected to treatment for VWD with von Willebrand concentrate, as in the case of our patient [10].
Treatment for patients with AVWS should be aimed at the underlying causative disease and remains difficult as there are no specific treatment guidelines. In some circumstances, treating the underlying disease is not possible or not practical as in the case of critical bleeding. In these situations, the underlying cause of the AVWS is essential to determine the most appropriate treatment to restore hemostasis. As in our case, patients with IgG MGUS often respond to treatment with IVIG (1g/kg daily for 2 days) with a correction of laboratory abnormalities and decreased bleeding complications [7, 11]. The 2g/kg of IVIG should be given over 2 days instead of being divided into smaller doses over a longer period of time to achieve maximum effects, but may need to be repeated as the average duration of effect from IVIG is three weeks [7]. The proposed mechanism of action of IVIG includes elimination of circulating immune complexes by monomeric Ig or the blockage of Fc-receptors on the reticuloendothelial system [7]. Systemic steroids have shown efficacy in AVWS associated with immunologic diseases but not with IgG MGUS because the monoclonal IgG protein continues to act as an anti-VWF autoantibody [1]. For IgM monoclonal gammopathies, IVIG has not been shown effective and plasmapharesis is recommended [7]. Other treatment modalities, although with less literature support, include Recombinant factor VIIa and antifibrinolytics (usually as adjuvant therapy with desmopressin or von Willebrand containing concentrates) [1].
Conclusion
While there is heterogeneity to the clinical presentation and laboratory evaluation between congenital VWD and AVWS, the latter should be considered when the onset of bleeding occurs later in life and without previous personal or family history of bleeding or when a patient has a known AVWS-associated disorder. Lack of standardization of laboratory methods for antibody identification and characterization makes diagnosis challenging, highlighting the need for clinical suspicion and careful history and physical examination. The goals of treatment should be aimed at resolving the underlying associated disorder, controlling acute bleeding, prevention of bleeding in high-risk situations and obtaining long-term remission.
Acknowledgements
We would like to acknowledge Dr. Allan Fleming at the University of Kansas Medical Center and Mayo Clinical laboratories for their assistance in the laboratory testing for diagnosis and treatment of this case.
Footnotes
Conflict of interest: All authors report no conflicts of interest.
Funding source: none
References
- 1.Tiede A, et al. How I treat the acquired von Willebrand syndrome. Blood. 2011;117(25):6777–6785. doi: 10.1182/blood-2010-11-297580. [DOI] [PubMed] [Google Scholar]
- 2.Michiels JJ, et al. Acquired von Willebrand syndromes: clinical features, aetiology, pathophysiology, classification and management. Best Practice & Research Clinical Haematology. 2001;14(2):401–436. doi: 10.1053/beha.2001.0141. [DOI] [PubMed] [Google Scholar]
- 3.De Meyer SF, Deckmyn H, Vanhoorelbeke K. von Willebrand factor to the rescue. Blood. 2009;113(21):5049–5057. doi: 10.1182/blood-2008-10-165621. [DOI] [PubMed] [Google Scholar]
- 4.James AH. Von Willebrand disease. Obstetrical & gynecological survey. 2006;61(2):136–145. doi: 10.1097/01.ogx.0000197818.94002.91. [DOI] [PubMed] [Google Scholar]
- 5.Sadler JE, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. Journal of Thrombosis and Haemostasis. 2006;4(10):2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 6.James AH, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. American Journal of Obstetrics & Gynecology. 2009;201(1):12.e1–12.e8. doi: 10.1016/j.ajog.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Federici AB, et al. Treatment of Acquired von Willebrand Syndrome in Patients With Monoclonal Gammopathy of Uncertain Significance: Comparison of Three Different Therapeutic Approaches. Blood. 1998;92(8):2707–2711. [PubMed] [Google Scholar]
- 8.Veyradier A, et al. Acquired von Willebrand syndrome: from pathophysiology to management. Thromb Haemost. 2000;84(2):175–82. [PubMed] [Google Scholar]
- 9.Tiede A, et al. Diagnostic workup of patients with acquired von Willebrand syndrome: a retrospective single-centre cohort study. Journal of Thrombosis and Haemostasis. 2008;6(4):569–576. doi: 10.1111/j.1538-7836.2008.02909.x. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Pruthi RK, Nichols WL. Acquired von Willebrand Disease. Mayo Clinic proceedings. 2002;77(2):181–187. doi: 10.4065/77.2.181. [DOI] [PubMed] [Google Scholar]
- 11.Federici A. Diagnosis of Inherited von Willebrand Disease: A Clinical Perspective. Semin Thromb Hemost. 2006;32(6):555–565. doi: 10.1055/s-2006-949661. [DOI] [PubMed] [Google Scholar]