Abstract
Porto-pulmonary hypertension (POPH), once considered an absolute contraindication for liver transplantation (LT), has become a more accepted indication because of the evolution of treatment with prostacyclin analogues, phosphodiesterase inhibitors and endothelin receptor antagonists. An exception MELD score of 22 is assigned to candidates with documentation of effective treatment. We examined the post-transplant outcomes of patients who received LT for POPH with exception.
Methods
Scientific Registry of Transplant Recipients data on 34318 adult (≥18years) deceased donor LT recipients transplanted between 3/1/2002 and 8/31/2010 were reviewed. The diagnosis of POPH was ascertained from MELD exception forms. Patients were followed from the time of transplant until the earlier of death or end of the follow-up period. Cox-regression was used to evaluate the predictors of post-LT mortality and graft failure.
Results
During the study period, 34318 patients received deceased donor LT. Seventy eight out of 34318 patients were transplanted for POPH with MELD exception. The 1-year adjusted risks of patient death and graft failure for patients transplanted under exception rules for POPH were significantly higher than with POPH adult recipients who did not receive exception points (death: hazard ratio [HR]=2.25, p=0.005); graft failure (HR=1.96, p=0.012).
Conclusions
This study of national data suggests that treated POPH continues to be associated with inferior early post-transplant outcomes.
Keywords: Porto-pulmonary hypertension, Mortality, Graft Failure, Liver Transplantation
Introduction
Pulmonary hypertension occurring in the setting of cirrhosis and portal hypertension is commonly referred to as porto-pulmonary hypertension (POPH). It is a relatively uncommon complication of cirrhosis occurring in an estimated 2-10% of all patients with advanced liver disease[1]. Diagnostic criteria for POPH include a mean pulmonary artery pressure (mPAP) ≥25 mmHg, an elevated pulmonary vascular resistance >240 dyne s/cm−5, and a normal pulmonary capillary wedge pressure.
Untreated moderate to severe POPH is considered a relative contraindication to liver transplantation (LT) due to high perioperative morbidity and mortality related to right heart failure[2-4]. The advent of various vasomodulating medications such as prostacyclin analogues, phosphodiesterase inhibitors and endothelin receptor antagonists to treat moderate to severe POPH has led to the ability to lower mean pulmonary artery pressures in some patients to a level below 35 mm Hg, which is considered acceptable for LT.[5-8]
LT candidates with POPH that meet the criteria of documentation of treatment and post-treatment mPAP <35 mmHg and PVR <400 dynes/sec/cm-5 are eligible for a MELD exception score[9]. Before 2007, patients with documentation of the successful treatment for POPH received the exception MELD score at the discretion of regional review boards. However, in 2007, the MELD Exceptional Case Study Group (MESSAGE) set the standardized MELD exception score to 22 for treated POPH with a 10% mortality-equivalent increase every three months, as long as mPAP remains below 35 mmHg as confirmed by repeat heart catheterization [10].
The aim of this retrospective cohort study was to examine the clinical characteristics of patients who received the MELD exception for treated POPH and to assess the impact of treated POPH on post-LT mortality and graft survival.
METHODS
Data Sources and Study Population
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR maintains a database of all candidates for and recipients of solid-organ transplants in the United States. This publically available data can be acquired under data use agreement from the SRTR. Candidates on the waiting list and recipients of solid organ transplants are tracked on a periodic basis; data are submitted to the Organ Procurement and Transplantation Network (OPTN)[11, 12]. The Health Resources and Services Administration (HRSA), United States Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The SRTR supplements information on vital status with data on deaths from the Social Security Death Master File and the Medicare Beneficiary Database.
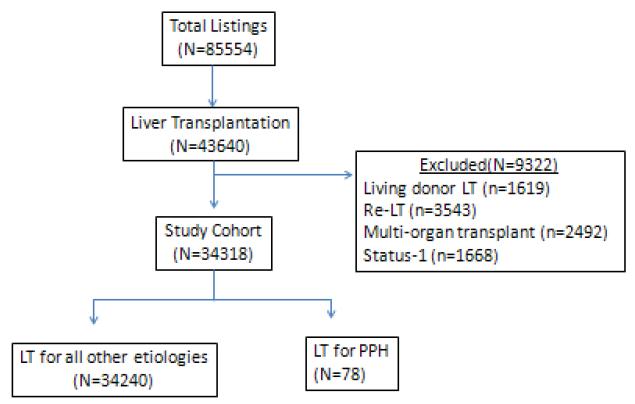
The study population included adult (age ≥18 years) deceased donor LT recipients that were listed and transplanted between March 1, 2002 and August 31, 2010. Recipients of living donor LT, re-LT, and multi-organ transplant, as well as transplants done as Status-1, were excluded.
Analytic Approach
Continuous variables were expressed as median (interquartile range) and categorical variables were expressed as proportions. The diagnosis of POPH was ascertained from MELD exception forms. Baseline characteristics of LT recipients with and without POPH were compared using Chi-square test for categorical variables and t-tests for continuous variables. The primary outcome was patient survival after LT. The secondary outcome was graft failure, defined as the earlier of death or re-LT. Time at risk began on the day of LT and extended until the recipient experienced an outcome or was censored at the end of the follow-up period. The median length of follow up after LT was four years (range: 0.5 – 8 years).
Cox regression was used to examine potential associations between POPH and early (within one year) post-transplant mortality and graft failure. The models were adjusted for recipient factors including recipient age, portal vein thrombosis, lab MELD, albumin, race/ethnicity, encephalopathy, diabetes, diagnosis, year of transplant as well as donor factors such as donor age, race, split liver, donation after cardiac death (DCD), cause of death, diabetes, organ location (local, regional, national) and cold ischemia time.
All statistical analyses were conducted using SAS v9.2 (SAS Institute; Cary, NC, USA).
RESULTS
Patient Characteristics and Outcomes
There were 85554 listings for LT during the study period, of which 43640 (51%) resulted in LT. After excluding recipients of living donor LT (n=1619), re-LT (n=3543), multi-organ transplant (n=2492), and status-1 (n=1668), a total of 34318 adult deceased donor LT recipients were included in the study data (Figure 1). One hundred twenty-three candidates were waitlisted and received an exception MELD score for POPH during the study period. Eleven candidates with POPH died on the waitlist and seven were removed from the list because they were too sick to be transplanted. Seventy eight (59%) received a deceased donor LT for POPH with MELD exception, of whom 22 (28%) were transplanted between 2002 and 2006 (before the implementation of MESSAGE) and 56 (72%) were transplanted between 2007 and 2010.
Figure 1.
Description of Cohort
Abbreviations: LT= Liver Transplantation; POPH=Portopulmonary hypertension
The baseline characteristics of LT recipients with and without exceptions for POPH are shown in Table 1. Compared to the LT recipients of all other etiologies, the group with POPH had lower mean age (52 years vs. 54 years, p=0.02) and lab MELD scores (14 vs. 18, p<0.001) and higher proportions of female recipients (47% vs. 30%, p<0.001) and recipients with hepatitis C as the etiology of liver disease (50% vs. 36%, p=0.02).
Table 1.
Baseline characteristics: POPH vs. Without POPH recipients
| Characteristic | POPH recipients (n=78) n (%) or median (quartile 1 – quartile 3) |
Without POPH (n=34,240) n (%) or median (quartile 1 – quartile 3) |
p-value* |
|---|---|---|---|
|
| |||
| Age at transplant (years) | 52 (48 – 56) | 54 (49 – 60) | 0.01 |
|
| |||
| Sex | <0.001 | ||
| Male | 41 (52.6%) | 23,955 (70.0%) | |
| Female | 37 (47.4%) | 10,285 (30.0%) | |
|
| |||
| Race | 0.37 | ||
| White | 58 (74.4%) | 25,068 (73.2%) | |
| Black | 5 (6.4%) | 2,952 (8.6%) | |
| Hispanic | 12 (15.4%) | 4,315 (12.6%) | |
| Asian | 1 (1.3%) | 1,586 (4.6%) | |
| Other | 2 (2.6%) | 319 (0.9%) | |
|
| |||
| Primary diagnosis | 0.02 | ||
| Hepatitis C | 39 (50.0%) | 12,325 (36.0%) | |
| Cryptogenic | 11 (14.1%) | 4,103 (12.0%) | |
| Alcoholic Cirrhosis | 14 (17.9%) | 4,702 (13.7%) | |
| Other diagnosis | 14 (17.9%) | 13,110 (38.3%) | |
|
| |||
| Lab MELD | 14 (11 – 18) | 18 (13 – 25) | <0.001 |
|
| |||
| Serum albumin (g/dL) | 3.0 (2.4 – 3.5) | 2.9 (2.5 – 3.4) | 0.49 |
|
| |||
| Year of transplant | <0.001 | ||
| 2002** | 0 (0%) | 1,529 (4.5%) | |
| 2003 | 1 (1.3%) | 3,241 (9.5%) | |
| 2004 | 8 (10.3%) | 3,983 (11.6%) | |
| 2005 | 12 (15.4%) | 4,289 (12.5%) | |
| 2006 | 1 (1.3%) | 4,598 (13.4%) | |
| 2007 | 15 (19.2%) | 4,485 (13.1%) | |
| 2008 | 14 (17.9%) | 4,485 (13.1%) | |
| 2009 | 14 (17.9%) | 4,572 (13.4%) | |
| 2010* | 13 (16.7%) | 3,058 (8.9%) | |
Chi–square test for categorical variables and t–tests for continuous variables
Available data cover only part of the year
The median total time from initial listing to LT for POPH group was 145 days (IQR 49-378). The median time on the waitlist from listing to receipt of a MELD exception was 29 days (IQR 6-285), and was 53 days (IQR 16-122) from receipt of a MELD exception to LT.
Unadjusted Patient and Graft Survival
The unadjusted 1- and 3-year patient survival for the recipients with POPH was 85% and 81%, respectively. This was similar to all other adult candidates without an exception for POPH with 1- and 3-year patient survivals of 88% and 79%, respectively. The numbers of patients still at risk were 57 and 27,976 at 1 year and 29 and 18,002 at 3 years after LT in the POPH and without POPH group, respectively.
The 1- and 3-year graft survival for the LT recipients with POPH was 82% and 78%, respectively. This was similar to all other LT recipients without an exception for POPH with 1- and 3-year patient survivals of 85% and 76%, respectively. The numbers of patients still at risk were 55 and 27,049 at 1 year and 27 and 17,198 at 3 years after LT in the POPH and without POPH group, respectively.
Predictors of Post-LT Mortality and Graft Failure
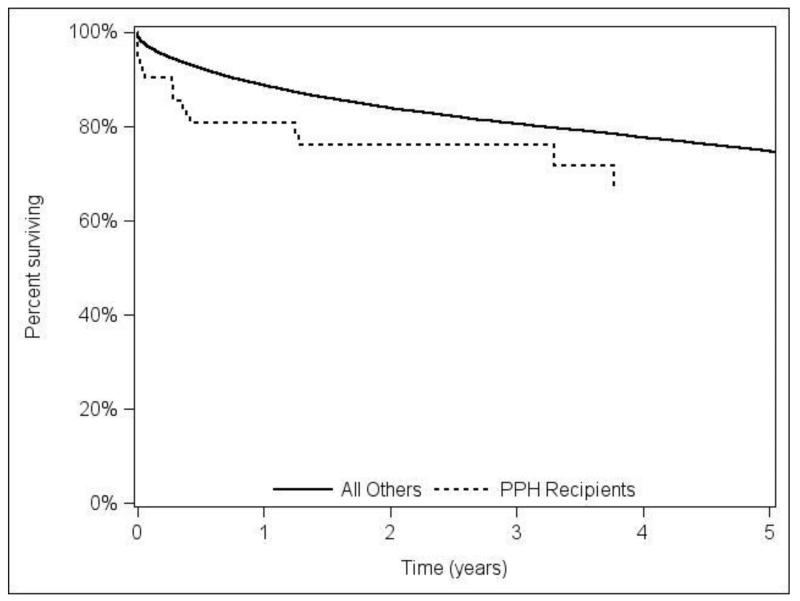
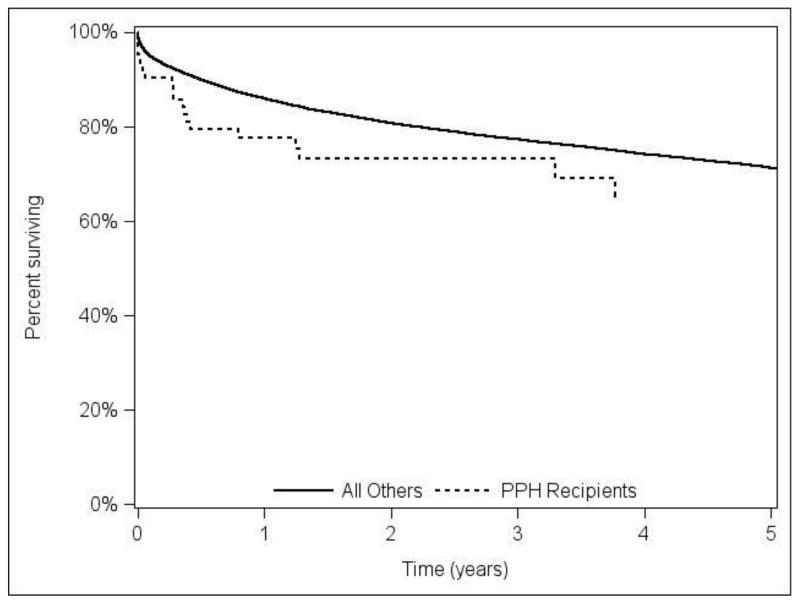
In the adjusted Cox model, POPH recipients had a trend towards higher risk of death (HR=1.56, p=0.07) and graft failure (HR=1.45, p=0.11), over all observed post-transplant follow-up. Figure 2A and 2B shows the adjusted patient and graft survival. The adjusted analysis using 3-year follow up resulted in similar hazard ratios and p-values to models using all available follow-up (HR=1.62, p=0.07 for post-LT mortality; HR=1.50, p=0.10 graft failure).
Figure 2A.
Adjusted overall patient survival comparing LT recipients with portopulmonary hypertension (POPH) versus without POPH.
Figure 2B.
Adjusted graft survival stratified by portopulmonary hypertension (POPH) versus without POPH LT recipients
Within the first post-transplant year, POPH recipients had a significantly higher risk of death (HR=2.25, p=0.005) and graft failure (HR=1.96; p=0.013). The complete Cox models for one-year mortality and graft failure are shown in Tables 2 and 3, respectively. We tested the interaction between hepatitis C and POPH to examine whether the effect of hepatitis C is different for LT recipients with and without POPH. This interaction was highly non-significant (P=0.75).
Table 2.
Cox regression model one-year post-transplant mortality
| Recipients Factors at LT | HR (95% CI) | p-Value |
|---|---|---|
|
| ||
| POPH (vs. No POPH) | 2.25 (1.27-3.97) | 0.005 |
|
| ||
| Age (Reference: 40-49 years) | ||
| 18-30 years | 0.99(0.76-1.30) | 0.97 |
| 30-39 years | 0.94(0.78-1.13) | 0.5 |
| 50-59 years | 1.23(1.13-1.35 | <0.001 |
| 60-69 years | 1.67(1.52-1.84) | <0.001 |
| ≥70 years | 2.08(1.75-2.48) | <0.001 |
|
| ||
| Portal Vein thrombosis (vs. not) | 1.62 (1.44-1,81) | <0.001 |
|
| ||
| MELD Score (Reference: 15-19) | ||
| 6-9 | 1.13(0.99-1.28) | 0.08 |
| 10-14 | 1.00 (0.91-1.11 | 0.93 |
| 20-24 | 1.20 (1.09-1.33) | <0.001 |
| 25-30 | 1.51 (1.34-1.69) | <0.001 |
| ≥31 | 2.17 (1.97-2.39) | <0.001 |
|
| ||
| Serum Albumin (per unit g/dl) | 0.91 (0.87-0.95) | <.001 |
|
| ||
| Race/Ethnicity (Reference: Caucasian) | ||
| Black | 1.16(1.05-1.29) | 0.006 |
| Hispanic | 0.88(0.80-0.97) | 0.012 |
| Others | 0.86(0.74-1.01) | 0.06 |
|
| ||
| Grade 1/II Encephalopathy (Reference: none) | 1.07 (1.00-1.16) | 0.06 |
| Grade III/IV Encephalopathy (Reference: none) | 1.38 (1.23-1.54) | <0.001 |
|
| ||
| Year of LT (per year) | 0.94 (0.93-0.95) | <.001 |
|
| ||
| Pre-LT Diabetes | 1.18(1.10-1.27) | <.001 |
|
| ||
| Diagnosis (Reference: Hepatitis C) | ||
| Hepatitis B | 0.65 (0.52-0.81) | <.001 |
| Cryptogenic | 0.85(0.77-0.95) | 0.003 |
| Alcoholic Cirrhosis | 0.75(0.68-0.83) | <0.001 |
| PBC | 0.72 (0.60-0.87) | <0.001 |
| PSC | 0.65(0.54-0.79) | <0.001 |
| HCC | 1.03 (0.93-1.14) | 0.60 |
| Autoimmune | 0.85(0.69-1.04) | 0.12 |
| Others | 1.03(0.92-1.15) | 0.61 |
|
| ||
| Donor Factors | HR (95% CI) | p-Value |
|
| ||
| Donor age | 1.01(1.01-1.01) | <.001 |
|
| ||
| Donor Race (Reference: Caucasian) | ||
| Black | 1.10(1.01-1.20) | 0.027 |
| Hispanic | 1.16 (1.06-1.28) | 0.002 |
| Other | 1.24 (1.05-1.46) | 0.01 |
|
| ||
| Split Liver (vs. not) | 1.47(1.13-1.91) | 0.004 |
|
| ||
| DCD (vs. not) | 1.58(1.38-1.80) | <0.001 |
|
| ||
| Cause of death (Reference: Others) | ||
| Anoxia | 1.09(0.99-1.20) | 0.08 |
| CVA | 1.14 (1.05-1.24) | <0.001 |
|
| ||
| Diabetes | 1.13(1.02-1.24) | 0.015 |
|
| ||
| Regional vs. Local | 1.07(0.99-1.15) | 0.085 |
|
| ||
| National vs. Local | 1.22 (1.08-1.37) | 0.001 |
|
| ||
| Cold Ischemia Time (per hour) | 1.02(1.01-1.03) | <0.001 |
Table 3.
Cox regression model of one-year post-transplant graft failure.
| Recipient factors | HR (95% CI) | p-value |
|---|---|---|
|
| ||
| POPH (vs. No POPH) | 1.96 (1.16-3.31) | 0.012 |
|
| ||
| Age (Reference: 40-49 years) | ||
| <30 years | 1.16 (0.93-1.43) | 0.18 |
| 30-39 years | 1.03(0.88-1.20) | 0.75 |
| 50-59 years | 1.09 (1.01-1.18) | 0.03 |
| 60-69 years | 1.35(1.24-1.47) | <0.001 |
| ≥70 years | 1.55(1.32-1.83) | <0.001 |
|
| ||
| Race/Ethnicity (Reference: Caucasian) | ||
| Black | 1.16 (1.06-1.28) | 0.002 |
| Hispanic | 0.90(0.82-0.98) | 0.02 |
| Other | 0.91 (0.79-1.04) | 0.16 |
|
| ||
| Portal vein thrombosis | 1.52 (1.37-1.69) | <0.001 |
|
| ||
| Lab MELD score (Reference: 15-19) | ||
| 6-9 | 1.06(0.95-1.19) | 0.32 |
| 10-14 | 1.00 | 0.98 |
| 20-24 | 1.17(1.07-1.27) | <0.001 |
| 25-30 | 1.28 (1.15-1.43) | <0.001 |
| ≥31 | 1.84 (1.68-2.00) | <0.001 |
|
| ||
| Albumin | 0.94 | 0.002 |
|
| ||
| Encephalopathy (Reference: none) | ||
|
| ||
| Grade I/II | 1.06 (0.99-1.14) | 0.08 |
|
| ||
| Grade III/IV | 1.32(1.19-1.46) | <0.001 |
|
| ||
| Transplant year (per year) | 0.94 (0.93-0.96) | <0.001 |
|
| ||
| Diabetes | 1.13 (1.06-1.21) | <0.001 |
|
| ||
| Primary diagnosis (Reference: HCV) | ||
| HBV | 0.0.67(0.55-0.81) | <0.001 |
| Cryptogenic/fatty liver disease | 0.86 (0.79-0.95) | 0.003 |
| Alcoholic liver disease | 0.77 (0.70-0.84) | <0.001 |
| PBC | 0.75 (0.63-0.89) | <0.001 |
| PSC | 0.79 (0.67-0.92) | 0.003 |
| HCC | 1.01 (0.93-1.11) | 0.76 |
| Autoimmune liver disease | 0.88 (0.73-1.06) | 0.17 |
| Others | 1.07 (0.97-1.18) | 0.17 |
|
| ||
| Donor factors | HR | p-value |
|
| ||
| Age (per year) | 1.01(1.01-1.01) | <0.001 |
|
| ||
| Race (Reference: Caucasian) | ||
| Black | 1.18(1.09-1.27) | <0.001 |
| Hispanic | 1.17 (1.08-1.28) | <0.001 |
| Other | 1.21 (1.04-1.41) | 0.01 |
|
| ||
| Split liver | 1.52 (1.20-1.92) | <0.001 |
|
| ||
| DCD | 20.3 (1.82-2.27) | <0.001 |
|
| ||
| Cause of death (reference: Other) | ||
| Anoxia | 1.03 (0.95-1.13) | 0.47 |
| CVA | 1.14 (1.06-1.22) | <0.001 |
|
| ||
| Donor history of diabetes | 1.14 (1.05-1.24) | 0.003 |
|
| ||
| Organ location (Reference: Local) | ||
| Regional | 1.08 (1.01-1.15) | 0.03 |
| National | 1.21(1.09-1.34) | <0.001 |
|
| ||
| Cold ischemia time (per hour) | 1.03(1.02-1.04) | <0.001 |
The MESSAGE criteria were implemented in 2007; therefore, we tested interactions between POPH and the era effect (2002-2006 vs. 2007-2010) on post-LT outcomes. The interaction terms were not significant (all p > 0.70), indicating that the relative risks of mortality and graft failure for LT recipients with POPH compared to those without POPH were not different before and after the standardized criteria were implemented.
DISCUSSION
This is the largest cohort study of national data to examine post-transplant outcomes of POPH patients who received LT under a MELD exception score. Despite documentation of pre-transplant treatment resulting in mPAP <35 mmHg and PVR <400 dynes/sec/cm−5, recipients with POPH had worse short-term patient and graft survival when compared to non-POPH non-exception recipients. Moreover, there was a trend towards lower long-term patient and graft survival for LT recipients with POPH.
Additionally, our study validated some of the previously described factors associated with poor post-LT survival including age, African American race, portal vein thrombosis, diabetes, high MELD score at transplant[13-15]. Advanced age at LT (50-59 years, 60-69 years and ≥70 years at LT vs. 40-49 years) was an independent risk factor of post-transplant mortality and graft failure.
Our study showed that LT recipients with hepatitis C had worse patient survival compared to other diagnoses. We further investigated the relationship of hepatitis C and POPH on mortality and graft failure. The interaction between POPH and HCV was not significant indicating that effect of HCV was not different for recipients with and without an exception for POPH.
The option to assign an exception MELD score was incorporated into MELD-based allocation to improve access to transplant for candidates in whom the MELD score does not reflect the disease severity and risk of death in the absence of transplant. This process was standardized in 2007 (MESSAGE criteria) for the indications requiring exception MELD points including POPH[10]. Our study showed the patients with treated POPH were getting MELD exception scores before the MESSAGE criteria were implemented. However, the post-LT outcomes were similar for POPH LT recipients before and after the implementation of MESSAGE criteria.
Some single center case series of patients with POPH, who responded to the vasodilatory or vasomodulating therapy and underwent LT, had favorable post-transplant survival[16, 17]. Our unadjusted analysis showed similar outcomes among POPH vs. without POPH recipients. However, after adjusting for various patient and donor factors, assignment of an exception MELD score for POPH was associated with higher risk of death and graft failure at one year after LT.
LT operation incurs acute right ventricular stress because of the acute increase in cardiac output at the time of reperfusion. Although MPAP < 35 mm Hg in the setting of a PVR < 400 dynes/sec/cm−5 may correspond to an adequate right ventricular reserve and sufficient compliance in the pulmonary vascular bed, acute POPH and acute right heart failure during or after LT can occur despite adequate pressure control, resulting in poor 1-year outcomes[18].
The limitations of this study include small sample size and retrospective design and its associated problems such as bias and confounding factors due to patient data that may be limited and/or immeasurable. Additionally, the SRTR registry data do not include specific information on right ventricular systolic pressure; mean pulmonary artery pressures, pulmonary vascular resistance as well as the specific details including the type and duration of pre-LT or post-LT treatment modality for POPH. Moreover, these results are only applicable to a selected subgroup of patients with POPH who had a satisfactory response to the vasomodulatory treatment and who were transplanted with a MELD exception score.
In conclusion, patients who received LT for treated POPH had lower 1-year patient and graft survival compared to transplant recipients without POPH. These results may have implications in patient counseling and management of POPH LT recipients. Strategies directed at pre-emptive risk modification, aggressive intraoperative and early post-transplant management may improve their post-transplant outcomes.
Acknowledgement
This research was presented, in part, as a poster of distinction at the American Transplant Congress, 2013, held in Seattle, Washington. Dr. Sharma is supported by National Institutes of Health (NIH) grant KO8 DK-088946 and research award from American College of Gastroenterology. The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Abbreviations
- LT
Liver Transplantation
- MELD
Model for End Stage Liver Disease
- POPH
Porto-pulmonary Hypertension
- mPAP
Mean Pulmonary Artery Pressure
Footnotes
Disclosures:
None
References
- 1.Bandara M, et al. Successful outcomes following living donor liver transplantation for portopulmonary hypertension. Liver Transpl. 2010;16(8):983–9. doi: 10.1002/lt.22107. [DOI] [PubMed] [Google Scholar]
- 2.Krowka MJ, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transpl. 2004;10(2):174–82. doi: 10.1002/lt.20016. [DOI] [PubMed] [Google Scholar]
- 3.Krowka MJ. Portopulmonary hypertension and the issue of survival. Liver Transpl. 2005;11(9):1026–7. doi: 10.1002/lt.20494. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Palli G, et al. Liver transplantation in high-risk patients: hepatopulmonary syndrome and portopulmonary hypertension. Transplant Proc. 2005;37(9):3861–4. doi: 10.1016/j.transproceed.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 5.Krowka MJ, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology. 1999;30(3):641–8. doi: 10.1002/hep.510300307. [DOI] [PubMed] [Google Scholar]
- 6.Hollatz TJ, et al. Treatment with sildenafil and treprostinil allows successful liver transplantation of patients with moderate to severe portopulmonary hypertension. Liver Transpl. 2012;18(6):686–95. doi: 10.1002/lt.23407. [DOI] [PubMed] [Google Scholar]
- 7.Halank M, et al. Use of oral endothelin-receptor antagonist bosentan in the treatment of portopulmonary hypertension. Transplantation. 2004;77(11):1775–6. doi: 10.1097/01.tp.0000122420.86904.89. [DOI] [PubMed] [Google Scholar]
- 8.Raevens S, et al. Oral vasodilator therapy in patients with moderate to severe portopulmonary hypertension as a bridge to liver transplantation. Eur J Gastroenterol Hepatol. 2013;25(4):495–502. doi: 10.1097/MEG.0b013e32835c504b. [DOI] [PubMed] [Google Scholar]
- 9.OPTN . 3.6.4.5 Liver Candidates with Exceptional Cases. 2005. [Google Scholar]
- 10.Freeman RB, Jr., et al. Model for end-stage liver disease (MELD) exception guidelines: results and recommendations from the MELD Exception Study Group and Conference (MESSAGE) for the approval of patients who need liver transplantation with diseases not considered by the standard MELD formula. Liver Transpl. 2006;12(12 Suppl 3):S128–36. doi: 10.1002/lt.20979. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson DM, et al. Transplant data: sources, collection, and caveats. Am J Transplant. 2004;4(Suppl 9):13–26. doi: 10.1111/j.1600-6135.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 12.Leppke S, et al. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando) 2013;27(2):50–6. doi: 10.1016/j.trre.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Englesbe MJ, et al. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl. 2010;16(8):999–1005. doi: 10.1002/lt.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayanan Menon KV, et al. MELD and other factors associated with survival after liver transplantation. Am J Transplant. 2004;4(5):819–25. doi: 10.1111/j.1600-6143.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, et al. Factors that affect deceased donor liver transplantation rates in the United States in addition to the model for end-stage liver disease score. Liver Transpl. 2012;18(12):1456–63. doi: 10.1002/lt.23548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sussman N, et al. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant. 2006;6(9):2177–82. doi: 10.1111/j.1600-6143.2006.01432.x. [DOI] [PubMed] [Google Scholar]
- 17.Ashfaq M, et al. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant. 2007;7(5):1258–64. doi: 10.1111/j.1600-6143.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- 18.Ramsay M. Portopulmonary hypertension and right heart failure in patients with cirrhosis. Curr Opin Anaesthesiol. 2010;23(2):145–50. doi: 10.1097/ACO.0b013e32833725c4. [DOI] [PubMed] [Google Scholar]