Abstract
Lipids from mycobacteria can be presented to human T cells by group 1 CD1 antigen-presenting molecules (CD1a, CD1b, and CD1c). Group 1 CD1-restricted T cells are activated by lipid antigens presented by myeloid dendritic cells, after which they generate anti-bacterial effector functions including IFNγ secretion and cytolysis. Thus, mycobacterial lipids are being investigated as components of novel vaccines for mycobacterial infections. Here we show that the mycobacterial lipid antigen C80 glucose-6-monomycolate (C80 GMM) can be delivered to human CD1b+ dendritic cells (DCs) via targeted liposomal nanoparticles, leading to robust group 1 CD1-restricted activation of T cells. Targeting was achieved by decorating the liposomes with a high affinity glycan ligand of Siglec-7, a siglec receptor expressed on DCs that mediates rapid endocytosis and transport of its cargo to lysosomes. An antibody to Siglec-7 completely blocked the binding of targeted liposomes to human monocyte-derived DCs (Mo-DCs), demonstrating their targeting specificity. Mo-DCs pulsed with targeted liposomes containing C80 GMM more potently activated CD1b-restricted T cell line relative to Mo-DCs pulsed with free lipid antigen or antigenic liposomes without Siglec-7 ligand. These data suggest that the endocytic function of Siglec-7 can be exploited to deliver glycolipids antigens to their target cell and increase the efficiency of display to T cells.
Keywords: CD1-restricted T cells, Antigen delivery, Siglecs, dendritic cell targeting
Introduction
T cells recognize mycobacterial lipids bound to group 1 CD1 antigen presenting molecules (CD1a, CD1b, and CD1c) (1–3). These studies represent an expansion of the known functions of human αβ T cells, which are now understood to recognize both peptide and lipid antigens. Published studies show that upon antigen recognition, group 1 CD1-restricted T cells produce IFNγ and TNF, which are key anti-mycobacterial effectors in human disease (4–7). Also, group 1 CD1-reactive T cells kill the Mycobacterium tuberculosis infected cells ex vivo (8, 9). Several studies show that group 1 CD1-restricted T cells expand and persist within individuals with tuberculosis (4, 5, 10), as well as animals vaccinated with the antigenic lipids (11, 12). These studies, along with the lack of common polymorphism of CD1 proteins in human populations, now provide the basis for considering lipid antigens as vaccines or immunodulatory agents that may provide protection from mycobacterial infections.
Glucose-6-monomycolates (GMMs), which have acyl chains attached to a glucose head group, are abundant lipid components present in the cell wall of all mycobacterial species studied to date (13). They bind to CD1b by their acyl chains, and although the acyl chains of GMMs vary by mycobacterial species, they are all completely buried in the lipophilic groove of CD1b (14). As a result, the glucose head group is exposed as a common antigenic epitope (14). Accordingly T cells which recognize GMM from one source as their matched antigen also react to GMM from other sources (9). Further, animal studies suggest that GMM is an immunodominant antigen during natural infection (15, 16), and recent studies with CD1b tetramers prove that polyclonal populations of GMM-reactive T cells exist in human tuberculosis patients (4, 7). Of note, conserved ‘germline-encoded, mycolyl lipid-reactive’ (GEM) T cells have been identified as high-affinity responders to GMM in humans (7). While GMM-specific T cells including GEM T cells are found at a low frequency in healthy individuals (0.002%), their expansion is commonly observed in active and latent tuberculosis infection, accounting for 0.01% of T cells (4, 7, 17). In addition, a second type of polyclonal GMM-reactive T cell type is known as LDN5-like T cells. LDN5 like T cells are so named because they express TCRs and cytokine patterns that are similar to those associated with a T cell clone named LDN5 (18). GEM T cells are defined by high affinity TRAV1-2+ TCRs, whereas TRBV4-1+ LDN5-like T cells have intermediate affinity for CD1b and GMM (7, 18). Following M. bovis Bacillus Calmette-Guerin (BCG)-vaccination GMM-reactive T cells produce IFNγ and TNF in a CD1b-restricted manner (6). Therefore, vaccination activating GMM-reactive T cells is now being studied as a new method to alter immunity to M. tuberculosis in vivo.
Current tuberculosis vaccine trials focus on mycobacterial protein subunit vaccines, and antigenic lipids including GMM are thought to comprise a next generation of vaccine candidates (19). To enhance the presentation of GMM, targeted delivery to the CD1b+ antigen presenting cells (APCs) is desired, since the cellular expression of CD1b is restricted to certain cell types. In the periphery, CD1b is exclusively expressed on dendritic cells (DCs) in healthy individuals (20) as well as patients with Mycobacterium leprae infection (21). Thus, as is also the case for MHC I and II, myeloid DCs are thought to be the main functionally important APC in the periphery (22). For DC-targeted antigen delivery, antibodies toward the cell surface receptors have been investigated for delivery of protein antigens conjugated to the antibody, some of which have been in human clinical trials for tumor and HIV vaccines (23, 24). However, more suitable delivery platforms for hydrophobic lipid antigens are yet to be developed and tested.
Previously we have developed a targeting platform based on liposomal nano-particles bearing glycan ligands of sialic acid-binding immunoglobulin-like lectins (siglecs) capable of in vivo delivery of both hydrophilic and hydrophobic agents to siglec-expressing immune cells (25–28). Siglecs are a cell surface lectin family that recognize sialic acids as ligands and are expressed on human leukocytes in a cell-type restricted manner (29–31). Among human siglecs, Siglec-7 is expressed on DCs as well as on other human leukocytes including natural killer (NK) cells, neutrophils, monocytes, and macrophages (31–33). Based on the restricted expression of Siglec-7, it has been proposed as an attractive target for cell-targeted therapies directed to myeloid cells (30, 34). We have recently developed a glycan ligand of high affinity and selectivity for Siglec-7 suitable for use for targeting cells expressing this siglec (35).
In this report, we investigated the potential for efficient delivery of GMM to CD1b+ human monocyte-derived DCs (Mo-DCs) using antigenic liposomes bearing ligands of Siglec-7. We found that targeted liposomes were captured by Mo-DCs and delivered to lysosomes in a Siglec-7 dependent manner. Mo-DCs pulsed with targeted liposomes containing C80 GMM, a GMM with long acyl chains (4, 14), potently activated the CD1b-restricted human T cell line LDN5, shown by IFNγ production. Thus, we conclude that the Siglec-7 endocytic pathway can be exploited for targeted delivery of mycobacterial lipid antigens to human DCs.
Materials and Methods
Cells
The CD1b-reactive human T cell line LDN5 cells were originally derived from a human skin lesion of a leprosy patient and maintained as described (9), and this T cell clone expresses a TRBV4-1+ TCR that is representative of a natural T cell population known as LDN5-like T cells (18). Human Mo-DCs were generated from the culture of peripheral blood monocytes in vitro. Briefly, monocytes were enriched with the CD14 magnetic beads (BD Biosciences) from buffy coats from peripheral blood obtained from healthy human donors according to Institutional Review Board at The Scripps Research Institute. After informed consent, 50 mL of blood were collected from asymptomatic tuberculin positive subjects with no clinical or radiographic evidence of active tuberculosis as approved by the institutional review boards of the Lemuel Shattuck Hospital and Partners Healthcare, and used for preliminary antigen presentation assay (data not shown). Isolated monocytes were cultured with the RPMI medium (Invitrogen) supplemented with 10% heat-inactivated FCS, 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM non-essential amino acids, 1 mM sodium pyruvate, and 50 μM 2-mercaptoethanol, and 20 ng/mL of human IL-4 and GM-CSF (Peprotech). At day 6, the floating cells were harvested and used as Mo-DCs.
Reagents
Antibodies used for flow cytometry and immunofluorescent microscopy included those against human CD1b (SN13, Biolegend), Siglec-7 (QA79, eBioscience), CD14 (HCD14, Biolegend), CD56 (HCD56, Biolegend), CD34 (HM34, Biolegend), CD3 (UCHT1, BD Biosciences), CD19 (HIB19, Biolegend), HLA-DR (L243, Biolegend), CD11c (3.9, Biolegend), CD123 (6H6, Biolegend), EEA-1 (ab2900, Abcam) and LAMP-1 (ab24170, Abcam). For the blocking of LDN5 cell activation and liposome binding, anti-CD1b (BCD1b.3) (36) and anti-Siglec-7 (S7.7, Biolegend) were used, respectively. C80 GMM was isolated as described (36). Ficoll Plaque plus (GE Healthcare) was used for the density gradient centrifugation to separate peripheral blood mononuclear cells. Human IFNγ ELISA kit (Biolegend) was used to measure human IFNγ.
Liposomes
The liposomes were prepared as described (25). Briefly, 4 mol% Siglec-7 targeted liposomes were composed of distearoyl phosphatidylcholine (DSPC, Avanti Polar Lipids): cholesterol (Chol, Sigma-Aldrich): polyethyleneglycol-distearoyl phosphoethanolamine (PEG-DSPE, NOF): Siglec-7 ligand-lipid (35) in a 58: 37: 1: 4 molar ratio. Naked liposomes were composed of 5mol% PEG-DSPE instead of the Siglec-7 ligand-lipid. Antigenic liposomes substituted 1 mol% DSPC for 1 mol% C80 GMM. Fluorescent liposomes contained 0.2 mol% of Alexa fluor 647-PEG-DSPE (28). For liposome preparation, lipid components in dimethyl sulfoxide (Siglec-7 ligand-lipid, C80 GMM, and Alexa 647-PEG-DSPE) were mixed and lyophilized in a glass tube. The other components in chloroform were then added to the tube and dried completely by air flow. The dried lipids were hydrated with 1 mL of PBS, sonicated, then extruded by an liposome extruder (Avanti Polar Lipids) until the size became around 100 nm measured by Zetasizer (Malvern). The GMM concentration in the antigenic liposomes was 10 μM.
Flow cytometry
Cells were washed with HBSS containing 0.1% BSA, 2 mM EDTA, and 0.1% NaN3 (FACS buffer), blocked with anti-human CD32 (3D3, BD Biosciences) for 5 min at 25°C, and stained with antibodies for 30 min at 4°C. Stained cells were washed once with FACS buffer and analyzed by FACS Calibur or LSR II (BD Biosciences). Propidium iodide (1 μg/mL) was added to the sample before the analysis for exclusion of the dead cells. Acquired data were analyzed with Flowjo (Tree Star). For the liposome binding analysis, cells were incubated with the liposomes for 30 min at 37°C, then washed and analyzed as above. To assess the Siglec-7 dependence in the liposome binding, cells were first incubated with 10 μg/mL of anti-Siglec-7 or mouse IgG1 isotype control antibody (Biolegend) for 30 min at 4°C followed by the addition of the liposomes.
LDN5 cell activation
Mo-DCs were pulsed with indicated reagents for 20 min at 37°C in the sterile FACS buffer and washed. To assess the antigen presentation activity of the cells, 5 × 104 of Mo-DCs was cultured with 5 × 104 of LDN5 cells. For the comparison of liposomal and free C80 GMM, same number of LDN5 cells was cultured with 2.5 × 104 of Mo-DCs. After 20 h, the culture supernatants were collected and human IFNγ was measured by ELISA. To assess the targeting specificity of the liposomes, Mo-DCs were first incubated with 10 μg/mL of anti-Siglec-7 or isotype control antibody for 30 min at 4°C prior to the addition of the liposomes. To determine the CD1b dependence in the LDN5 activation, 10 μg/mL of anti-CD1b antibody was added to the culture. For some experiments, Mo-DCs mixed with peripheral blood mononuclear cells (PBMCs) were pulsed as described above. The Mo-DCs were isolated by CD209 magnetic beads (Miltenyi Biotech) and used for the antigen presentation assay.
Microscopy
Mo-DCs on the cover slide were incubated with 100 μM of Alexa 647-labeled Siglec-7 targeted or naked liposomes in the culture medium for 90 min at 37°C. The cells were washed with PBS, fixed with 4% PFA in PBS for 5 min at 25°C, and permeabilized with PBS containing 0.1% Saponin and 0.1% BSA for 5 min at 25°C. The cells were then blocked with the culture medium for 1 h at 25°C and stained with 1 μg/mL of anti-LAMP1 and EEA-1 in the culture medium for 18 h at 4°C. The cells were washed with PBS and stained with 1 μg/mL of anti-rabbit IgG-Alexa 555 (Lifetechnologies) in the culture medium containing 1 μg/mL of DAPI for 30 min at 25°C. The cells were washed with PBS and mounted with anti-fade mounting solution (Lifetechnologies). Images were obtained on a Bio-Rad (Zeiss) Radiance 2100 Rainbow laser scanning confocal microscope (LSCM). The acquired images were analyzed using the WCIF ImageJ processing program (Wright Cell Imaging Facility at University Health Network, Toronto, Canada). The degree of co-localization between Siglec-7 targeted liposome and LAMP-1 or EEA-1, respectively, was evaluated using the intensity correlation analysis plug-in of the co-localization module of the program (37). Analysis was conducted on three individual cells, each showing good expression of both antigens from different images. The calculated Pearson’s correlation coefficients (Rr) obtained from each cell were averaged and expressed as mean ± standard deviation.
Statistical analysis
Student’s t test was used for statistical analysis on Prism software (GraphPad). P < 0.05 was considered as statistically significant.
Results
Siglec-7 targeted liposomes bind specifically to Siglec-7 on human Mo-DCs
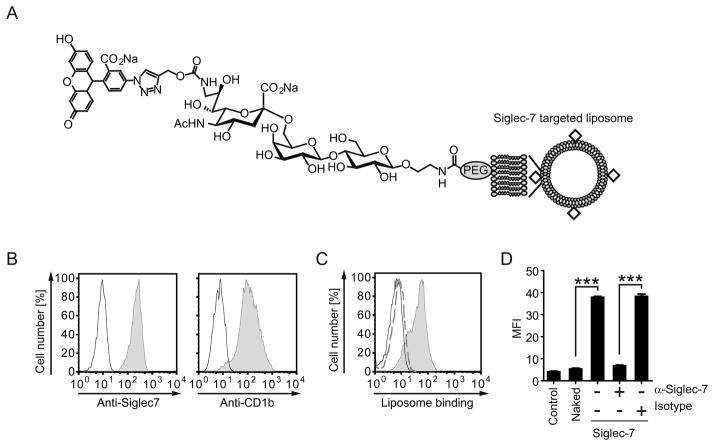
Recent screening of a sialic acid analog glycan library identified a high affinity glycan ligand for Siglec-7, and liposomes bearing this ligand specifically bound to Siglec-7 bearing cells, but not cells bearing any other human siglec (Fig. 1A) (35). To test if these Siglec-7 targeted liposomes bound to human DCs via Siglec-7, we assessed the binding of Alexa 647-labeled liposomes to human Mo-DCs, which express Siglec-7 as well as the antigen presenting molecule CD1b (Fig. 1B). As shown in Fig 1C, the Alexa 647-labeled Siglec-7 targeted liposomes bound to Mo-DCs, whereas ‘naked’ untargeted liposomes did not. This binding was completely Siglec-7 dependent since anti-Siglec-7 Ab abolished the binding of the liposomes (Fig. 1D). Although other siglecs including Siglec-1, 3, 5, and 9 are known to be expressed on Mo-DCs (Supplemental Fig. 1) (33), these data demonstrate that Siglec-7 targeted liposomes specifically target Siglec-7 expressed on Mo-DCs.
Figure 1. A glycan ligand decorated liposome targets to Siglec-7 on human DCs.
(A) Structure of the Siglec-7 targeted liposomes. (B) The expression of Siglec-7 and CD1b on human Mo-DCs is shown. Human Mo-DCs were generated by in vitro culture of peripheral blood monocytes. The cells were stained with anti-Siglec-7 and CD1b (gray) or isotype control Abs (black), then washed and analyzed by flow cytometry. (C) Alexa 647-labeled Siglec-7 targeted liposomes bind to Mo-DCs. Mo-DCs were incubated with 10 μM of targeted liposomes (gray, filled), naked liposomes (broken line), or buffer only (control, solid line). Cells were washed and analyzed by flow cytometry. (D) The binding of Siglec-7 targeted liposomes to the Mo-DCs is Siglec-7 dependent. Mo-DCs were first incubated with 10 μg/mL of indicated Abs or buffer only for 30 min at 4°C, and then incubated with the liposomes (10 μM) or buffer only (control). The cells were washed and analyzed by flow cytometry. The binding of liposomes is shown as mean fluorescence intensity (MFI). Error bars indicate standard deviation (SD). Statistical analyses were performed by Student’s t-test. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Data show the results of one out of two experiments with similar results.
Siglec-7 targeted liposomes are delivered to lysosomes in Mo-DCs
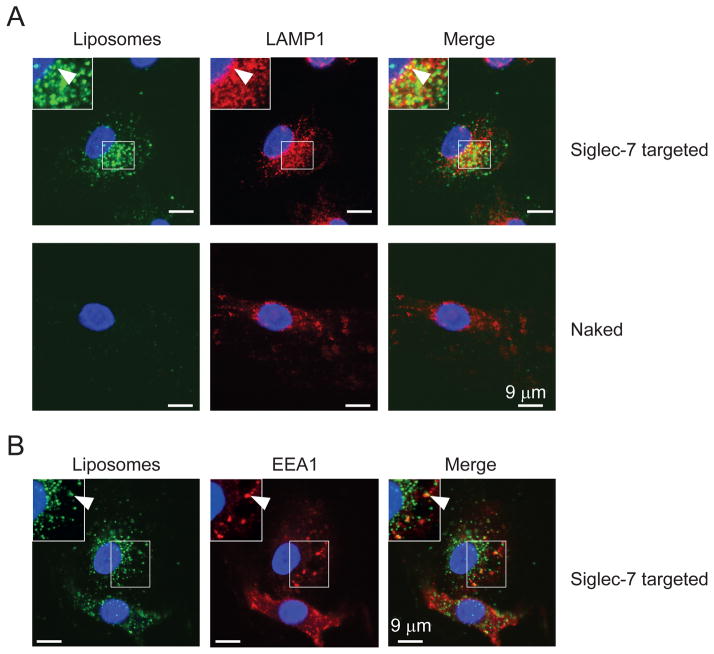
Next, we asked if the Siglec-7 targeted liposomes were internalized by Mo-DCs upon binding to Siglec-7. We incubated Mo-DCs with Alexa 647-labeled liposomes for 90 min at 37°C. As shown in Fig. 2A, Siglec-7 targeted liposomes showed a punctate staining pattern, typical for the staining of intracellular small vesicles, and were partially co-localized with the lysosomal marker LAMP-1 (Pearson’s correlation coefficient Rr = 0.35 ± 0.09, Fig. 2A). In contrast, little or no binding of naked liposomes was detected (Rr = 0.006 ± 0.02, Fig. 2A). We also observed a co-localization of Siglec-7 targeted liposomes with the early endosomal marker EEA-1 to a similar extent (Rr = 0.32 ± 0.04, Fig. 2B). These data confirm that Siglec-7 is an endocytic receptor (34), and is capable of delivering ligand-decorated liposomes to lysosomes via an endocytic pathway involving early endosomes.
Figure 2. Siglec-7 targeted liposomes are delivered to lysosomes in Mo-DCs.
Mo-DCs adhered on a cover slip were incubated with 100 μM of Alexa 647-labeled Siglec-7 targeted or naked liposomes for 90 min at 37°C. Cells were then washed, fixed, and permeabilized. The permeabilized cells were stained with anti-LAMP1 (A) and EEA-1 (B) to visualize lysosomes and early endosomes respectively. Data show representative results of two experiments.
Siglec-7 endocytic pathway leads to lipid antigen presentation to CD1b-restricted T cells
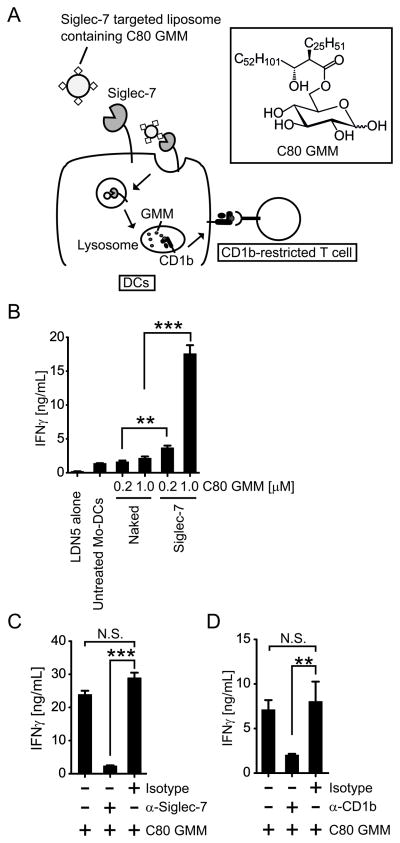
Since the mycobacterial lipid antigen C80 GMM has been shown to be loaded onto CD1b in acidic lysosomal compartments (38), we hypothesized that targeted delivery of antigenic liposomes to lysosomes of DCs would lead to presentation of C80 GMM to CD1b-restricted T cells (Fig. 3A). To test this, we incorporated C80 GMM into the Siglec-7 targeted liposomes or naked liposomes. Mo-DCs were then pulsed with liposomes containing C80 GMM, washed and cultured with the human CD1b-restricted T cell clone LDN5 to assess the antigen presenting activity. The LDN5 reporter cell line was used because it has a type of TCR expressed by a recently discovered population of polyclonal T cells named after this cell line, known as LDN5-like T cells (18). Antigen presentation assays using primary polyclonal CD1b-restricted T cells can be confounded by concurrent allogeneic responses to MHC unmatched DCs, whereas LDN5 clone sensitively detects CD1b-GMM complexes on MHC unmatched primary Mo-DCs used in these assays (4, 7). Also, in attempts to conduct antigen presentation using polyclonal peripheral blood T cells obtained from tuberculosis patients, high background responses of non-antigen specific T cells in the presence of medium alone obscured the GMM-specific response (data not shown). However, the LDN5 T cell clone, which was originally derived from a leprosy patient (9) and has been used extensively as an in vitro reporter for human CD1b-restricted T cells (4, 9, 13, 38) overcame both technical limitations. Upon GMM recognition, the LDN5 T cell clone produces IFNγ, which is a common CD1b-restricted T cell responses seen in tuberculosis patients, polyclonal LDN5-like T cells, and BCG-vaccinated healthy individuals (4–7, 18). As shown in Fig. 3B, Mo-DCs pulsed with Siglec-7 targeted liposomal C80 GMM induced significantly more IFNγ from LDN5 cells than those pulsed with naked liposomal C80 GMM. This activation was abolished by the treatment of Mo-DCs with anti-Siglec-7 Ab prior to the addition of the liposomes (Fig. 3C). As a further control, addition of anti-CD1b blocking antibody into the co-culture of Mo-DCs with the T cells inhibited this activation (Fig. 3C). Taken together, these data with the LDN5 clone clearly demonstrate that the Siglec-7 endocytic pathway is able to effectively deliver lipid antigen for presentation to CD1b-restricted T cells.
Figure 3. Siglec-7 endocytic pathway is linked to antigen presentation to CD1b-restricted T cells.
(A) Siglec-7 targeted liposomes with C80 GMM activate CD1b-restricted T cells via Siglec-7-mediated endocytosis. (B) Mo-DCs pulsed with Siglec-7 targeted liposomal C80 GMM activate CD1b-restricted T cell line LDN5. Mo-DCs were incubated with indicated amount of C80 GMM formulated in naked and targeted liposomes or buffer only (untreated). The cells were washed and cultured with LDN5 cells. After 20 h, IFNγ in the culture supernatants was measured by ELISA. (C) The LDN5 activation by Mo-DCs is Siglec-7 dependent. Mo-DCs were incubated first with 10 μg/mL of indicated Abs, followed by the addition of Siglec-7 targeted liposomes containing C80 GMM. Mo-DCs were washed and assessed its antigen presenting activity as above. (D) The LDN5 activation is CD1b dependent. Mo-DCs were incubated with Siglec-7 targeted liposomes containing C80 GMM and cultured with LDN5 cells in the presence of 10 μg/mL of anti-CD1b or isotype control Ab. After 20 h, IFNγ in the culture supernatants was measured as above. Statistical analyses were performed by Student’s t-test. *, p < 0.05; **, p < 0.01; ***, p < 0.001. N.S. statistically not significant. Data show the results of one out of two experiments with similar results.
Delivery of the mycobacterial antigen to DCs via Siglec-7 enhances CD1b-restricted T cell activation
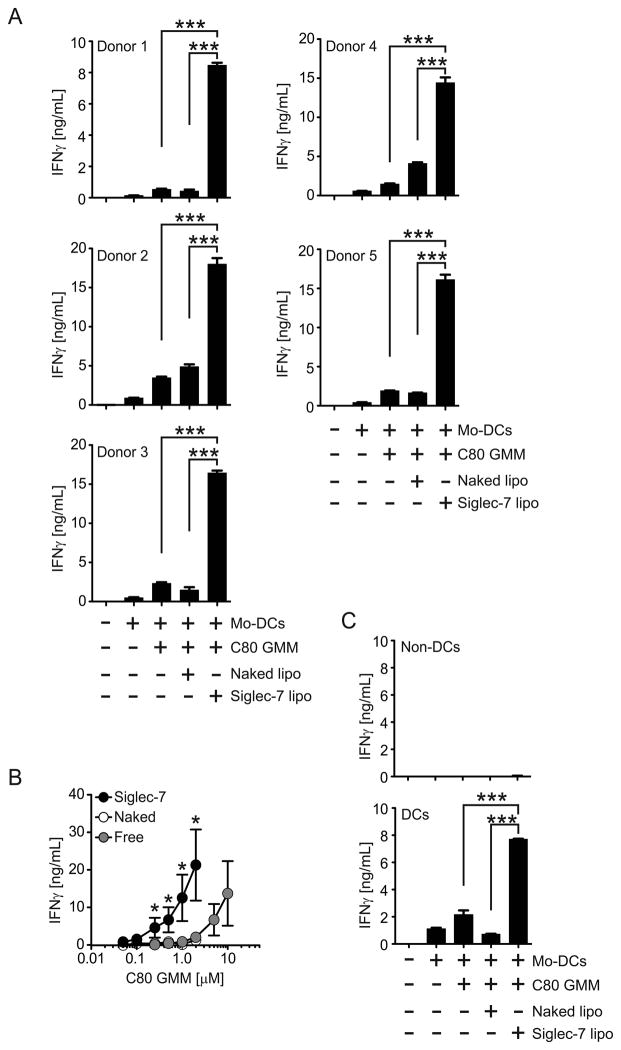
To assess the efficiency of Siglec-7-mediated delivery of lipid antigens we compared the ability of Mo-DCs pulsed with 1 μM C80 GMM in targeted antigenic liposomes or the free lipid to activate CD1b-restricted T cells. Mo-DCs pulsed with Siglec-7 targeted liposomal C80 GMM consistently activated LDN5 cells more efficiently than those pulsed with free C80 GMM, with Mo-DCs prepared from blood of five human donors (Fig. 4A). Titration of C80 GMM showed that Siglec-7 targeted liposomal antigen produced equivalent activation of LDN5 cells at a 10-fold lower dose than free C80 GMM (Fig 4B).
Figure 4. Siglec-7 mediated delivery of GMM induces robust CD1b-restricted T cell activation.
(A) Siglec-7 liposomal C80 GMM activates LDN5 cells more efficiently than free antigens. Mo-DCs were incubated with 1 μM of C80 GMM formulated in Naked and targeted liposomes or buffer only (untreated). The cells were washed and cultured with LDN5 cells. After 20 h, IFNγ in the culture supernatants was measured by ELISA. Data show the results using Mo-DCs obtained from five different donors. (B) Siglec-7 liposomal C80 GMM is approximately 10-fold more effective than free antigen. Mo-DCs were treated with indicated GMM reagents and LDN5 cell activation was analyzed as in (A). Note that naked and free lines overlap. (C) Siglec-7 targeted liposomes deliver C80 GMM to human DCs in the mixed cell population. Mo-DCs were mixed with PBMCs and incubated with the reagents as in (A). DCs were then isolated by Anti-CD209 magnetic beads and DCs and non-DCs were tested its antigen presenting activity. Statistical analyses were performed by Student’s t-test. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Data show the results of one out of two experiments with similar results.
Since other leukocytes express Siglec-7, such as NK cells and monocytes, we sought to determine if a mixed population of cells expressing Siglec-7 would impact presentation by Mo-DCs. To this end we mixed the Mo-DCs with PBMCs and incubated the cell mixture with the liposomal C80 GMM. Mo-DCs as well as non-DC populations were then separated and co-cultured with LDN5 cells respectively to test antigen-presenting activity. We found that DCs isolated from the cell mixture (Supplemental Fig. 2) activated LDN5 cells whereas remaining non-DCs did not support activation, confirming the unique ability of DCs to present antigen and demonstrating robust Siglec-7 mediated targeting to DCs in a mixed population of cells (Fig. 4C).
Discussion
Several vaccination studies have assessed the ability of mycobacterial lipids, including GMM formulated in liposomes, to generate T cell responses in vivo (12, 39). In the guinea pig model of tuberculosis, animals vaccinated with bacterial lipid extracts showed reduced bacterial burden and pathology in the lung (39), which is presumed to be due to activation of CD1-restricted T cells based on mechanistic studies with the same vaccine (12). Although a detailed analysis of the cell types that captured antigen from the liposomal vaccine was not performed, it is notable that the formulation used were ‘non-stealth’ liposomes, well-known to be targeted to primarily macrophages, which in humans typically do not express CD1a, CD1b or CD1c (20, 21). Nonetheless, the protective effects are encouraging, setting the stage for improved lipid antigen vaccines targeting CD1+ DCs.
Delivery of antigen to the desired APC is a fundamental principle for successful vaccination. Although CD1d is constitutively expressed on B cells and myeloid cells, the group1 CD1 isoforms, including CD1b, show more restricted expression on DCs (20–22), providing a specific cellular target for vaccine delivery. A major challenge for cell-type specific therapies such as targeted vaccines is to identify a receptor that is specific for the targeted cell, and a corresponding ligand or antibody that can be incorporated into the targeting platform. In this report we have used Siglec-7 as the targeted receptor due to its known restricted expression on human myeloid cells. A recent study has shown that around 75% of Siglec-7+ myeloid cells in human gut lamina propria express CD11c, a dendritic cell marker, indicating the presence of Siglec-7+ DCs in human tissue (32). Furthermore, Lundberg et al have shown that human tonsillar DCs and blood DCs express the mRNA transcript of Siglec-7 (40). Consistent with this, we found that human peripheral blood myeloid DCs, but not plasmacytoid DCs, express Siglec-7 protein (Supplemental Fig. 3). Several studies also show constitutive expression of Siglec-7 on monocytes and DCs during differentiation and maturation in vitro (33, 41). These data support the potential for use of Siglec-7 targeted liposomes for delivery of vaccines to DCs.
While we have successfully targeted Siglec-7 expressed on DCs in this study, other siglecs may also represent attractive targets for human DC-targeting. Transcript expression analysis suggests that Siglec-10 is highly expressed on various human DCs in skin, tonsil, and blood (40). Siglec-3, 5, and 9 have also been shown to be expressed on Mo-DCs (33), and Siglec-1 is induced on Mo-DCs in response to IFNγ (27). Given that both glycan ligand and antibody-based targeting technologies for siglecs are being actively developed (29, 30), verification of the in situ expression of these receptors in APCs of relevant human tissues will be needed to select the appropriate siglec to target.
For targeting DCs, we have employed a PEGylated liposomal platform, wherein liposomes are decorated with glycan ligands of Siglec-7, and have established that these lead to robust delivery of mycobacterial lipid antigens to DCs. PEGylation is a well-established modification of liposomes, which confers ‘stealth’ properties and avoids non-specific uptake by phagocytes (25). As a result, PEGylated liposomes without the targeting glycan ligand (naked liposomes) were invisible to DCs and other Siglec-7 negative leukocytes (Fig. 1) (35), leading to exquisite DC-targeting specificity of the Siglec-7 targeted liposomes.
Key to this strategy is the fact that, like most other siglecs, Siglec-7 is an endocytic receptor, capable of efficiently internalizing targeted antigenic liposomes. The partial co-localization of Siglec-7 targeted liposomes with early endosomes and lysosomes is consistent with previous reports showing that poly(lactide-co-glycolide) nano-particles decorated with an anti-Siglec-7 Ab are internalized and delivered to acidic endocytic compartments (34). The fact that co-localization is observed in both early endosomal and lysosomal compartments is consistent with a stepwise endocytic mechanism wherein endosomes precede, and eventually fuse with, lysosomes.
Delivery of lipid antigens to Mo-DCs results in robust activation of CD1b-restricted T cells relative to Mo-DCs exposed to free antigen or antigen in non-targeted liposomes. Further, human myeloid DCs also express CD1a, CD1c and CD1d, so the proof of principle for lectin targeting shown here has potential applications for other lipid antigens delivered in liposomes. Although cross-linking of Siglec-7 by immobilized anti-Siglec-7 Ab has been reported to stimulate secretion of inflammatory cytokines by monocytes (42), it is notable that activation of T cells is antigen dependent, since there is no activation with Siglec-7 targeted liposomes formulated without C80 GMM (Supplemental Fig. 4), excluding bystander effects resulting from induction of inflammatory cytokines. Thus we conclude that Siglec-7 is a promising target for selective delivery of mycobacterial lipid antigens to DCs for improved vaccines against tuberculosis.
Supplementary Material
Acknowledgments
We would like to thank Dr. R. Nedellec and B. C. Arlian for technical assistance; Dr. T. Lowary for reagents; University of California at San Diego Moore Cancer Center for Zeta sizer instrument; members in Paulson laboratory for fruitful discussion, and Anna Tran-Crie for her help in manuscript preparation.
This work was supported by NIH grants CA138891, AI50143 and HL107151 to J.C.P., T32AI007606 to C.D.R and AI R01 049313 to D.B.M.
Abbreviations
- APC
antigen presenting cell
- BCG
M. bovis Bacillus Calmette-Guerin
- DC
dendritic cells
- GMM
glucose-6-monomycolates
- Mo-DCs
monocyte-derived DC
- Siglec
sialic acid-binding immunoglobulin-like lectin
References
- 1.De Libero G, Mori L. How the immune system detects lipid antigens. Progress in lipid research. 2010;49:120–127. doi: 10.1016/j.plipres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Kasmar A, Van Rhijn I, Moody DB. The evolved functions of CD1 during infection. Current opinion in immunology. 2009;21:397–403. doi: 10.1016/j.coi.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annual review of immunology. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 4.Kasmar AG, van Rhijn I, Cheng TY, Turner M, Seshadri C, Schiefner A, Kalathur RC, Annand JW, de Jong A, Shires J, Leon L, Brenner M, Wilson IA, Altman JD, Moody DB. CD1b tetramers bind alphabeta T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. The Journal of experimental medicine. 2011;208:1741–1747. doi: 10.1084/jem.20110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montamat-Sicotte DJ, Millington KA, Willcox CR, Hingley-Wilson S, Hackforth S, Innes J, Kon OM, Lammas DA, Minnikin DE, Besra GS, Willcox BE, Lalvani A. A mycolic acid-specific CD1-restricted T cell population contributes to acute and memory immune responses in human tuberculosis infection. The Journal of clinical investigation. 2011;121:2493–2503. doi: 10.1172/JCI46216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Layre E, Collmann A, Bastian M, Mariotti S, Czaplicki J, Prandi J, Mori L, Stenger S, De Libero G, Puzo G, Gilleron M. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chemistry & biology. 2009;16:82–92. doi: 10.1016/j.chembiol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, de Vries N, Godfrey DI, Altman JD, de Jager W, Rossjohn J, Moody DB. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nature immunology. 2013;14:706–713. doi: 10.1038/ni.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Bohmer G, Prandi J, Mori L, Puzo G, De Libero G. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. The Journal of experimental medicine. 2004;199:649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong ST, Ye S, Reinhold VN, Sieling PA, Modlin RL, Besra GS, Porcelli SA. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- 10.Ulrichs T, Moody DB, Grant E, Kaufmann SH, Porcelli SA. T-cell responses to CD1-presented lipid antigens in humans with Mycobacterium tuberculosis infection. Infection and immunity. 2003;71:3076–3087. doi: 10.1128/IAI.71.6.3076-3087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TK, Koets AP, Santema WJ, van Eden W, Rutten VP, Van Rhijn I. The mycobacterial glycolipid glucose monomycolate induces a memory T cell response comparable to a model protein antigen and no B cell response upon experimental vaccination of cattle. Vaccine. 2009;27:4818–4825. doi: 10.1016/j.vaccine.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiromatsu K, Dascher CC, LeClair KP, Sugita M, Furlong ST, Brenner MB, Porcelli SA. Induction of CD1-restricted immune responses in guinea pigs by immunization with mycobacterial lipid antigens. J Immunol. 2002;169:330–339. doi: 10.4049/jimmunol.169.1.330. [DOI] [PubMed] [Google Scholar]
- 13.Moody DB, Guy MR, Grant E, Cheng TY, Brenner MB, Besra GS, Porcelli SA. CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection. The Journal of experimental medicine. 2000;192:965–976. doi: 10.1084/jem.192.7.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moody DB, Zajonc DM, Wilson IA. Anatomy of CD1-lipid antigen complexes. Nature reviews. Immunology. 2005;5:387–399. doi: 10.1038/nri1605. [DOI] [PubMed] [Google Scholar]
- 15.Van Rhijn I, Nguyen TK, Michel A, Cooper D, Govaerts M, Cheng TY, van Eden W, Moody DB, Coetzer JA, Rutten V, Koets AP. Low cross-reactivity of T-cell responses against lipids from Mycobacterium bovis and M. avium paratuberculosis during natural infection. European journal of immunology. 2009;39:3031–3041. doi: 10.1002/eji.200939619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen TKA, Koets AP, Santema WJ, van Eden W, Rutten VPMG, Van Rhijn I. The mycobacterial glycolipid glucose monomycolate induces a memory T cell response comparable to a model protein antigen and no B cell response upon experimental vaccination of cattle. Vaccine. 2009;27:4818–4825. doi: 10.1016/j.vaccine.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kronenberg M, Zajonc DM. A ‘GEM’ of a cell. Nature immunology. 2013;14:694–695. doi: 10.1038/ni.2644. [DOI] [PubMed] [Google Scholar]
- 18.Van Rhijn I, Gherardin NA, Kasmar A, de Jager W, Pellicci DG, Kostenko L, Tan LL, Bhati M, Gras S, Godfrey DI, Rossjohn J, Moody DB. TCR Bias and Affinity Define Two Compartments of the CD1b–Glycolipid-Specific T Cell Repertoire. J Immunol. 2014 doi: 10.4049/jimmunol.1400158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann SHE. Tuberculosis vaccines: Time to think about the next generation. Seminars in Immunology. 2013;25:172–181. doi: 10.1016/j.smim.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Ochoa MT, Loncaric A, Krutzik SR, Becker TC, Modlin RL. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages. The Journal of investigative dermatology. 2008;128:2225–2231. doi: 10.1038/jid.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, Bloom BR, Modlin RL. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nature medicine. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moody DB. TLR gateways to CD1 function. Nature immunology. 2006;7:811–817. doi: 10.1038/ni1368. [DOI] [PubMed] [Google Scholar]
- 23.Morse MA, Chapman R, Powderly J, Blackwell K, Keler T, Green J, Riggs R, He LZ, Ramakrishna V, Vitale L, Zhao B, Butler SA, Hobeika A, Osada T, Davis T, Clay T, Lyerly HK. Phase I study utilizing a novel antigen-presenting cell-targeted vaccine with Toll-like receptor stimulation to induce immunity to self-antigens in cancer patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:4844–4853. doi: 10.1158/1078-0432.CCR-11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trumpfheller C, Longhi MP, Caskey M, Idoyaga J, Bozzacco L, Keler T, Schlesinger SJ, Steinman RM. Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity. Journal of internal medicine. 2012;271:183–192. doi: 10.1111/j.1365-2796.2011.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, Paulson JC. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood. 2010;115:4778–4786. doi: 10.1182/blood-2009-12-257386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nycholat CM, Rademacher C, Kawasaki N, Paulson JC. In silico-aided design of a glycan ligand of sialoadhesin for in vivo targeting of macrophages. Journal of the American Chemical Society. 2012;134:15696–15699. doi: 10.1021/ja307501e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawasaki N, Vela JL, Nycholat CM, Rademacher C, Khurana A, van Rooijen N, Crocker PR, Kronenberg M, Paulson JC. Targeted delivery of lipid antigen to macrophages via the CD169/sialoadhesin endocytic pathway induces robust invariant natural killer T cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7826–7831. doi: 10.1073/pnas.1219888110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen WC, Kawasaki N, Nycholat CM, Han S, Pilotte J, Crocker PR, Paulson JC. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PloS one. 2012;7:e39039. doi: 10.1371/journal.pone.0039039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacology & therapeutics. 2012;135:327–336. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends in pharmacological sciences. 2009;30:240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nature reviews. Immunology. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki K, Sakuma K, Kawamura YI, Izawa M, Ohmori K, Mitsuki M, Yamaji T, Hashimoto Y, Suzuki A, Saito Y, Dohi T, Kannagi R. Colonic epithelial cells express specific ligands for mucosal macrophage immunosuppressive receptors siglec-7 and -9. J Immunol. 2012;188:4690–4700. doi: 10.4049/jimmunol.1100605. [DOI] [PubMed] [Google Scholar]
- 33.Lock K, Zhang J, Lu J, Lee SH, Crocker PR. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology. 2004;209:199–207. doi: 10.1016/j.imbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Scott CJ, Marouf WM, Quinn DJ, Buick RJ, Orr SJ, Donnelly RF, McCarron PA. Immunocolloidal targeting of the endocytotic siglec-7 receptor using peripheral attachment of siglec-7 antibodies to poly(lactide-co-glycolide) nanoparticles. Pharmaceutical research. 2008;25:135–146. doi: 10.1007/s11095-007-9400-7. [DOI] [PubMed] [Google Scholar]
- 35.Rillahan CD, Schwartz E, Rademacher C, McBride R, Rangarajan J, Fokin VV, Paulson JC. On-Chip Synthesis and Screening of a Sialoside Library Yields a High Affinity Ligand for Siglec-7. ACS Chemical Biology. 2013;8:1417–1422. doi: 10.1021/cb400125w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moody DB, Briken V, Cheng TY, Roura-Mir C, Guy MR, Geho DH, Tykocinski ML, Besra GS, Porcelli SA. Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nature immunology. 2002;3:435–442. doi: 10.1038/ni780. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A Syntaxin 1, Gαo, and N-Type Calcium Channel Complex at a Presynaptic Nerve Terminal: Analysis by Quantitative Immunocolocalization. The Journal of Neuroscience. 2004;24:4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng TY, Relloso M, Van Rhijn I, Young DC, Besra GS, Briken V, Zajonc DM, Wilson IA, Porcelli S, Moody DB. Role of lipid trimming and CD1 groove size in cellular antigen presentation. The EMBO journal. 2006;25:2989–2999. doi: 10.1038/sj.emboj.7601185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dascher CC, Hiromatsu K, Xiong X, Morehouse C, Watts G, Liu G, McMurray DN, LeClair KP, Porcelli SA, Brenner MB. Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. International immunology. 2003;15:915–925. doi: 10.1093/intimm/dxg091. [DOI] [PubMed] [Google Scholar]
- 40.Lundberg K, Albrekt A-S, Nelissen I, Santegoets S, de Gruijl TD, Gibbs S, Lindstedt M. Transcriptional Profiling of Human Dendritic Cell Populations and Models - Unique Profiles of In Vitro Dendritic Cells and Implications on Functionality and Applicability. PloS one. 2013;8:e52875. doi: 10.1371/journal.pone.0052875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bax M, Kuijf ML, Heikema AP, van Rijs W, Bruijns SC, Garcia-Vallejo JJ, Crocker PR, Jacobs BC, van Vliet SJ, van Kooyk Y. Campylobacter jejuni lipooligosaccharides modulate dendritic cell-mediated T cell polarization in a sialic acid linkage-dependent manner. Infection and immunity. 2011;79:2681–2689. doi: 10.1128/IAI.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varchetta S, Brunetta E, Roberto A, Mikulak J, Hudspeth KL, Mondelli MU, Mavilio D. Engagement of Siglec-7 receptor induces a pro-inflammatory response selectively in monocytes. PloS one. 2012;7:e45821. doi: 10.1371/journal.pone.0045821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.