Abstract
The anti-apoptotic protein Bcl-2 is a versatile regulator of cell survival. Its interactions with its own pro-apoptotic family members are widely recognized for their role in promoting the survival of cancer cells. These interactions are thus being targeted for cancer treatment. Less widely recognized is the interaction of Bcl-2 with the inositol 1,4,5-trisphosphate receptor (InsP3R), an InsP3-gated Ca2+ channel located on the endoplasmic reticulum. The nature of this interaction, the mechanism by which it controls Ca2+ release from the ER, its role in T-cell development and survival, and the possibility of targeting it as a novel cancer treatment strategy are summarized in this review.
Keywords: Bcl-2; inositol 1,4,5-trisphosphate receptor; apoptosis; cancer; chronic lymphocytic leukemia; lymphocyte
1. Introduction
It has been almost thirty years since Bcl-2 was discovered [1, 2] and found to be a positive regulator of cell survival [3]; twenty years since the first indication that Bcl-2 regulates intracellular Ca2+ dynamics [4, 5], and ten years since an interaction of Bcl-2 and its close relative Bcl-xl with the inositol 1,4,5-trisphosphosphate receptor (InsP3R) was discovered [6, 7]. An inhibitor of the Bcl-2-InsP3R interaction has recently been developed and shown to induce the death of primary human chronic lymphocytic leukemia (CLL) cells [8, 9], raising the possibility that targeting this interaction may become a novel treatment strategy for Bcl-2-positive malignancies. This review will discuss our understanding of how the Bcl-2-InsP3R interaction promotes cell survival, the evidence that Bcl-2-positive cancer cells exploit this mechanism to avoid cell death, and the efforts to therapeutically target the Bcl-2-InsP3R interaction. Readers are referred to a number of excellent reviews for expanded information about InsP3Rs and Ca2+ signaling [10, 11] and the Bcl-2 protein family [12, 13].
2. Bcl-2 Family Members and Functions
Bcl-2 is a 26 kDa integral membrane protein that resides on the outer mitochondrial membrane and endoplasmic reticulum (ER) membrane. It is anchored on these membranes by a C-terminal hydrophobic tail and is mainly cytoplasmic in location. Bcl-2 excited great interest when it was discovered to promote cell survival by inhibiting apoptosis [3]. One after another, Bcl-2 relatives were identified, elevating Bcl-2 to pater familias stature. Proteins in the Bcl-2 family share sequence motifs referred to as Bcl-2 Homology Domains (BH domains), of which four are recognized (Figure 1). From a functional standpoint, members generally fall into two opposing groups: anti-apoptotic proteins and pro-apoptotic proteins. Anti-apoptotic members such as Bcl-2 typically have four BH domains (BH1-4). Pro-apoptotic members fall into two subgroups: those with three BH domains (BH1-3), such as Bax and Bak, and those with only a BH3 domain, the ‘BH3-only proteins’, including for example Bim, Bad, and PUMA. These distinctions are useful from an operational standpoint, but are undergoing significant revision and clarification as an increasing number of proteins are found to have BH3-like domains but not all are Bcl-2 family members [14]. Moreover, only anti-apoptotic members were originally considered to have BH4 domains, but this distinction is eroding as BH4-domain-like structures become recognized in certain pro-apoptotic family members [14].
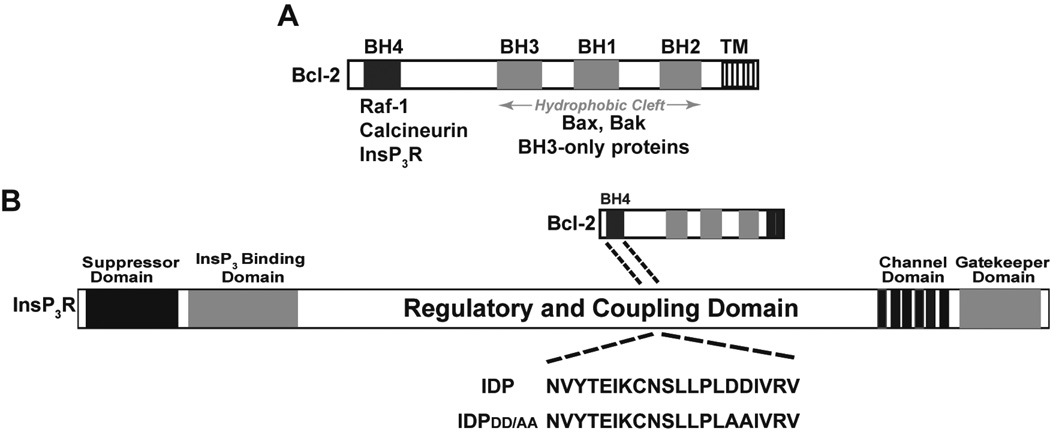
Figure 1. Bcl-2-InsP3R interaction and its peptide inhibitor.
(A) The location of Bcl-2 homology (BH) domains is shown, together with proteins known to interact with the BH4 domain and with a hydrophobic cleft composed of BH 1-3 domains. (B) The diagram illustrates the interaction of the BH4 domain of Bcl-2 with a region within the regulatory and coupling domain of the InsP3R. IDP is a synthetic peptide corresponding to a 20 amino acid sequence within the Bcl-2 binding site on the InsP3R. IDPDD/AA is a modification of IDP that eliminates a predicted cleavage site and increases the peptide’s activity when introduced into cells by fusion with the cell penetrating peptide of HIV TAT.
One of the most remarkable features of Bcl-2 is its lack of an obvious inherent function, such as kinase, phosphatase or enzymatic activity. Bcl-2 and its anti-apoptotic relatives nevertheless exert widespread influence over many cell functions, ultimately influencing cell survival. The main modus operandi involves engagement in diverse interactions. These include homomeric and heteromeric oligomerization involving both Bcl-2 family members and non-family proteins. A major activity of Bcl-2 involves interaction with its pro-apoptotic family members, including Bax, Bak and the BH3-only members. By binding pro-apoptotic family members, Bcl-2 prevents these proteins from oligomerizing and forming pores in the outer mitochondrial membrane, thus releasing cytochrome c and activating a cascade of caspase activation, ultimately leading to apoptosis. Bcl-2’s site of interaction with pro-apoptotic proteins is located in a hydrophobic cleft composed of BH1-3 domains (Figure 1). This cleft is occupied by small molecule BH3-mimetics such as ABT-737 that displace pro-apoptotic proteins from Bcl-2 and thus trigger apoptosis [15, 16]. For an in-depth explanation of how Bcl-2 interacts with its pro-apoptotic relatives, thereby preserving outer mitochondrial membrane integrity, the reader is referred to publications by Llambi et al [17] and Shamas-Din et al [13].
Bcl-2 family proteins also regulate cell survival through their localization to the ER [18, 19]. Moreover, Bcl-2 interacts with a number of proteins in addition to Bcl-2 protein family members, indicating that Bcl-2 has functional significance beyond its direct control of pro-apoptotic relatives (Figure 1). These interactions are mediated through the BH4 domain and include binding of the BH4 domain to InsP3Rs, the serine/threonine protein kinase Raf-1 [20] and the serine/threonine protein phosphatase calcineurin (CaN) [21]. Raf-1 phosphorylates and thereby inhibits the pro-apoptotic protein Bad [20]. The interaction of the Bcl-2 BH4 domain with CaN and the InsP3R regulates intracellular Ca2+ dynamics and cell survival, as addressed in this review.
3. Regulation of Cell Survival and Cell Death by InsP3R-mediated Ca2+ Elevation
InsP3Rs are InsP3-gated Ca2+ channels located mainly on the ER [10, 11] (Figure 2). Their central function is to release Ca2+ ions from the ER lumen, where Ca2+ is stored at high concentration. Ca2+ release induces highly regulated and systematic elevations of cytoplasmic Ca2+ concentration. InsP3R-mediated Ca2+ elevation regulates the activity of many fundamental cellular processes including fertilization, cell cycle entry, cell division, metabolism, and transcription [10, 22]. Ca2+ information is encoded in the frequency and amplitude of Ca2+ oscillations and decoded by Ca2+-sensitive kinases and phosphatases, in turn regulating the activity of target proteins as diverse as transcription factors, endonucleases, proteases and metabolic enzymes [23, 24].
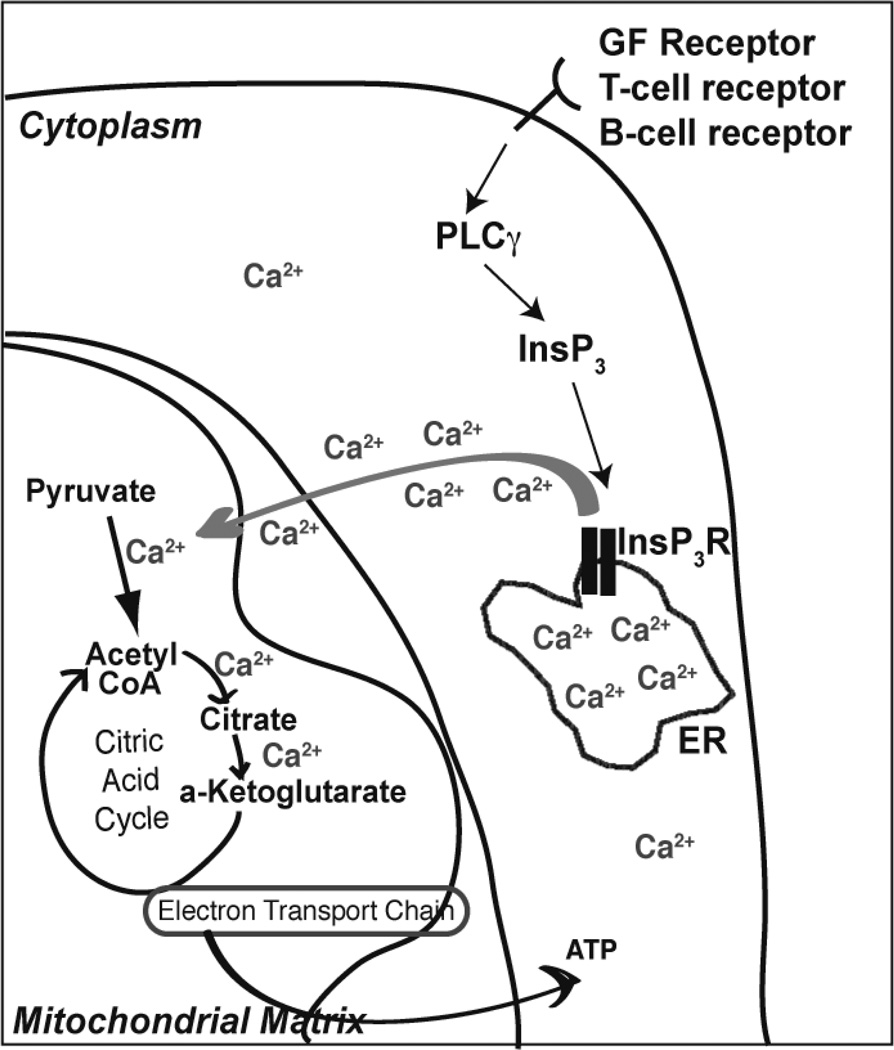
Figure 2. ER-mitochondria Ca2+ transfer.
InsP3R-mediated transfer of Ca2+ from the ER lumen into the mitochondrial matrix is of vital importance since Ca2+ activates multiple steps in the citric acid cycle, promoting ATP production.
One of the most important functions of InsP3R-mediated Ca2+ signaling is the promotion of cell survival by supporting mitochondrial Ca2+ uptake and mitochondrial metabolism. The close proximity of ER-localized InsP3Rs to mitochondria facilitates Ca2+ transfer from the ER lumen into mitochondria [25, 26] (Figure 2). This calcium transfer promotes mitochondrial ATP production by catalyzing the conversion of pyruvate to acetyl-CoA and by activating multiple Ca2+-sensitive enzymes in the citric acid cycle [27, 28]. Insufficient ER-mitochondrial Ca2+ transfer results in autophagy, a survival mechanism through which cells digest intracellular components in order to produce ATP [29], but which may lead to cell death if prolonged (Figure 3). Conversely, excessive transfer of Ca2+ to mitochondria induces Ca2+ overload, resulting in loss of mitochondrial membrane potential, cytochrome c release and apoptosis [30, 31] (Figure 3). Therefore, although Ca2+ elevation plays a critical role in promoting cell survival, it is also a known inducer of cell death [32, 33] and for this reason InsP3R-mediated Ca2+ release from the ER must be tightly regulated. Ca2+ elevation triggers apoptosis through a number of different pathways in addition to mitochondrial Ca2+ overload. These include activation of Ca2+-sensitive proteases and endonucleases, activation of the pro-apoptotic Bcl-2 family member Bad, and inducing expression of pro-apoptotic Bcl-2 family member Bim [33].
Figure 3. Control over InsP3R-mediated Ca2+ release and its importance.
The consequences of insufficient or excessive Ca2+ transfer from ER to mitochondria illustrate why cells have developed a number of mechanisms, including interaction with Bcl-2, to control InsP3R channel opening and Ca2+ release.
4. Bcl-2 Regulation of InsP3R-mediated Ca2+ Elevation in T-cells
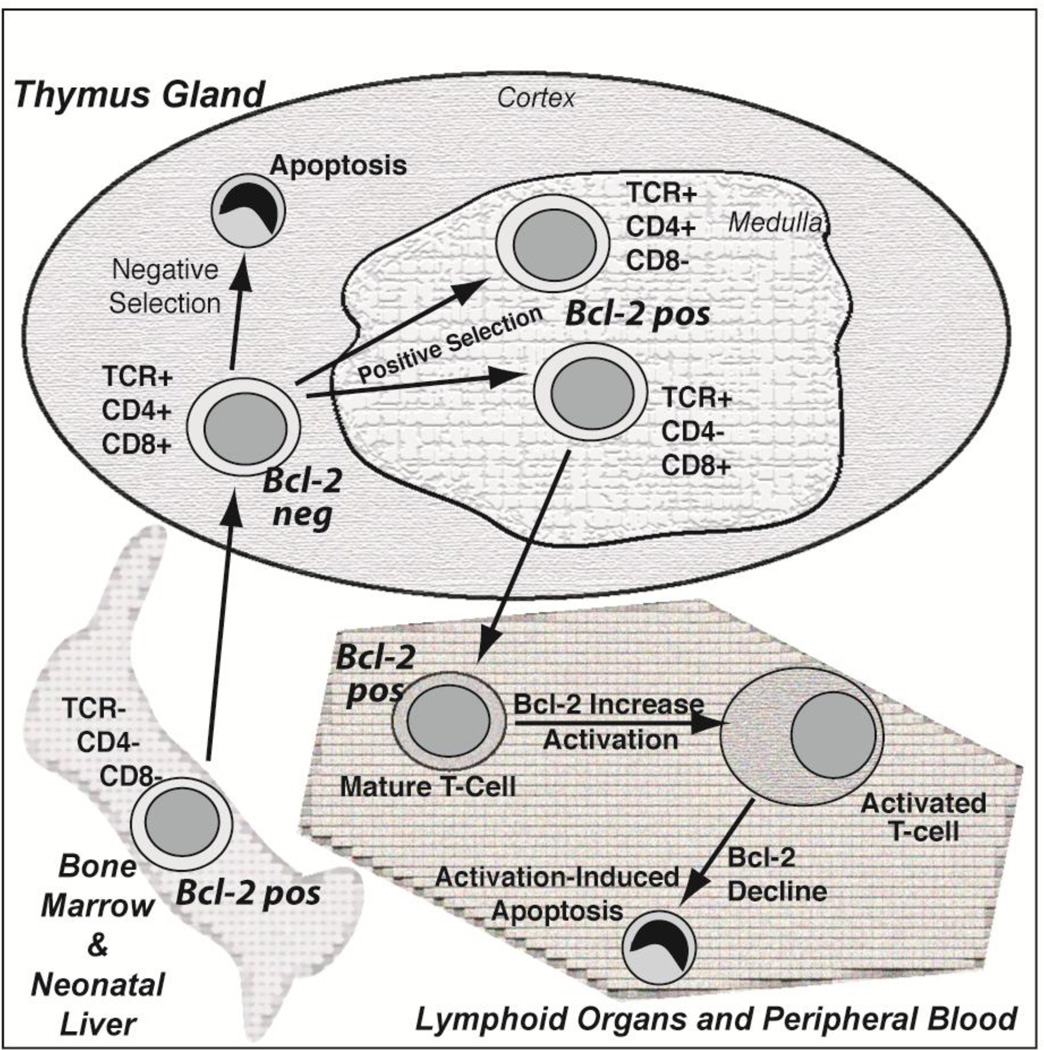
Our work has focused on the role of Bcl-2 in regulating InsP3R-mediated Ca2+ signals in T-cells. Bcl-2 is of critical importance in T-cell development and survival. The developing T-cell passes through successive maturational stages within the thymus [34] and Bcl-2 levels vary considerably throughout these different developmental stages [4, 35, 36] (Figure 4). The earliest precursors from the bone marrow or fetal liver do not express either the TCR or the CD4 and CD8 antigens (i.e., ‘double negative stage’), but do express Bcl-2: this provides an element of protection from apoptosis en route to the thymus gland. In the cortex of the thymus these immature T-cells express the TCR and both CD4 and CD8 antigens (i.e., ‘double positive stage’). Bcl-2 levels are down-regulated at this stage, increasing their sensitivity to Ca2+-induced apoptosis [4]. This facilitates a stringent test of whether or not the T-cells respond to self-antigens, with strong responders undergoing apoptosis (‘negative selection’) and weak responders avoiding apoptosis (‘positive selection’) [37, 38]. During negative selection, apoptosis is induced by Ca2+-dependent up-regulation of the pro-apoptotic Bcl-2 family member Bim [39]. Surviving cells advance to the ‘single positive stage’ (CD4+/CD8−, CD4−/CD8+), where Bcl-2 levels are increased, and enter the circulation to mount immune responses to foreign antigens as mature T cells. Bcl-2 levels are further elevated when a T-cell responds to antigenic stimulation and proliferates, but them decline as the immune response wanes and the T-cell dies, a process referred to as activation-induced cell death.
Figure 4. Dynamic fluctuations in Bcl-2 levels throughout T-cell development and adult life.
The complexity of the immune system is dependent, in part, upon variation in Bcl-2 expression. Bcl-2 levels are sufficient to repress apoptosis during the earliest stages of lymphocyte precursor development in the fetal liver and bone marrow, and during the journey from these distant organs to the thymus gland. Bcl-2 levels then decline upon entry into the thymic cortex, allowing for apoptotic death of most young thymocytes through negative selection, a process that deletes thymocytes that strongly react with self-antigens. Thymocytes that only weakly react with self-antigens are allowed survive in a process of positive selection and enter the thymic medulla where Bcl-2 levels become elevated in preparation for survival of mature lymphocytes in peripheral organs including blood and lymph nodes. Bcl-2 levels further increase when a mature lymphocyte is activated to proliferate in response to antigenic stimulation, but then decline later as the immune response declines, an apoptotic process referred to as activation-induced cell death.
The role of Bcl-2 in T-cell development was elegantly demonstrated by Bcl-2 knockout and over-expression strategies. The Bcl-2 knockout mouse, developed in the laboratory of Stanley Korsmeyer, demonstrated extensive lymphoid apoptosis [40]. Conversely, enforced expression of Bcl-2 in transgenic mice inhibited negative selection, causing excessive accumulation of thymocytes [41, 42]. Transgenic Bcl-2 inhibits negative selection by a mechanism independent of its ability to antagonize Bax, suggesting a role for Bcl-2-mediated regulation of Ca2+ in this process [43, 44].
The InsP3R-mediated Ca2+ signals induced by T-cell receptor (TCR) activation are of critical physiological importance in the developing immune system [39, 45, 46]. Positive versus negative selection decisions in the thymus may be encoded by distinct Bcl-2-regulated Ca2+ signaling patterns [47]. T-cell activation by antigen binding to the TCR triggers a signaling cascade that activates phospholipase C-γ, generating InsP3. InsP3 binds to the InsP3R, inducing channel opening and Ca2+ release from the ER, thus stimulating T-cell proliferation [45, 48–50]. Depending on the strength of TCR activation, a variety of Ca2+ response patterns are generated, including transient Ca2+ elevation, sustained Ca2+ elevation, or Ca2+ oscillations [51, 52]. TCR activation by physiologically-relevant antigenic peptides in immature thymocytes produce a similar effect: negatively-selecting antigenic peptides induce a strong Ca2+ flux, whereas positively-selecting peptides induce a smaller Ca2+ flux [53]. Additionally, earlier studies in thymocytes demonstrated that cytoplasmic Ca2+ elevation following strong TCR activation by a high concentration of anti-CD3 antibody in vitro induces apoptosis, whereas cytoplasmic Ca2+ elevation induced by weak TCR activation using lower concentrations of anti-CD3 antibody do not trigger apoptosis [54].
5. Effect of Bcl-2 on Ca2+ signaling
We investigated the effect of Bcl-2 on Ca2+ signaling patterns in the murine thymocyte line, WEHI7.2. These cells correspond to the double positive stage of thymocyte development and thus have very low levels of Bcl-2. We find that strong TCR activation by a high concentration of anti-CD3 antibody induces a large transient Ca2+ elevation, whereas weak TCR activation by a low concentration of anti-CD3 antibody induces sustained Ca2+ oscillations [47]. These Ca2+ signaling patterns differ in two ways: high anti-CD3 induces a much more prolonged Ca2+ elevation than low anti-CD3 (> 4 min versus < 1 min); and high anti-CD3 triggers a higher peak Ca2+ amplitude than low anti-CD3 [47]. High amplitude Ca2+ elevation, particularly if continuous and sustained, triggers cell death [32, 33]. Thus, consistent with the earlier findings of other investigators, the critical determinant of whether or not TCR stimulation induces apoptosis appears to lie in both the duration and amplitude of the Ca2+ elevation.
We find that Bcl-2 selectively inhibits the pro-apoptotic Ca2+ elevation induced by strong TCR activation while enhancing the pro-survival Ca2+ oscillations induced by weak TCR activation [47]. The positive effect of Bcl-2 on Ca2+ oscillations and its pro-survival effects are consistent with a number of other findings. For example, Ca2+ oscillations regulate thymocyte motility during positive selection, thereby modulating interactions with stromal cells [55]. Ca2+ oscillations also lead to a sustained activation of CaN [38], which dephosphorylates and thereby activates NFAT, increasing expression of the gene encoding the cytokine interleukin-2 [48, 51, 56].
Moreover, although this review emphasizes Bcl-2-InsP3R interaction in the context of regulating cell death, the regulatory role of this interaction extends far beyond cell death to include many processes in which Ca2+ signaling plays important roles. As an example, elegant experiments by Gillet and coworkers [57] indicate that the zebrafish homolog of Bcl-2, Nrz, interacts with InsP3Rs and controls cytoskeletal dynamics via the regulation of Ca2+ trafficking. Cytoskeletal dynamics are important in cancer cell migration and metastasis and Bcl-2, by regulating Ca2+, plays an important role in tumor metastasis and increased tumor vascularity [58].
How Bcl-2 regulates InsP3R-mediated Ca2+ release is a major focus in our laboratory. We discovered that Bcl-2 interacts with the InsP3R [6] and that this interaction involves binding of the BH4 domain of Bcl-2 to a region located within the regulatory and coupling domain of the InsP3R [59, 60] (Figure 1). We synthesized a 20 amino acid peptide corresponding to the Bcl-2 interaction site on the InsP3R and found that this peptide, which we refer to as InsP3R-Derived Peptide (IDP), functions as a decoy peptide that binds to Bcl-2 and inhibits Bcl-2-InsP3R interaction. This peptide, we find, reverses the inhibitory effect of Bcl-2 on InsP3R-mediated Ca2+ elevation in T-cells treated with high concentrations of anti-CD3 antibody [59, 60]. This peptide has proven to be a valuable tool in studies of Bcl-2-InsP3R interaction.
Other anti-apoptotic Bcl-2 family members, including Bcl-xl and Mcl-1, also interact with InsP3Rs and regulate InsP3R-mediated Ca2+ release [50, 61]. Although these anti-apoptotic family members, and Bcl-2, decrease ER luminal Ca2+ concentration, this has not been observed in our studies [6, 47, 62]. A recent report also indicates that Bcl-2 may not interact with InsP3Rs in all circumstances or cell types [63], raising the important question of what actually regulates the Bcl-2-InsP3R interaction in different types of cells.
Bcl-2 may also regulate ER Ca2+ release through other mechanisms besides its interaction with the InsP3R. One proposed mechanism involves Bcl-2 interaction with Sarcoplasmic/Endoplasmic Reticulum-associated Ca2+-ATPases (SERCA). These proteins pump Ca2+ ions from the cytoplasm into the ER lumen, maintaining large ER luminal Ca2+ stores. This steep Ca2+ concentration gradient from ER lumen to cytoplasm facilitates Ca2+ efflux from the ER lumen via InsP3R channel opening, leading to cytoplasmic Ca2+ elevation. Bcl-2’s interaction with SERCA attenuates ER Ca2+ filling, indirectly diminishing InsP3R-mediated Ca2+ release and Ca2+-mediated apoptosis [64, 65]. Recent findings indicate that HSP70 regulates the Bcl-2-SERCA interaction, maintaining SERCA in an active state that may be essential for apoptosis regulation [66]. Accordingly, an earlier report of the Bcl-2-SERCA interaction finds that Bcl-2 increases the ER Ca2+ pool, promoting the high luminal Ca2+ concentration required for normal cell function [67].
6. How Bcl-2-InsP3R Interaction Regulates InsP3-mediated Ca2+ Release
The preceding findings illustrate the importance of InsP3R-mediated Ca2+ signals in T-cells, and the role of Bcl-2 in regulating these signals. However, it has not been determined how Bcl-2 regulates InsP3R-mediated Ca2+ elevation through its interaction with the InsP3R. Oakes et al [68] show that Bcl-2 regulates InsP3R phosphorylation at serine 1755 within the regulatory and coupling domain of the InsP3R in murine embryonic fibroblasts. Protein kinase A (PKA) phosphorylates serine 1755 and serine 1589 of the InsP3R, increasing InsP3-mediated channel opening and Ca2+ release [64, 69]. We previously reported that Bcl-2 decreases InsP3R phosphorylation, although a specific phosphorylation site was not identified [6]. In recent work, we find that Bcl-2 inhibits InsP3R phosphorylation at serine 1755, correlating with its inhibition of anti-CD3-induced Ca2+ elevation.
The mechanism underlying Bcl-2 regulation of InsP3R phosphorylation following TCR activation is under investigation in our laboratory. PKA-mediated protein phosphorylation is typically regulated by PP1α [70]. Tang et al [71] discovered a direct association between PP1α and InsP3R-1 and established that the association with PP1α reverses PKA-mediated InsP3R-1 phosphorylation. Similarly, others have shown that AKAP9, a multifunctional PKA anchoring protein, docks both PKA and PP1α to InsP3R-1 [72]. Moreover, an InsP3-RPP1α complex has been implicated in Bcl-2-mediated suppression of ER Ca2+ release in breast cancer cells [70]. Bcl-2 also binds CaN [21] and increases the association of CaN with InsP3Rs [72, 73]; this has a neuroprotective effect in primary neuronal cells [73]. Knowledge that Bcl-2 binds CaN, together with evidence that PP1α reverses PKA-mediated InsP3R-1 phosphorylation, stimulated us to hypothesize a role for DARPP-32 (dopamine- and c-AMP-regulated phosphoprotein of 32 kDa) in the regulation of InsP3R-mediated Ca2+ elevation by Bcl-2.
DARPP-32 is a PKA-activated and CaN-deactivated PP1α inhibitor studied extensively in the brain [74]. In experiments with medium spiny neurons from DARPP-32 knockout mice, DARPP-32 was shown to regulate dopamine-induced Ca2+ oscillations [75]. However, very little is known about the role of DARPP-32 in peripheral tissues, including lymphocytes, although DARPP-32 has been shown to increase the phosphorylation and activity of various ion channels [76]. We recently found that Bcl-2 prevents exaggerated InsP3R-mediated Ca2+ elevation in T-cells by decreasing InsP3R phosphorylation through a feedback mechanism involving DARPP32 and CaN [77].
Although these recent findings establish a role for Bcl-2 in regulating InsP3R phosphorylation, other potential mechanisms by which Bcl-2 and/or other anti-apoptotic members of the Bcl-2 family regulate InsP3R-mediated Ca2+ signaling should be considered also. For example, one report suggested that the Bcl-2 homologue Bcl-xl, affects Ca2+ homeostasis by altering InsP3Rs levels [78]. More recent evidence indicates that the Bcl-2 protein family member Bok binds to InsP3Rs and protects them from proteolytic cleavage, although not governing the ability of InsP3Rs to release Ca2+ [63]. Also, the possibility that certain Bcl-2 family members may regulate InsP3 binding affinity should be considered [79].
7. Exploitation of the Bcl-2-InsP3R Interaction by Cancer Cells and Targeting the Bcl-2-InsP3R Interaction for Cancer Treatment
Anti-apoptotic Bcl-2 family members such as Bcl-2, Bcl-xl and Mcl-1 play major roles in tumorigenesis by prolonging cancer cell survival (reviewed in 80). Anti-apoptotic Bcl-2 family members also regulate the migration and invasion of colorectal cancer cells [81]. In general, Bcl-2 is prominently expressed in leukemia and lymphoma cells, whereas Mcl-1 is highly expressed in solid tumors in addition to lymphoid malignancies [82]. Moreover, ion channels such as the InsP3R play extensive roles in regulating cell proliferation and cell death, and are thus emerging as promising targets for cancer treatment [83]. Importantly, cancer cells remodel Bcl-2-regulated intracellular Ca2+ fluxes to promote cell proliferation and avoid cell death [58, 84]. This remodeling has important therapeutic implications.
Our work indicates that Bcl-2 promotes cancer cell survival by interacting with InsP3Rs to prevent pro-apoptotic Ca2+ elevation (Figure 1), in addition to its known role in binding and inhibiting pro-apoptotic family members. Small molecules that bind to the hydrophobic cleft formed by the BH1-3 domains of Bcl-2 displace pro-apoptotic proteins from Bcl-2 and thus trigger apoptosis [15, 16]. These molecules, including the Bcl-2 selective and platelet-sparing ABT-199, are already in clinical trials for cancers as diverse as lymphoid malignancies, myeloid malignancies and breast cancer [15, 16, 85]. However, cancer cells become resistant to virtually any single therapeutic approach. Therefore, it is essential to target cancer cells from multiple angles if one hopes to achieve a cure. For this reason, efforts are underway to target the Bcl-2-InsP3R interaction. As summarized above, we have developed a synthetic peptide corresponding to the InsP3R binding site for Bcl-2 [59, 60] (Figure 1). This InsP3R-Derived Peptide (IDP) inhibits the Bcl-2-InsP3R interaction by binding to the BH4 domain of Bcl-2, destabilizing Bcl-2’s alpha-helical structure [59, 60, 86]. By inhibiting the Bcl-2-InsP3R interaction, IDP and its protease resistant analog IDPDD/AA decreases Bcl-2’s control over InsP3R-mediated Ca2+ elevation.
Bcl-2 elevation is a hallmark of chronic lymphocytic leukemia (CLL), the most common form of leukemia in the Western world. Initially, CLL runs an indolent clinical course, providing a unique opportunity to investigate primary CLL cells before they are subjected to chemotherapy. We find that IDPDD/AA-mediated inhibition of Bcl-2-InsP3R interaction induces marked Ca2+ elevation and Ca2+-mediated apoptosis in primary human CLL cells, with minimal if any effect on the viability of normal human lymphocytes [9]. IDPDD/AA also induces apoptosis in Bcl-2-positive cell lines representing the B-cell malignancies, including diffuse large cell lymphoma [87]. Also, in a preliminary study in CLL cells, IDPDD/AA and the BH3-mimetic ABT-737 displayed synergistic cytotoxicity [8]. If confirmed by additional in vivo testing, these findings will underscore the value of simultaneously targeting Bcl-2’s interaction with both InsP3Rs and pro-apoptotic family members for cancer treatment.
The usefulness of targeting the Bcl-2-InsP3R interaction may be dependent upon a number of factors. One factor is the InsP3R isoform expressed in different types of malignant cells. There are three InsP3R isoforms, which vary in both tissue distribution and in sensitivity to Ca2+ and InsP3 regulation [88]. A recent study discovered that the sensitivity of lymphoma cells to IDPDD/AA-induced apoptosis correlated with InsP3R-2 isoform rather than InsP3R-1 or InsP3R-3 [87], which suggests the InsP3R isoform expression in the malignancy being treated will need to be considered in order to yield optimal therapeutic success. Another factor is the level of Bcl-2 in different types of cancer and the reliance of various cancers on Bcl-2 for their survival [82]. Bcl-2 is typically expressed in lymphoid malignancies, whereas other anti-apoptotic Bcl-2 family members, such as Mcl-1, predominate in non-lymphoid malignancies. Individual anti-apoptotic family members may also differ in their interaction with InsP3Rs [89]. Also, recent evidence indicates that the BH4 domain of Bcl-xL binds to a different region on the InsP3R than Bcl-2 [86]. Therefore, IDPDD/AA is more likely to be effective in killing malignant cells where Bcl-2 levels predominate over Bcl-xL levels.
8. Summary and Future Directions
This review has emphasized the role of the Bcl-2 protein in regulating InsP3R-mediated Ca2+ elevation, both that which mediates normal cell function and that which can induce cell death. Moreover, the review has focused primarily on this function of Bcl-2 in the contexts of lymphocyte function and lymphoid malignancy. This focus should not be interpreted as an indication, or even a suggestion, that Bcl-2 is the only family member that regulates InsP3R-mediated Ca2+ release, as indeed a number of Bcl-2 family members, including both anti-apoptotic and pro-apoptotic, are known to regulate Ca2+ release from the ER. Similarly, the main emphasis on lymphoid malignancies should not suggest that these are the only malignancies in which Bcl-2 family members and Ca2+ regulation are important. The reader is referred to an extensive review by Roderick and Cook [84] of the many ways that Ca2+ signaling toolkit is remodeled by cancer cells to promote their proliferation and survival. In addition, the role of Ca2+ signaling in tumor cell migration and metastasis, mentioned earlier in this review, has been thoughtfully reviewed [58].
In sum, the discovery that Bcl-2 and its family members interact with InsP3Rs and regulate InsP3-induced Ca2+ signals has brought widespread attention to Ca2+ signaling and its exploitation by cancer cells. Yet, this area of research deserves more recognition among cancer investigators than it seems to garner. This is likely due to the complexity of Ca2+ signaling and the specialized nature of techniques employed to study Ca2+ and Ca2+ signaling. With this oversight comes a loss of opportunity to target Ca2+ signaling pathways for cancer treatment. The presently narrow emphasis of the cancer research field on genomics is sure to wane as the rate of progress plateaus and the interest turns back to biology and physiology, since it may prove difficult to correct defective genes and a better investment of time and resources to develop therapies based on the output of defective genes. We already witness this trend with the skyrocketing interest in autophagy and metabolism, processes involving InsP3Rs and Ca2+ signaling.
For the immediate future, in depth analysis of the differences between Bcl-2 family members in terms of their sites of interaction with InsP3Rs and their differential effects on Ca2+ signaling is likely to generate new ideas and understanding, and to better elucidate the various roles of Ca2+ in cancer. In addition, the next steps need to move beyond the present emphasis on Ca2+ in processes such as apoptosis and autophagy, to expand our knowledge of the role of Ca2+ in metastasis, which remains the major obstacle in our quest for cancer cure.
Highlights.
Marked elevation of intracellular calcium induces apoptosis.
Inositol 1,4,5-trisphosphate receptors mediate calcium elevation.
Bcl-2 binds to inositol 1,4,5-trisphosphate receptors to control calcium elevation.
Bcl-2 inhibits pro-apoptotic calcium elevation in cancer cells.
The control of calcium by Bcl-2 is a potential target for cancer therapy.
Acknowledgements
This work was supported by NIH grants RO1 CA085804 (CWD), 5T32HL007147 (AL) and 5T32GM007250 (AL). EG is supported by the Physician-Scientist Program in the Department of Medicine, MetroHealth Medical Center and Case Western Reserve University School of Medicine.
Abbreviations
- Bcl-2
B-cell leukemia/lymphoma-2
- BH
Bcl-2 homology
- CaN
calcineurin
- ER
endoplasmic reticulum
- IDP
inositol 1,4,5-trisphosphate receptor-derived peptide
- InsP3
inositol 1,4,5-trisphosphate
- InsP3R
inositol 1,4,5-trisphosphate receptor
- SERCA
Sarcoplasmic/Endoplasmic Reticulum-associated Ca2+-ATPase
- TCR
T-cell receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Edward F. Greenberg, Email: e.f.greenberg@gmail.com.
Andrew R. Lavik, Email: arl18@case.edu.
References
- 1.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 2.Tsujimoto Y, Croce CM. Proc. Natl. Acad. Sci. USA. 1986;83:5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaux DL, Cory S, Adams J. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 4.Andjelic S, Jain N, Nikolic-Zugic J. J. Exp. Med. 1993;178:1745–1751. doi: 10.1084/jem.178.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zornig M, Busch G, Beneke R, Gulbins E, Lang F, Ma A, Korsmeyer S, Moroy T. Oncogene. 1995;11:2165–2174. [PubMed] [Google Scholar]
- 6.Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, Velez P, Distelhorst CW. J Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. Nature Cell Biology. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rong Y, Barr P, Yee VC, Distelhorst CW. Biochim Biophys Acta. 2009;1793:971–978. doi: 10.1016/j.bbamcr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong F, Harr MW, Bultynck G, Monaco G, Parys JB, DeSmedt H, Rong Y-P, Molitoris JK, Lam M, Ryder C, Matsuyama S, Distelhorst CW. Blood. 2011;117:2924–2934. doi: 10.1182/blood-2010-09-307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berridge MJ, Bootman MD, Roderick HL. Nature Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 11.Parys JB, De Smedt H. Adv Exp Med Biol. 2012;740:255–279. doi: 10.1007/978-94-007-2888-2_11. [DOI] [PubMed] [Google Scholar]
- 12.Youle RJ, Strasser A. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 13.Shamas-Din A, Kale J, Leber B, Andrews DW. Cold Spring Harb Perspect Biol. 2013;5:a008714. doi: 10.1101/cshperspect.a008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aouacheria A, Rech de Laval V, Combet C, Hardwick JM. Trends Cell Biol. 2013;23:103–111. doi: 10.1016/j.tcb.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billard C. Mol Cancer Ther. 2013;12:1691–1700. doi: 10.1158/1535-7163.MCT-13-0058. [DOI] [PubMed] [Google Scholar]
- 16.Vandenberg CJ, Cory S. Blood. 2013;121:2285–2288. doi: 10.1182/blood-2013-01-475855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annis MG, Yethon JA, Leber B, Andrews DW. Biochim. Biophys. Acta. 2004;1644:115–123. doi: 10.1016/j.bbamcr.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Thomenius MJ, Wang NS, Reineks EZ, Wang Z, Distelhorst CW. J Biol Chem. 2003;278:6243–6250. doi: 10.1074/jbc.M208878200. [DOI] [PubMed] [Google Scholar]
- 20.Wang H-G, Rapp UR, Reed JC. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 21.Shibasaki F, Kondo E, Akagi T, McKeon F. Nature. 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- 22.Clapham DE. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 24.Hajnoczky G, Csordas G, Krishnamurthy R, Szalai G. J Bioenerg Biomembr. 2000;32:15–25. doi: 10.1023/a:1005504210587. [DOI] [PubMed] [Google Scholar]
- 25.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 26.Drago I, Pizzo P, Pozzan T. EMBO J. 2011;30:4119–4125. doi: 10.1038/emboj.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutter GA, Rizzuto R. Trends Biochem Sci. 2000;25:215–221. doi: 10.1016/s0968-0004(00)01585-1. [DOI] [PubMed] [Google Scholar]
- 28.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardenas C, Miller RA, Simth I, Bui T, Molgo J, Muller M, Vais H, Cheung K-H, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szalai G, Krishnamurthy R, Hajnoczky G. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha RS, Yi M. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orrenius S, Zhivotovsky B, Nicotera P. Nature Rev. Mol. Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 33.Joseph SK, Hajnoczky G. Apoptosis. 2007;12:951–968. doi: 10.1007/s10495-007-0719-7. [DOI] [PubMed] [Google Scholar]
- 34.Takahama Y. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 35.Hockenberry DM, Zutter M, Hickey W, Nahm M, Korsmeyer SJ. Proc. Natl. Acad. Sci. USA. 1991;88:6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 37.Hogquist KA. Curr Opinion Immunol. 2001;13:225–231. doi: 10.1016/s0952-7915(00)00208-9. [DOI] [PubMed] [Google Scholar]
- 38.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 39.Cante-Barrett K, Gallo EM, Winslow MM, Crabtree GR. J Immunol. 2006;176:2299–2306. doi: 10.4049/jimmunol.176.4.2299. [DOI] [PubMed] [Google Scholar]
- 40.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 41.Strasser A, Harris AW, Cory S. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 42.Strasser A, Harris AW, von Boehmer H, Cory S. Proc Natl Acad Sci U S A. 1994;91:1376–1380. doi: 10.1073/pnas.91.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St Clair EG, Anderson SJ, Oltvai ZN. J Biol Chem. 1997;272:29347–29355. doi: 10.1074/jbc.272.46.29347. [DOI] [PubMed] [Google Scholar]
- 44.Williams O, Halligey NTM, Kioussis D, Brady HJM. J Exp Med. 1998;188:1125–1133. doi: 10.1084/jem.188.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berridge MJ. Critical Reviews in Immunology. 1997;17:155–178. doi: 10.1615/critrevimmunol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- 46.Gallo EM, Cante-Barrett K, Crabtree GR. Nat Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- 47.Zhong F, Davis MC, McColl KS, Distelhorst CW. J Cell Biol. 2006;172:127–137. doi: 10.1083/jcb.200506189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis RS. Biochem Soc Transactions. 2003;31:925–929. doi: 10.1042/bst0310925. [DOI] [PubMed] [Google Scholar]
- 49.Fracchia KM, Pai CY, Walsh CM. Front Immunol. 2013;4:324. doi: 10.3389/fimmu.2013.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erin N, Billingsley ML. Brain Res. 2004;1014:45–52. doi: 10.1016/j.brainres.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 51.Randriamampita C, Trautmann A. Biology of the Cell. 2003;96:69–78. doi: 10.1016/j.biolcel.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Donnadieu E, Bismuth G, Trautmann A. J Biol Chem. 1992;267:25864–25872. [PubMed] [Google Scholar]
- 53.Mariathasan S, Bachmann MF, Bouchard D, Ohteki T, Ohashi PS. J Immunol. 1998;161:6030–6037. [PubMed] [Google Scholar]
- 54.McConkey DJ, Hartzell P, Amador-Perez JF, Orrenius S, Jondal M. J Immunol. 1989;143:1801–1806. [PubMed] [Google Scholar]
- 55.Bhakta NR, Oh DY, Lewis RS. Nat Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 56.Tomida T, Hirose K, Takizawa A, Shibasaki f, Lino M. Embo J. 2003;22:3825–3832. doi: 10.1093/emboj/cdg381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Popgeorgiev N, Bonneau B, Ferri KF, Prudent J, Thibaut J, Gillet G. Dev Cell. 2011;20:663–676. doi: 10.1016/j.devcel.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 58.Prevarskaya N, Skryma R, Shuba Y. Nat Rev Cancer. 2011;11:609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 59.Rong Y, Aromolaran AS, Bultynck G, Zhong F, Li X, McColl KS, Herlitze S, Matsuyama S, Roderick HL, Bootman MD, Mignery GA, Parys JB, DeSmedt H, Distelhorst CW. Mol Cell. 2008;31:255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rong Y, Bultynck G, Aromolaran AS, Zhong F, Parys JB, De Smedt H, Mignery GA, Roderick HL, Bootman MD, Distelhorst CW. Proc Natl Acad Sci. 2009;106:14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palmer AE, Jin C, Reed JC, Tsien RY. Proc Natl Acad Sci U S A. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanson CJ, Bootman MD, Distelhorst CW, Wojcikiewicz RJ, Roderick HL. Cell Calcium. 2008;44:324–338. doi: 10.1016/j.ceca.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Schulman JJ, Wright FA, Kaufmann T, Wojcikiewicz RJ. J Biol Chem. 2013;288:25340–25349. doi: 10.1074/jbc.M113.496570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Volpe P, Alderson-Lang BH. Am J Physiol. 1990;258:C1086–C1091. doi: 10.1152/ajpcell.1990.258.6.C1086. [DOI] [PubMed] [Google Scholar]
- 65.Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schoneich C. Biochem J. 2004;383:361–370. doi: 10.1042/BJ20040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dremina ES, Sharov VS, Schoneich C. Biochem J. 2012;444:127–139. doi: 10.1042/BJ20111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuo TH, Kim H-RC, Zhu L, Yu Y, Lin H-M, Tsang W. Oncogene. 1998;17:1903–1910. doi: 10.1038/sj.onc.1202110. [DOI] [PubMed] [Google Scholar]
- 68.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proc Natl Acad Sci U S A. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wagner LE, Joseph SK, Yule DI. J Physiol. 2008;586 doi: 10.1113/jphysiol.2008.152314. 357-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu L, Kong D, Zhu L, Zhu W, Andrews DW, Kuo TH. Mol Cell Biochem. 2007;295:153–165. doi: 10.1007/s11010-006-9285-5. [DOI] [PubMed] [Google Scholar]
- 71.Tang TS, Tu H, Wang Z, Bezprozvanny I. J Neurosci. 2003;23:403–415. doi: 10.1523/JNEUROSCI.23-02-00403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tu H, Tang T-s, Wang Z, Bezprozvanny I. J Biol Chem. 2004;279:19375–19382. doi: 10.1074/jbc.M313476200. [DOI] [PubMed] [Google Scholar]
- 73.Erin N, Lehman RAW, Boyer PJ, Billingsley ML. Neuroscience. 2003;117:557–565. doi: 10.1016/s0306-4522(02)00934-x. [DOI] [PubMed] [Google Scholar]
- 74.Walaas SI, Hemmings HC, Jr, Greengard P, Nairn AC. Front Neuroanat. 2011;5:1–17. doi: 10.3389/fnana.2011.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang TS, Bezprozvanny I. J Biol Chem. 2004;279:42082–42094. doi: 10.1074/jbc.M407389200. [DOI] [PubMed] [Google Scholar]
- 76.Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 77.Chang MJ, Zhong F, Lavik AR, Parys JB, Berridge MJ, Distelhorst CW. Proc Natl Acad Sci U S A. 2014;111:1186–1191. doi: 10.1073/pnas.1323098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li C, Fox CJ, Master SR, Bindokas VP, Chodosh LA, Thompson CB. Proc Natl Acad Sci U S A. 2002;99:9830–9835. [Google Scholar]
- 79.Bonneau B, Nougarede A, Prudent J, Popgeorgiev N, Peyrieras N, Rimokh R, Gillet G. Sci Signal. 2014;7:ra14. doi: 10.1126/scisignal.2004480. [DOI] [PubMed] [Google Scholar]
- 80.Kelly PN, Strasser A. Cell Death Differ. 2011;18:1414–1424. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koehler BC, Scherr AL, Lorenz S, Urbanik T, Kautz N, Elssner C, Welte S, Bermejo JL, Jager D, Schulze-Bergkamen H. PLoS One. 2013;8:e76446. doi: 10.1371/journal.pone.0076446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Placzek WJ, Wei J, Kitada S, Zhai D, Reed JC, Pellecchia M. Cell Death Dis. 2010;1:e40. doi: 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leanza L, Biasutto L, Manago A, Gulbins E, Zoratti M, Szabo I. Front Physiol. 2013;4:227. doi: 10.3389/fphys.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roderick HL, Cook SJ. Nat Rev Cancer. 2008;8:361–365. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 85.Juin P, Geneste O, Gautier F, Depil S, Campone M. Nat Rev Cancer. 2013;13:455–465. doi: 10.1038/nrc3538. [DOI] [PubMed] [Google Scholar]
- 86.Monaco G, Decrock E, Akl H, Ponsaerts R, Vervliet T, Luyten T, De Maeyer M, Missiaen L, Distelhorst CW, De Smedt H, Parys JB, Leybaert L, Bultynck G. Cell Death Differ. 2012;19:295–309. doi: 10.1038/cdd.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akl H, Monaco G, La Rovere R, Welkenhuyzen K, Kiviluoto S, Vervliet T, Molgo J, Distelhorst CW, Missiaen L, Mikoshiba K, Parys JB, De Smedt H, Bultynck G. Cell Death Dis. 2013;4:e632. doi: 10.1038/cddis.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thrower EC, Hagar RE, Ehrlich BE. TRENDS in Pharmacological Sciences. 2001;22:580–586. doi: 10.1016/s0165-6147(00)01809-5. [DOI] [PubMed] [Google Scholar]
- 89.Eckenrode EF, Yang J, Velmurugan GV, Foskett JK, White C. J Biol Chem. 2010;285:13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]