Abstract
The essential role of transferrin in mammalian iron metabolism is firmly established. Integral to our understanding of transferrin, studies in hpx mice, a model of inherited transferrin deficiency, have demonstrated that transferrin is essential for iron delivery for erythropoiesis and in the regulation of expression of hepcidin, a hormone that inhibits macrophage and enterocyte iron efflux. Here we investigate a potential role for transferrin in the distribution of three other physiologic metals, manganese, copper and zinc. We first assessed metal content in transferrin-rich fractions of wild-type mouse sera and demonstrate that while both iron and manganese cofractionate predominantly with transferrin, the absolute levels of manganese are several orders of magnitude lower than those of iron. We next measured metal content in multiple tissues in wild-type and hpx mice at various ages. Tissue metal imbalances were severe for iron and minimal to moderate for some metals in some tissues in hpx mice. Measurement of metal levels in a transferrin-replete yet hepcidin-deficient and iron-loaded mouse strain suggested that the observed imbalances in tissue copper, zinc and manganese levels were not all specific to hpx mice or caused directly by transferrin deficiency. Overall, our results suggest that transferrin does not have a primary role in the distribution of manganese, copper or zinc to tissues and that that the abnormalities observed in tissue manganese levels are not attributable to a direct role for transferrin in manganese metabolism but rather to an indirect effect of transferrin deficiency on hepcidin expression and/or iron metabolism.
Keywords: transferrin, iron, copper, zinc, manganese
Introduction
Our understanding of the role of the serum protein transferrin in mammalian iron metabolism is based partly on studies of a condition of inherited transferrin deficiency known as hypotransferrinemia [1]. Human cases of hypotransferrinemia are documented yet exceedingly rare. A murine model of hypotransferrinemia arose spontaneously in a BALB/cJ line several decades ago [2]. Hypotransferrinemic (hpx) mice are homozygous for a splice-site mutation in the transferrin gene that renders them transferrin-deficient [3, 4]. Hpx mice develop a profound anemia which necessitates treatment with exogenous transferrin prior to weaning to ensure their survival to adulthood. This anemia highlights the essential role of transferrin in iron delivery for erythropoiesis.
Transferrin is also an essential regulator of iron metabolism. This is best demonstrated by the iron overload that develops in transferrin-deficient patients and mice that do not receive transferrin treatment. Iron overload is most severe in the liver but also has been documented in other organs including the heart, kidneys and pancreas [2, 4–6]. Iron overload stems from deficiency in hepcidin, a hormone secreted mainly by the liver that inhibits enterocyte and macrophage iron efflux. Hepcidin deficiency develops in the context of transferrin deficiency for two reasons [7]. First, transferrin directly stimulates hepcidin expression by the liver independently of transferrin’s role in erythropoiesis. In conditions of transferrin deficiency, there is minimal transferrin-mediated stimulation of hepcidin expression. Second, transferrin is essential for iron delivery to erythroid precursors. In the absence of transferrin-mediated erythroid iron delivery, the resulting anemia and/or hypoxia inhibit hepatic hepcidin expression, although the mechanism of inhibition is not well understood.
While an essential role for transferrin in mammalian iron metabolism is firmly established, a role for transferrin in the distribution of other physiologic metals has not been thoroughly explored. Transferrin can bind a variety of physiologic and non-physiologic metals in vitro, although the relevance of transferrin binding to physiologic metals other than iron is not well established in vivo [8]. Perhaps the most direct evidence of a role for transferrin in distribution of other metals is the demonstration that 54Mn cofractionates with transferrin in plasma harvested from rodents injected with 54Mn [9, 10]. To our knowledge, there is no in vivo data suggesting that transferrin binds or trafficks copper or zinc. Therefore, we hypothesized that analysis of serum and tissue distribution of iron, manganese, copper and zinc in hpx mice would demonstrate severe imbalances for iron, possible imbalances for manganese and no imbalances for copper or zinc relative to wild-type mice. If aberrant copper or zinc levels would be observed, it would occur after the onset of severe iron overload, possibly reflecting a secondary effect of severe iron overload on tissue distribution of these metals. To test this hypothesis, we assessed metal content in transferrin-rich fractions of wild-type mouse sera and analogous fractions of hpx mouse sera. We also measured metal content in multiple tissues in wild-type and hpx mice at various ages. Here we present the results of these experiments and discuss possible implications of our findings.
Materials and Methods
Animal studies were performed under an Institutional Animal Care and Use Committee-approved protocol. Care and characterization of BALB/cJ hpx and C57BL/6J Tmprss6-deficient mice has been described[7, 11, 12]. Generation of hpx Hjv mice from BALB/cJ hpx and C57BL6/J Hjv mice and their characterization has been described [12]. For harvest, mice were anesthetized and blood collected by retro-orbital puncture. Mice were euthanized and tissues immediately harvested and frozen in liquid nitrogen. Serum was isolated from blood using Microtainer Serum Separator Tubes (Becton Dickinson).
To fractionate serum, serum was isolated from 5–10 two- to four-month-old wild-type and hpx mice, pooled and frozen at −80 °C. Frozen serum was thawed, filtered and diluted fivefold with 20 mM Tris pH 8.0 wash buffer. Columns were washed with 10 column volumes (CV) wash buffer. Five mL diluted serum were applied at 1 mL/min to GE HiTrapBlue (5 mL CV), two Pierce Protein A/G (1 mL CV each) and GE HiTrap Q (1 mL CV)columns in series. Columns were washed with 10 CV wash buffer. Bound proteins were eluted with 10 CV from the Blue column with wash buffer/2 M NaCl, from the A/G columns with 0.1 M glycine pH 2.7 and from the Q column with wash buffer/100 mM NaCl and wash buffer/1000 mM NaCl. Flow through and eluates were collected, washed with wash buffer three times and concentrated to 1 mL volume with 10 kDa molecular weight cut-off Millipore Centrifugal Filter Units. Fractions were electrophoresed on NuPAGE 10% Bis-Tris Gels (Life Technologies) and gels were stained with SimplyBlueSafeStain (Life Technologies) or immunoblotted with transferrin-specific antibodies as described below.
To determine metal content by graphite furnace atomic absorption spectroscopy (GF-AAS) in serum fractions, 0.2 mL of each fraction were digested with 1 mL trace metal grade 70% nitric acid at 65 °C for two hours, diluted 1:25 in trace metal grade water and analyzed in triplicate on a Perk in Elmer A Analyst 600 in the Environmental Chemistry Facility at Brown University. National Institutes of Standards and Technology certified reference material 1640a and chromatography buffers were also measured to ensure run consistency and assess background metal levels. Iron levels were also measured in undiluted serum using a bathophenanthrolinedisulfonate (BPS)-based assay[7] and a ferrozine-based Iron/TIBC Reagent Set (Pointe Scientific). To determine metal levels in unfractionated wild-type and hpx serum, 50 μL of mouse serum were digested with 0.25 mL nitric acid, diluted 1:25 in water then analyzed by GF-AAS.
To measure tissue metal levels, 50–200 mg of frozen tissues were thawed, weighed and digested in 1 mL trace metal grade 70% nitric acid at 65 °C for two hours, diluted 1:25 in trace metal grade water and analyzed in triplicate on a JY2000 Ultrace Inductively Coupled Plasma Atomic Emission Spectrometer(ICP-AES) or by GF-AAS in the Environmental Chemistry Facility. ICP-AES and GF-AAS were used to analyze concentrated and dilute samples respectively. National Institutes of Standards and Technology certified reference material 1640a was also measured to ensure run consistency.
For gene expression and activity analysis, RNA was extracted from thawed tissues using Trizol then subjected to DNase I treatment and reverse transcription using High Capacity cDNA Reverse Transcription Kit (Life Technologies). Gene expression was measured using TaqMan Gene Expression Master Mix and Assays Mm00444148 (Ccs), Mm01344233 (Sod1), Mm01313000 (Sod2) and Mm99999915 (Gapdh) (Life Technologies) on a ViiA 7 System (Applied Biosystems) using a relative standard curve approach. Proteins were extracted from thawed tissues by Dounce homogenization in 20 mM Tris pH 7.5/1% Triton X-100/1 mM EDTA with protease inhibitors, centrifuged to pellet debris then immunoblotted using NuPAGE 10% Bis-Tris Gels (Life Technologies) and ECL Western Blotting Analysis System (GE Life Sciences). The following antibodies (at indicated dilutions) were used: rabbit anti-CCS (1:1000)[13]kindly provided by Joe Prohaska at the University of Minnesota, Duluth, MN, Proteintech rabbit anti-Sod1 (1:1000), Novus rabbit anti-Sod2 (1:1000), Cell Signaling rabbit anti-Gapdh (1:5000) and Proteintech rabbit anti-transferrin (1:500). Superoxide dismutase activity was analyzed using Novex Tris-Glycine Gels (Life Technologies) and a nitrobluetetrazolium-based assay[14]. Bovine Sod1 was purchased from Sigma. Sod1-deficient mouse tissues were kindly provided by Rick Eisenstein at the University of Wisconsin-Madison, Madison, WI. Densitometry was performed using ImageJ software [15].
Results
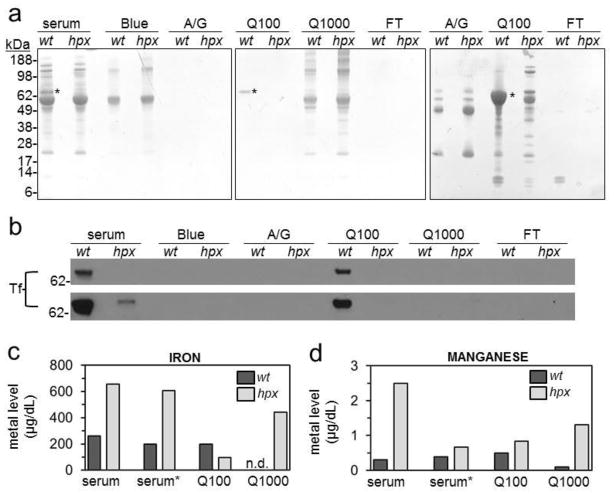
To explore a potential role for transferrin in distribution of manganese, copper and zinc, we first estimated the iron, manganese, copper and zinc content of transferrin. We rationalized that metal content in transferrin-rich fractions of wt mouse sera would represent the maximal amount of metal potentially bound to transferrin. Given that manganese cofractionates with transferrin in vivo, we predicted that both iron and manganese would cofractionate with transferrin while copper and zinc would not. To isolate transferrin-rich fractions, we employed a protocol that segregates transferrin into a low-salt anion exchange fraction [1]. We analyzed pooled serum samples from wt and hpx mice given that our fractionation protocol behaved more consistently with larger starting volumes and serum volumes from individual mice were inadequate. Pooled serum samples were diluted then applied to an albumin affinity, immunoglobulin affinity and anion exchange Q column in series. Proteins were eluted from each column separately. Proteins were eluted from the Q column with 100 mM NaCl(“Q100 fraction”) then 1000 mM NaCl (“Q1000 fraction”). Eluates were washed and concentrated to the original serum volume by centrifugal filtration. Equal volumes of each fraction were analyzed by SDS-PAGE and Coomassie staining (Figure 1a, two left panels). A band in wt but not hpx sera and Q100 fractions migrated to a position consistent with transferrin’s 75 kDa molecular mass (*, Figure 1a). SDS-PAGE and Coomassie staining of a larger volume of specific fractions revealed the presence of multiple other bands of much lesser abundance in both wt and hpx Q100 fractions (Figure 1a, right panel). Transferrin immunoblot detected a single band in wt but not hpx sera or Q100 fractions migrating to a similar position as the Coomassie-stained band in the wt Q100 fraction (Figure 1b). With our previous mass spectrometric analysis identifying the most prominent band in low-salt Q fractions as transferrin[1], our data indicated that the prominent band in the wt Q100 fraction was transferrin.
Figure 1. Metal content in transferrin-rich fractions of mouse sera.
Wild-type (wt) and hypotransferrinemic (hpx) sera were pooled from five to ten two- to four-month-old mice. Sera were applied to albumin affinity (Blue), immunoglobulin affinity(A/G) and anion exchange (Q) columns in series then eluted from individual columns. Proteins were eluted from the Q column with 100 mM NaCl (Q100) then 1000 mM NaCl (Q1000). Column flow through (FT) and all eluates were washed and concentrated to original serum volume. (a) Fractions (left two panels) and larger volumes of select fractions (right panel) were electrophoresed under denaturing, reducing conditions on polyacrylamide gels then stained to visualize proteins. * indicates transferrin band. (b) Fractions were immunoblotted with a transferrin (Tf)-specific antibody. Upper and lower images correspond to short and long exposures respectively. Iron (c) and manganese (d) levels in wt and hpx sera and fractions were measured by GF-AAS in triplicate. Serum* indicates sera diluted then concentrated without fractionation. ‘n.d.’ indicates value was below the lower limit of detection.
GF-AAS was next used to measure metal content in wt and hpx sera, Q100 and Q1000 fractions and in sera diluted and concentrated without any fractionation (indicated as serum*) to assess the loss of metal during sample dilution and concentration(Figure 1c,d; Figure S1a,b). Iron levels were markedly increased in hpx sera relative to wt sera and were unaffected by dilution and concentration (Figure 1c). In wt and hpx sera, most iron was contained in the Q100 and Q1000 fractions respectively, suggesting a redistribution of iron to non-transferrin species in hpx mice. The increased iron levels in hpx sera were striking, given that a commercial ferrene-based assay previously yielded decreased iron levels[7]. To address this, we measured iron levels in wt and hpx sera using two other methods, a BPS-based assay and a commercial ferrozine-based assay—the ferrene-based assay was no longer available for purchase. While the absolute iron levels in wt sera were similar in all three assays, both GF-AAS and the BPS-based assay indicated increased iron levels in hpx sera while the ferrozine-based assay, like the ferrene-based assay before, indicated decreased iron levels (data not shown). This discrepancy has been noted before in hpx sera[16] and suggests that not all iron assays measure non-transferrin bound iron equivalently. To determine if this discrepancy was specific to hpx sera, we measured serum iron levels in pooled sera from wild-type and Tmprss6-deficient mice. Tmprss6 is a membrane-bound serine protease essential for down-regulation of hepcidin expression [17]. Mice deficient in Tmprss6 develop hepcidin excess and systemic iron deficiency. While absolute iron levels in wild-type and Tmprss6-deficient mouse sera differed between assays, levels were greater in wild-type than mutant sera in all assays (data not shown), suggesting that the discrepancy noted above for hpx sera is specific to non-transferrin bound iron.
Manganese was most abundant in the wt Q100 and hpx Q1000 fractions (Figure 1d). Copper and zinc were below the level of detection or low in Q100 fractions but abundant in Q1000 fractions (Figure S1a,b). Hpx sera manganese levels and wt sera zinc levels decreased with dilution and concentration (serum*, Figure 1d, S1b), suggesting that some serum manganese and zinc were associated with low molecular-weight species, as 10 kDa cut-off centrifugal filter units were used to concentrate samples. Overall, our data is consistent with transferrin as the main iron- and manganese-, but not copper- or zinc-, binding protein in the serum. However, the absolute manganese levels in unfractionated sera and Q100 fractions were two orders of magnitude below iron levels. If transferrin is the main serum manganese-binding protein, iron-replete transferrin is much more abundant than manganese-replete transferrin.
Above, we pooled sera from several mice—our fractionation protocol behaved more consistently with larger starting volumes. This did not permit us to examine metal content in sera of individual mice. To address this, we analyzed metal content individually in smaller volumes of serum from wt and hpx mice by GF-AAS (Figure S1c). Serum iron and zinc levels were decreased in hpx relative to wt mice. Serum copper and manganese levels did not differ between genotypes. The observation that serum iron levels were decreased in hpx mice in Figure S1c contrasts with our observation that iron levels were increased in pooled hpx sera in Figure 1c. Serum iron levels in Figure S1c and 1c respectively represent one-month-and two- to four-month-old mice. Hpx mice receive weekly intraperitoneal transferrin injections prior to weaning to ensure their survival to weaning age. While all hpx mice were anemic at the one-month time of harvest (data not shown) and iron loading worsens progressively in hpx mice (see below), the difference in hpx serum iron levels in Figure S1c and 1c most likely reflects the fact that non-transferrin-bound iron levels had not yet started to increase at one months of age. We presume that with age and continued hepcidin deficiency in hpx mice, excessive dietary iron absorption eventually increases non-transferrin-bound iron levels to levels above that of wt mice.
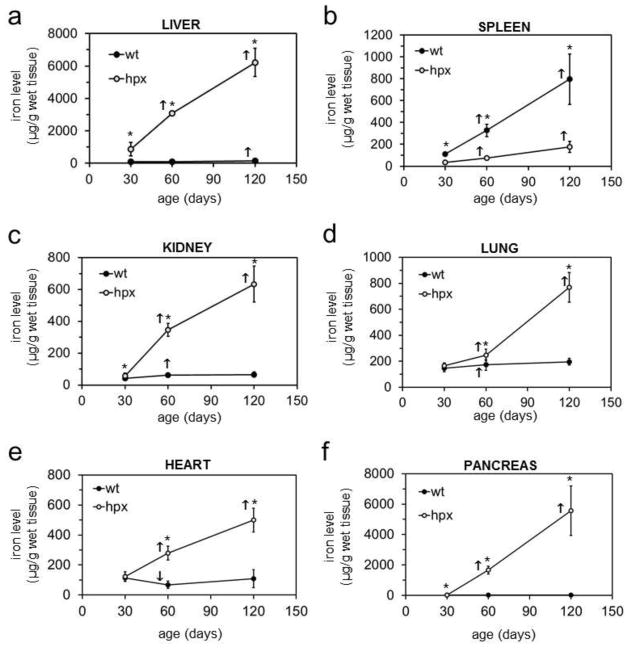
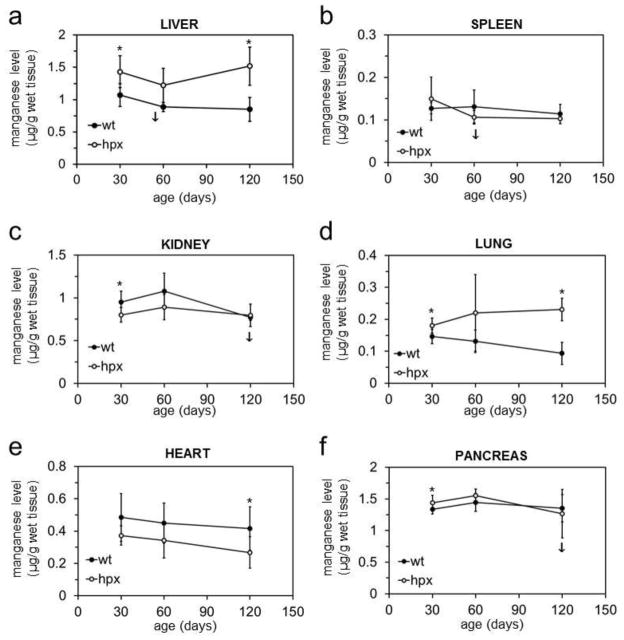
The above results suggested that transferrin binds iron predominantly. However, hpx mice could still display a defect in distribution of manganese, copper and zinc if transferrin deficiency has an indirect effect on those metals. To examine this, we measured wt and hpx tissue metal levels by ICP-AES and GF-AAS with the former and latter used for more concentrated and diluted samples respectively. Iron levels increased with age in liver, spleen, kidney, lung, heart and pancreas of hpx mice and were greater at all ages in hpx than wt mice for all tissues except spleen (Figure 2). Manganese levels were increased significantly or near significantly at all ages in hpx liver and lung relative to wt tissues (Figure 3). Copper levels decreased in hpx liver with age and were decreased relative to wt liver at 120 days (Figure S2). Copper levels were greater in hpx spleen and lung than wt at all ages (Figure S2). Similar to copper, zinc levels decreased in hpx liver with age and were decreased relative to wt liver at 60 and 120 days (Figure S3). Decreased zinc levels were noted at 120 days in hpx heart and pancreas relative to wt organs (Figure S3). We also measured metal levels in quadriceps muscle and femur from four-month-old wt and hpx mice. While iron levels were significantly elevated in hpx muscle and bone, there were no differences in manganese, copper or zinc levels in these tissues between wt and hpx mice (Figure 4, S4). Overall, hpx tissue metal imbalances were most prominent for iron, while much less prominent defects were noted for copper, zinc and manganese in some tissues at some ages.
Figure 2. Tissue iron levels in hpx mice.
Iron levels were measured in nitric acid-digested liver (a), spleen (b), kidney (c), lung (d), heart (e) and pancreas (f) from wt (filled circles) and hpx (open circles) mice by ICP-AES and GF-AAS and expressed as μg metal per g wet tissue relative to mouse age in days.* indicates statistically significant difference between wt and hpx values at same age. ↑ and ↓ indicate increase or decrease respectively in level at indicated age vs. one time point earlier for mouse of same genotype. Statistical significance determined by P<0.05 (Student’s t-test, two-tailed, unequal variance). Error bars indicate standard deviation. Each point represents 5–12 mice. All values were measured in triplicate.
Figure 3. Tissue manganese levels in hpx mice.
Manganese levels in liver (a), spleen (b), kidney (c), lung (d), heart (e) and pancreas (f) were measured and plotted as described in Figure 2.
Figure 4. Iron and manganese levels in hpx muscle and bone.
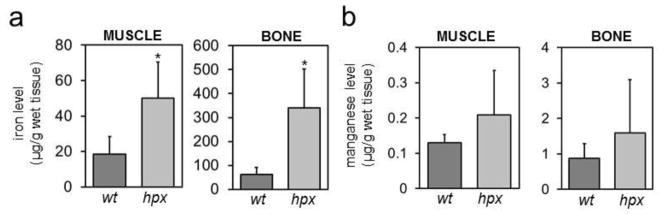
Iron (a) and manganese (b) levels were measured by GF-AAS in quadriceps muscle and femurs from five four-month-old wt and hpx mice. * indicates statistically significant difference between wt and hpx mice. Statistical significance determined by P<0.05 (Student’s t-test, two-tailed, unequal variance). Error bars indicate standard deviation.
To determine if the observed tissue metal imbalances were unique to hpx mice, we analyzed livers from mice deficient in hemojuvelin (Hjv). Hjv is a bone morphogenetic co-receptor essential for hepcidin expression. Like hpx mice, Hjv-deficient mice develop hepcidin deficiency and iron overload. Unlike hpx mice, Hjv-deficient mice do not have transferrin deficiency. We took advantage of a previous report in which we generated mice deficient in both transferrin and Hjv (hpx Hjv) mice and analyzed samples from these mice to demonstrate that Hjv is essential for transferrin-dependent hepcidin expression [12]. Measurement of liver metal levels by ICP-AES demonstrated increased iron levels in hpx, Hjv and hpx Hjv mice, as previously demonstrated by BPS-based iron assay (Figure 5a). Manganese levels were also increased in hpx mice and Hjv mice relative to wt mice and in hpx Hjv mice relative to Hjv mice (Figure 5b). No significant changes in copper or zinc levels were detected in these mice (Figure S4). These data suggested that the increased manganese levels observed in hpx liver do not directly reflect transferrin deficiency but rather consequences of transferrin deficiency such as hepcidin deficiency or iron overload.
Figure 5. Liver metal levels in Hjv mice.
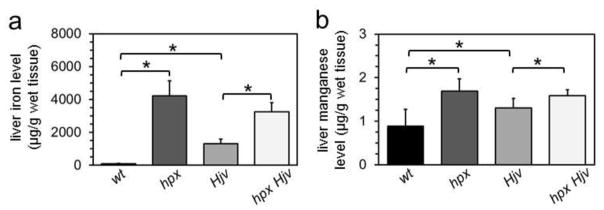
Liver iron (a) and manganese (b) levels were measured by ICP-AES in five to six two-month-old wild-type (wt), transferrin-deficient (hpx), Hjv-deficient (Hjv) and transferrin-/Hjv-deficient (hpx Hjv) mice. Levels were expressed as μg metal per g wet tissue. * indicates statistically significant difference between bracketed groups. Statistical significance determined by P<0.05 (Student’s t-test, two-tailed, unequal variance). Error bars indicate standard deviation.
To explore the hpx tissue metal imbalances further, we interrogated the Ccs-Sod1 pathway, a relatively well characterized pathway of mammalian metal biology. The copper chaperone for superoxide dismutase (Ccs) is essential for copper incorporation into the antioxidant protein copper/zinc superoxide dismutase (Sod1) [18]. Mice with copper deficiency induced by dietary restriction possess increased Ccs protein levels, decreased Sod1 protein levels but unchanged Ccs and Sod1 RNA levels [13, 19]. We measured hepatic expression of Ccs, Sod1 and manganese superoxide dismutase (Sod2) by quantitative PCR, immunoblots and activity gels. The specificity of Sod1 activity bands was demonstrated by analysis of wild-type and Sod1-deficient mouse lysates; Sod activity gel linearity was demonstrated by analysis of increasing amounts of purified bovine Sod1 (Figure S5a,b). Ccs and Sod1 RNA levels were decreased in hpx relative to wt livers by 120 days of age (Figure S5c). Ccs and Sod2 protein levels were unchanged but Sod1 protein levels were decreased in hpx relative to wt livers at 30 and 120 days of age (Figure S5d,e). Sod1 activity levels were decreased in hpx relative to wt livers only at 120 days of age (Figure S5d,e).
The cause of decreased Sod1 protein in 30- and 120-day-old hpx liver and decreased Sod1 activity only in 120-day-old hpx liver is unclear. Notably, Sod1 activity levels were unchanged and Sod1 protein levels were decreased in 30-day-old hpx mice relative to wt mice, suggesting that Sod1 protein was more active in hpx than wt mice at this age. The percent decrease in Sod1 activity was less than the percent decrease in protein levels in 120-day-old hpx liver relative to wt mice, also suggesting that Sod1 protein was more active in hpx and wt mice at this age. We speculate that hpx iron overload impacts steady-state Sod1 protein and activity levels, although there is no literature precedent to currently support this. Overall, other than the finding of decreased Sod1 activity at 30 days of age, all noted defects in the Ccs-Sod1 pathway had a later age of onset than iron overload in hpx mice.
Discussion
Perhaps the most direct experimental data supporting an in vivo link between transferrin and an essential metal other than iron are the demonstrations that transferrin is the main manganese-binding protein in the circulation[8]. Biochemical fractionation of plasma harvested from rodents intraperitoneally or intravenously injected with 54Mn indicates that manganese cofractionates with transferrin[9, 10]. While hpx mice are a potentially productive model for studies of the role of transferrin in distribution of metals other than iron, studies in these mice have focused predominantly on the tissue uptake and distribution of injected 54Mn. One study demonstrated that transferrin is required for distribution of intravenously injected 54Mn within but not to the brain [20]. Another showed that transferrin is not required for distribution of subcutaneously injected 54Mn to brain, heart and bone, although increased 54Mn uptake was observed in liver soon after injection [21].
To our knowledge, no study has yet to examine the relative abundance of iron versus manganese, copper and zinc in transferrin-rich serum or plasma fractions or the absolute tissue levels of manganese, copper or zinc in hpx mice, as we performed above. Our data recapitulate the above findings that manganese fractionates predominantly with transferrin. However, given our observed concentrations of manganese and iron in transferrin-rich serum fractions, manganese-replete transferrin is much less abundant than iron-replete transferrin, assuming that manganese present in transferrin-rich fractions represents transferrin-bound manganese. This finding, together with the lack of prominent imbalances in tissue manganese levels in hpx mice, implies that transferrin is not essential for manganese distribution to various tissues under conditions present in hpx mice. However, these data do not exclude the possibility that transferrin is essential for manganese distribution under conditions of manganese deficiency or overload or distribution of manganese, copper or zinc within tissues.
While hpx mice do exhibit some mild alterations in tissue content of manganese, copper and zinc, our analyses suggest that these alterations may not be specific to hpx mice and/or reflect the secondary effects of severe iron overload or other consequences of transferrin deficiency—the observed increases in liver manganese levels in hpx and hpx Hjv mice relative to wild-type mice may simply reflect a redistribution of manganese from serum to liver. There is literature supporting a link between iron overload and perturbations in levels of other metals. Aberrant intestinal manganese absorption and intracellular manganese distribution have been demonstrated in a mouse model of hemochromatosis, an inherited disease of iron overload due to mutations in genes required for hepcidin expression [22, 23]. Increased hepatic zinc content was observed after iron loading of mice by intraperitoneal iron dextran injections [24]. Mild copper deficiency in the heart and liver developed in rats fed a high iron diet [25]. Increased manganese and zinc content in the liver and spleen was noted after iron loading of rats by diet or injection[26]. Whether these findings reflect shared physiologic pathways underlying iron and manganese transport or solely reflect the pathophysiologic effect of iron overload on other metals remains to be determined.
In this paper, we initially predicted that hpx mice would not display aberrant levels of copper or zinc but may display aberrant levels of tissue manganese. While our data do not suggest an essential role for transferrin in the distribution of manganese in hpx mice, several other overlaps between iron and manganese transport have already been established or suggested. Divalent metal transporter 1 (Dmt1) was first characterized in vivo in studies of microcytic mice and Belgrade rats, two rodent models that harbor missense Dmt1 mutations [27]. These animal models exhibit a microcytic anemia indicative of Dmt1’s role in intestinal iron absorption and erythroid iron uptake. These animals also display similar defects in manganese transport, indicating that manganese and iron share some similar transport mechanisms. Less well explored than the role of Dmt1 in manganese metabolism is the putative role of the cellular iron exporter ferroportin. A membrane protein expressed largely in enterocytes and macrophages, ferroportin is internalized and degraded after binding by hepcidin[17]. Hepcidin-dependent regulation of ferroportin serves as the mechanism by which hepcidin limits dietary iron absorption and macrophage iron release. In vitro studies have suggested that ferroportin mediates cellular manganese efflux [28, 29]. Increased 54Mn absorption occurs after intragastric administration of 54Mn in a mouse model of hemochromatosis, a disease of primary hepcidin deficiency, although a role for ferroportin or Dmt1 in this process was not formally demonstrated [22].
One key difference between iron and manganese metabolism is the means of elimination of each metal from the body. Unlike iron, manganese undergoes hepatobiliary excretion. While the molecular mechanism of hepatobiliary manganese excretion has yet to be established, mutations in SLC30A10, a putative metal transporter expressed in liver and brain, were recently identified in patients with inherited manganese excess, Parkinson-like neurologic defects and liver dysfunction [30–32]. If SLC30A10 does mediate the transport of manganese out of hepatocytes into bile, it is unlikely that it also transports iron, given that iron does not undergo hepatobiliary excretion under homeostatic conditions. This suggests that while iron and manganese share some transport pathways, they do not share all transport pathways.
Supplementary Material
Acknowledgments
This work was supported by NIH grant K99/R00 DK084122 to TBB. We thank Joseph Orchardo and David Murray at the Environmental Chemistry Facility at Brown University for providing assistance with ICP-AES and GF-AAS, Joe Prohaska for providing an aliquot of rabbit anti-CCS antibody for use in immunoblots and Rick Eisenstein for providing samples of Sod1-deficient mouse tissue.
Abbreviations
- wt
wild-type
- hpx
hypotransferrinemic
- CV
column volume
- GF-AAS
graphite furnace atomic absorption spectrometry
- ICP-AES
inductively coupled plasma atomic emission spectroscopy
- BPS
bathophenanthrolinedisulfonic acid
- Ccs
copper chaperone for superoxide dismutase
- Sod1
copper/zinc superoxide dismutase
- Dmt1
divalent metal transporter 1
References
- 1.Bartnikas TB. Known and potential roles of transferrin in iron biology. Biometals Int J Role Met Ions Biol Biochem Med. 2012 doi: 10.1007/s10534-012-9520-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein SE. Hereditary hypotransferrinemia with hemosiderosis, a murine disorder resembling human atransferrinemia. J Lab Clin Med. 1987;110:690–705. [PubMed] [Google Scholar]
- 3.Huggenvik JI, Craven CM, Idzerda RL, et al. A splicing defect in the mouse transferrin gene leads to congenital atransferrinemia. Blood. 1989;74:482–486. [PubMed] [Google Scholar]
- 4.Trenor CC, Campagna DR, Sellers VM, et al. The molecular defect in hypotransferrinemic mice. Blood. 2000;96:1113–1118. [PubMed] [Google Scholar]
- 5.Simpson RJ, Konijn AM, Lombard M, et al. Tissue iron loading and histopathological changes in hypotransferrinaemic mice. J Pathol. 1993;171:237–244. doi: 10.1002/path.1711710313. [DOI] [PubMed] [Google Scholar]
- 6.Beard JL, Wiesinger JA, Li N, Connor JR. Brain iron uptake in hypotransferrinemic mice: influence of systemic iron status. J Neurosci Res. 2005;79:254–261. doi: 10.1002/jnr.20324. [DOI] [PubMed] [Google Scholar]
- 7.Bartnikas TB, Andrews NC, Fleming MD. Transferrin is a major determinant of hepcidin expression in hypotransferrinemic mice. Blood. 2011;117:630–637. doi: 10.1182/blood-2010-05-287359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent JB, Love S. The binding and transport of alternative metals by transferrin. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagen.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Davidsson L, Lönnerdal B, Sandström B, et al. Identification of transferrin as the major plasma carrier protein for manganese introduced orally or intravenously or after in vitro addition in the rat. J Nutr. 1989;119:1461–1464. doi: 10.1093/jn/119.10.1461. [DOI] [PubMed] [Google Scholar]
- 10.Jursa T, Smith DR. Ceruloplasmin alters the tissue disposition and neurotoxicity of manganese, but not its loading onto transferrin. Toxicol Sci Off J Soc Toxicol. 2009;107:182–193. doi: 10.1093/toxsci/kfn231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartnikas TB, Steinbicker AU, Campagna DR, et al. Identification and characterization of a novel murine allele of Tmprss6. Haematologica. 2013 doi: 10.3324/haematol.2012.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartnikas TB, Fleming MD. Hemojuvelin is essential for transferrin-dependent and -independent hepcidin expression in mice. Haematologica. 2011 doi: 10.3324/haematol.2011.054031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prohaska JR, Geissler J, Brokate B, Broderius M. Copper, zinc-superoxide dismutase protein but not mRNA is lower in copper-deficient mice and mice lacking the copper chaperone for superoxide dismutase. Exp Biol Med Maywood NJ. 2003;228:959–966. doi: 10.1177/153537020322800812. [DOI] [PubMed] [Google Scholar]
- 14.Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson RJ, Cooper CE, Raja KB, et al. Non-transferrin-bound iron species in the serum of hypotransferrinaemic mice. Biochim Biophys Acta. 1992;1156:19–26. doi: 10.1016/0304-4165(92)90090-h. [DOI] [PubMed] [Google Scholar]
- 17.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347–360. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 18.Wong PC, Waggoner D, Subramaniam JR, et al. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc Natl Acad Sci U S A. 2000;97:2886–2891. doi: 10.1073/pnas.040461197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prohaska JR, Broderius M, Brokate B. Metallochaperone for Cu, Zn-superoxide dismutase (CCS) protein but not mRNA is higher in organs from copper-deficient mice and rats. Arch Biochem Biophys. 2003;417:227–234. doi: 10.1016/s0003-9861(03)00364-3. [DOI] [PubMed] [Google Scholar]
- 20.Malecki EA, Cook BM, Devenyi AG, et al. Transferrin is required for normal distribution of 59Fe and 54Mn in mouse brain. J Neurol Sci. 1999;170:112–118. doi: 10.1016/s0022-510x(99)00203-8. [DOI] [PubMed] [Google Scholar]
- 21.Dickinson TK, Devenyi AG, Connor JR. Distribution of injected iron 59 and manganese 54 in hypotransferrinemic mice. J Lab Clin Med. 1996;128:270–278. doi: 10.1016/s0022-2143(96)90028-1. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Buckett PD, Wessling-Resnick M. Absorption of manganese and iron in a mouse model of hemochromatosis. PloS One. 2013;8:e64944. doi: 10.1371/journal.pone.0064944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jouihan HA, Cobine PA, Cooksey RC, et al. Iron-mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis. Mol Med Camb Mass. 2008;14:98–108. doi: 10.2119/2007-00114.Jouihan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Li B, Chen C, Gao Z. Hepatic distribution of iron, copper, zinc and cadmium-containing proteins in normal and iron overload mice. Biometals Int J Role Met Ions Biol Biochem Med. 2009;22:251–259. doi: 10.1007/s10534-008-9161-8. [DOI] [PubMed] [Google Scholar]
- 25.Klevay LM. Iron overload can induce mild copper deficiency. J Trace Elem Med Biol Organ Soc Miner Trace Elem GMS. 2001;14:237–240. doi: 10.1016/S0946-672X(01)80009-2. [DOI] [PubMed] [Google Scholar]
- 26.Vayenas DV, Repanti M, Vassilopoulos A, Papanastasiou DA. Influence of iron overload on manganese, zinc, and copper concentration in rat tissues in vivo: study of liver, spleen, and brain. Int J Clin Lab Res. 1998;28:183–186. doi: 10.1007/s005990050041. [DOI] [PubMed] [Google Scholar]
- 27.Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology. 2008;29:569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin Z, Jiang H, Lee E-SY, et al. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J Neurochem. 2010;112:1190–1198. doi: 10.1111/j.1471-4159.2009.06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madejczyk MS, Ballatori N. The iron transporter ferroportin can also function as a manganese exporter. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamem.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quadri M, Federico A, Zhao T, et al. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet. 2012;90:467–477. doi: 10.1016/j.ajhg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stamelou M, Tuschl K, Chong WK, et al. Dystonia with brain manganese accumulation resulting from SLC30A10 mutations: a new treatable disorder. Mov Disord Off J Mov Disord Soc. 2012;27:1317–1322. doi: 10.1002/mds.25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuschl K, Clayton PT, Gospe SM, Jr, et al. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet. 2012;90:457–466. doi: 10.1016/j.ajhg.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.