Abstract
An individual’s susceptibility to psychological and physical disorders associated with chronic stress exposure e.g., cardiovascular and infectious disease, may also be predicted by their reactivity to acute stress. One factor associated with both stress resilience and health outcomes is personality. An understanding of how personality influences responses to acute stress may shed light upon individual differences in susceptibility to chronic stress-linked disease.
This study examined relationships between personality and acute responses to stress in 125 healthy adults, using hierarchical linear regression. We assessed personality traits using the Multidimensional Personality Questionnaire (MPQ-BF), and responses to acute stress (cortisol, heart rate, blood pressure, mood) using a standardised laboratory psychosocial stress task, the Trier Social Stress Test (TSST).
Individuals with high Negative Emotionality exhibited greater emotional distress and lower blood pressure responses to the TSST. Individuals with high Agentic Positive Emotionality exhibited prolonged heart rate responses to stress, whereas those with high Communal Positive Emotionality exhibited smaller cortisol and blood pressure responses.
Separate personality traits differentially predicted emotional, cardiovascular, and cortisol responses to a psychosocial stressor in healthy volunteers. Future research investigating the association of personality with chronic stress-related disease may provide further clues to the relationship between acute stress reactivity and susceptibility to disease.
Keywords: personality, stress, cortisol, mood, heart rate, blood pressure, human
Introduction
Reactivity to acute stress has been linked to certain illnesses usually associated with longer term stress exposure including cardiovascular and infectious disease (Cohen et al. 1991; Chida and Steptoe 2010). In addition, maladaptive responses to acute stress have been associated with risk factors for psychological disorders, including depression and anxiety (Nemeroff 1988; Koob 1999; Bale 2005; Bale 2006). Since there is considerable inter-individual variation in psycholobiological responses to an acute stressor, factors that influence an individual’s reactivity to acute stress may also influence their susceptibility to these disorders. In this study, we examined individual differences in reactivity to a psychosocial stressor in relation to personality.
Personality can influence stress responses in several ways. Personality traits may shape how individuals perceive a given situation (Magnus et al. 1993; Kaplan 1996) or how they cope with and recover from a stressor (Strutton et al. 1995). In addition, personality may be related to stress responses because of shared underlying neurobiological systems (Gray 1970; Chida and Hamer 2008). Thus a better understanding of how personality predicts responses to acute stress may advance our understanding of the basic mechanisms that underlie the relationship between stress and health.
Responses to acute stress are multidimensional in nature; stress exposure activates the sympathetic nervous system and hypothalamic pituitary adrenal axis producing hormonal, cardiovascular and emotional responses. It is unclear how these components are related to each other because few studies have measured multiple components simultaneously in the same subjects (Thiagarajan et al. 1989; Britton et al. 1992; McCann et al. 1993; Gerra et al. 2001; Schommer et al. 2003). With regard to personality, existing data suggest that hormonal, cardiovascular and emotional responses to psychosocial stress may be predicted by different, non-overlapping factors. For instance, strong cortisol responses to stress have been predicted by high trait perfectionism and low novelty seeking, emotional intelligence and self-esteem (Kirschbaum et al. 1995; Pruessner et al. 1997; Oswald et al. 2006; Mikolajczak et al. 2007; Tyrka et al. 2007; Wirtz et al. 2007). Cardiovascular responses to stress have been predicted by Type-D personality (e.g. negative affectivity and social inhibition), aggression and Type-A behavior (e.g. hostility, competitiveness, time-urgency, Fichera and Andreassi 2000; Habra et al. 2003). Negative mood after stress has been predicted by high trait neuroticism and low trait novelty seeking and emotional intelligence (Bolger 1990; Bolger and Zuckerman 1995; Mikolajczak et al. 2007; Tyrka et al. 2007; Schneider et al. 2011). A recent meta-analysis of studies that assessed psychosocial factors and physiological stress responses (Chida and Hamer 2008) concluded that positive psychological traits (e.g. emotional intelligence, openness, self-esteem, religiosity/spirituality) protected against large hormonal stress responses, whereas Type-A behavior positively predicted and negative psychological traits (e.g. anxiety, neuroticism, negative affect) inversely predicted cardiovascular responses. Thus, overall the data suggest that personality modulates responses to acute stress and that the separate stress response components may be differentially influenced by distinct traits (Arnetz and Fjellner 1986; Bossert et al. 1988; Bohnen et al. 1991; Kirschbaum et al. 1992, 1995; van Eck et al. 1996; Schommer et al. 1999; Takahashi et al. 2005). However, despite observations that the different components of responses to acute stress may vary independently, many studies examine only a single measure of the stress response. Therefore, it is necessary to investigate personality influences on multiple measures of the stress response in the same subjects.
Here, we examined several facets of personality (Multidimensional Personality Questionnaire Brief Form, MPQ-BF; Patrick et al. 2002) in relation to responses to the Trier Social Stress Test (TSST; Kirschbaum et al. 1993) using a within-subject, multi-measure approach in healthy adults. The MPQ-BF and TSST are both standardised and widely used in personality and stress research respectively. The TSST is a psychosocial stress task that reliably induces changes in physiological and psychological dimensions (Dickerson and Kemeny 2004). We also included demographic characteristics that are known to influence acute stress responses (i.e., sex, menstrual cycle phase and race) in the analyses. We hypothesised, based on previous findings, that 1) Negative Emotionality would predict greater emotional distress and smaller cardiovascular responses, 2) Positive Emotionality would predict smaller cortisol responses to stress and 3) Constraint would positively predict cortisol and emotional responses.
Methods
Subjects
Data were taken from three studies that employed the TSST (Childs et al. 2010; Hamidovic et al. 2010; Dlugos et al. 2012; Total N=125, Samples 1-3 of Table 1). The studies used the same experimental methods, recruitment and screening procedures. Participants were healthy adults, aged 18-32, with body mass index 19-29 kg/m2. Exclusion criteria included smoking >20 cigarettes/week, a serious medical condition, a current or past year diagnosis of a Major Axis I psychiatric disorder (American Psychiatric Association, 1994), a history of substance dependence (including nicotine), an abnormal electrocardiogram, use of prescription medications including, in women, oral contraceptives, or night shift work. Women were assigned to participate in study sessions during either the follicular (days 3 and 10) or luteal (days 2-10 after a positive urine ovulation test) phase of the menstrual cycle. A 7-day time window was used for each phase so that women could schedule both experimental sessions within a single cycle and sessions were scheduled as near to the beginning of the time window as possible.
Table 1.
Demographic characteristics of study participants.
| Sample 137 | Sample 238 | Sample 339 | Total n | Significance | |
|---|---|---|---|---|---|
| N (Male/Female) | 51 (18/33) | 24 (8/16) | 50 (30/20) | 125 (56/69) | 3>1,2* |
|
| |||||
| Race (N / %) | |||||
| European American | 30 | 12 | 36 | 78 | |
| African American | 9 | 8 | 5 | 22 | |
| Other | 12 | 4 | 9 | 25 | |
|
| |||||
| Age (yrs) | 20.6±0.4 | 23.9±0.9 | 20.8±0.3 | 21.3±0.3 | 2>1,3* |
|
| |||||
| BMI (kg/m2) | 22.1±0.3 | 22.6±0.4 | 22.8±0.4 | 22.5±0.2 | |
|
| |||||
| Current Drug Use | |||||
| Caffeine (drinks/wk) | 6.1±1.2 | 6.3±1.4 | 7.3±0.9 | 6.6±0.7 | |
| Alcohol (drinks/wk) | 3.1±0.5 | 2.2±0.6 | 7.7±1.1 | 4.8±0.5 | 3>1,2* |
| Cigarettes (per wk) | 0.5±0.4 | 0.2±0.2 | 8.2±1.3 | 3.5±0.6 | 3>1,2* |
|
| |||||
| Personality | |||||
| Absorption | 6.3±0.4 | 6.3±0.6 | 7.5±0.4 | 6.8±0.3 | |
| Agenic PEM | 62.4±1.9 | 64.8±1.7 | 60.7±2.0 | 62.2±1.1 | |
| Communal PEM | 64.0±2.2 | 72.5±2.8 | 69.8±2.0 | 67.9±1.3 | |
| NEM | 28.7±1.5 | 20.9±1.3 | 33.7±2.3 | 29.2±1.2 | 2<1, 3* |
| CON | 70.9±2.3 | 74.3±4.0 | 64.3±2.7 | 68.9±1.7 | |
Values indicate mean±SD. Asterisks indicate a significant difference between the studies (p<0.05, assessed using one factor (Group) ANOVA for continuous variables or Chi-squared analysis for categorical variables).
Procedure
The University of Chicago Hospital’s Institutional Review Committee for the use of human subjects approved the study protocols. Participants completed two sessions at least 48h apart, one with the TSST and another with a non-stressful control task, in randomized order. Each task lasted 10 min. The TSST consisted of a 5min speech and 5min mental arithmetic (serial subtraction) performed before a panel of interviewers (mixed sex) who were unknown to the participant and who provided no positive feedback. Participants performed a non-stressful control task on a separate day to account for diurnal rhythms in mood and physiology (Childs and de Wit 2009; Het et al. 2009; Childs et al. 2010; Lovallo et al. 2010). During the control task, participants spoke to the research assistant (female) for 10min about neutral topics e.g., their interests and hobbies. Before and at repeated times after the tasks, participants rated their mood, saliva samples were obtained for cortisol analysis and vital signs were obtained. Two of the studies were conducted in the morning (Childs et al. 2010; Dlugos et al. 2012) and one was conducted in the afternoon (Hamidovic et al. 2010); therefore, Time of Day was included as a variable in the analysis.
Dependent Measures
Saliva samples were collected using Salivette® cotton wads (Sarstedt Inc., Newton, NC) at −20, 0, 10, 20 and 30min after the tasks and were analysed by the Core Laboratory at the University of Chicago Hospitals General Clinical Research Center for levels of cortisol (Salimetrics LLC, State College, PA, sensitivity=0.003 ug/dL). Heart rate (HR) was measured continuously throughout the experimental session (one reading per minute) using a Polar chest band and monitor (Mini-Logger, Mini Mitter/Respironics, Bend, OR). Scores were averaged over consecutive 10min periods. Blood pressure was measured using a monitor (Critikon Dinamap Plus Vital Signs Monitor, GE Healthcare Technologies, Waukesha, WI) at −20, 0, 10, 20 and 30min after the tasks and mean arterial pressure (MAP) was calculated using the formula MAP=2/3*Diastolic + 1/3*Systolic. Self-reported mood was measured using the Positive Mood scale of the Profile of Mood States (POMS, McNair, Lorr et al. 1971) at −20, 0, 10 and 30min after the task. This measure is a sum of two primary scales measuring positive and negative affect; Positive Mood = Elation (e.g. happy, satisfied) minus Depression (e.g. unhappy, discouraged). The TSST typically decreases Positive Mood scores, i.e. it increases emotional distress.
Two markers of stress reactivity were calculated for the outcome measures; 1) peak change from pre-task baseline, termed the peak response = maximum value post-task – pre-task value, and 2) area under the curve relative to the pre-task baseline, termed the total response (Pruessner et al. 2003) calculated using the trapezoidal method (Altman 1991). The peak response provides an indication of system reactivity while the total response provides information about the duration and recovery of homeostasis after stress exposure. For each marker, we calculated the net response e.g., Peak ResponseTSST minus Peak ResponseControl which were entered as the dependent variable in separate regression models.
Personality Measures
We assessed personality traits using the MPQ-BF (Patrick et al. 2002). This questionnaire is an empirically-derived personality instrument with an orthogonal factor structure that yields 11 well-defined primary trait scores and three super-factors termed Positive Emotionality (PEM), Negative Emotionality (NEM) and Constraint (CON). PEM measures an individual’s tendency to experience positive emotions and to actively engage with social and work environments (i.e., extraversion). It is primarily composed of the Social Potency, Achievement, Social Closeness and Well Being scales. PEM may be divided into Agentic (AgPEM) and Communal (ComPEM) components which reflect tendencies toward social dominance and affiliation respectively, and we assessed the influences of these two facets of PEM separately. NEM taps an individual’s tendency to negative emotions including anxiety and anger, and largely consists of the Stress Reaction, Alienation and Aggression scales (i.e., neuroticism). The CON super-factor indicates an individual’s tendency to behavioural spontaneity, including cautiousness, planning and conventional tendencies, and is predominantly composed of the Control, Harm Avoidance and Traditionalism scales (i.e., inverse of impulsivity). The Absorption scale (ABS) provides an index of mental spontaneity and openness to absorbing and self-altering experiences. It is considered orthogonal to the three super-factors. We included the superfactors AgPEM, ComPEM, NEM, CON, and the ABS scale in the analyses as they represent summary outcomes of the primary trait scales and reflect constructs of emotion and temperament with a significant psychobiological basis (Patrick et al. 2002).
Statistical Analysis
1) Sample Characteristics
We compared participant demographics and personality characteristics between the three studies, and also between men and women using independent samples t-test for continuous variables and chi-squared analysis for categorical variables.
2) Manipulation Check
We confirmed the efficacy of the stress manipulation by assessing task effects upon the outcome measures using two-factor (Task*Time) repeated-measures analysis of variance. Cortisol data were transformed prior to analysis using natural logarithms to better approximate a normal distribution.
3) Relationships between Personality and Stress Responses
We first conducted simple correlations between baseline values for each outcome measure (mean of pre-TSST and pre-Control task values) and the personality super-factors (AgPEM, ComPEM, NEM, CON and ABS) to determine any relationships present before behavioural manipulations. We then used hierarchical linear modeling to regress AgPEM, ComPEM, NEM, CON and ABS on stress reactivity on each outcome measure, controlling for time of day (am vs. pm), sex and menstrual cycle phase (men vs. follicular vs. luteal), and race. Control variables that did not contribute significant variance were removed from the final models. Continuous variables were centred prior to entry in the models. We conducted analyses for each outcome separately because it is unclear how the measures relate to each other and they may be considered distinct components (Schommer et al. 2003). The personality super-factors that were a significant predictor of stress responses were further explored by examining simple correlations between the primary personality traits contributing to that super-factor and the outcome measure. All analyses were conducted using SPSS v19 for windows. Effect sizes are reported using Cohen’s f2; 0.02, 0.15 and 0.35 represent small, medium and large effects respectively.
Personality information was not available for thirteen participants and the validity scale of the MPQ-BF indicated inconsistent responses for three participants, thus the total sample size for the study was N=125. Some data points were missing due to sample loss or equipment failure, thus there were minor variations in samples sizes between analyses.
Results
Sample characteristics
Most participants were European American (78%) light or non-smokers (3.6 ± 0.7 cigarettes.wk−1) in their early twenties (21.4 ± 0.3 years, see Table 1). Men and women did not differ significantly on any of the personality super-factors. At baseline, ratings of Positive Mood were positively correlated with AgPEM and ComPEM scores and inversely correlated with NEM scores. That is, individuals who scored higher on PEM and lower on NEM tended to rate their mood more positively at baseline. Also, baseline HR was negatively correlated with ABS scores. That is individuals with higher ABS scale scores tended to exhibit low resting HR.
Manipulation check
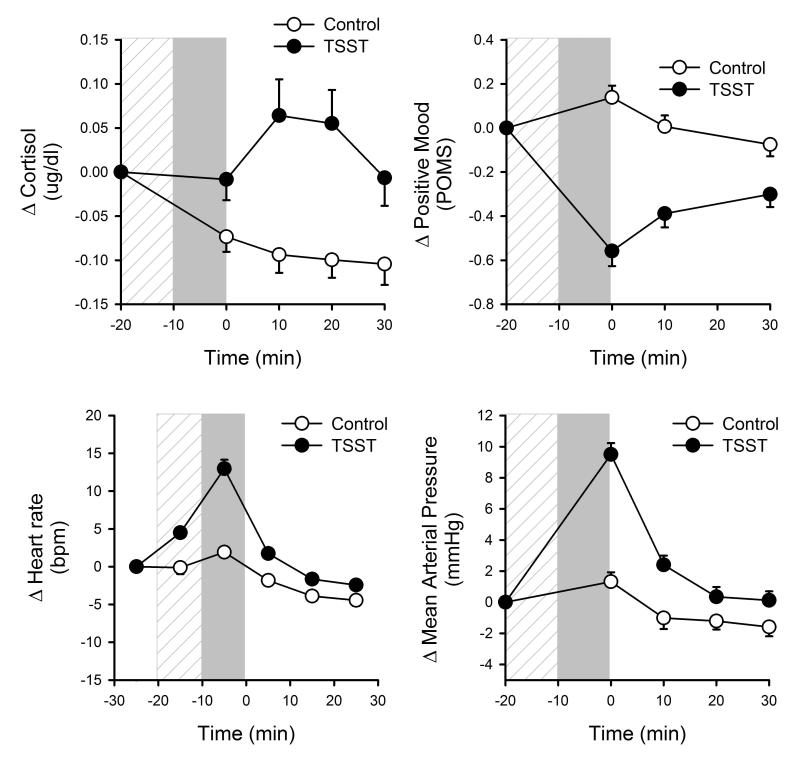
The TSST significantly increased participants’ salivary cortisol, heart rate and mean arterial pressure, and decreased positive mood, compared to the control task (Figure 1). Changes in cortisol, mood and heart rate after stress did not differ between the three studies. MAP response was greater when the TSST was performed in the afternoon i.e., in Study 3 [Task*Time*Time of Day, F(3,118)=3.64 p<0.02]. There is a well-known diurnal rhythm in blood pressure which peaks in the morning; thus, in the afternoon baseline blood pressure is lower and the stress effect is greater [Morning: Task*Time, F(3,72)=8.18 p<0.001, Afternoon: Task*Time, F(3,44)=21.69 p<0.001].
Figure 1.
Changes in salivary cortisol, POMS Positive Mood, heart rate and mean arterial pressure across time in relation to completion of the TSST and control tasks. Hatched bar indicates preparatory period and filled bar indicates task period.
Relationships between stress and personality
Table 2 shows the final models for regression of personality factors, controlling for demographic and experimental variables, upon cortisol, cardiovascular and emotional responses to the TSST.
Table 2.
Final regression models of personality factors upon physiological and psychological responses to acute psychosocial stress, controlling for demographic and experimental and variables.
| Predictor | r | β | t | R2 | R2 Change | FChange | |
|---|---|---|---|---|---|---|---|
| Peak Change Cortisol | |||||||
| Step 1 | Sex | 0.05 | 0.05 | 3.27* | |||
| Men vs. Foll | −0.22 | −0.23 | −2.41* | ||||
| Men vs. Lut | −0.18 | −0.20 | −1.96* | ||||
| Step 2 | Race | 0.09 | 0.04 | 2.38 | |||
| EA vs. AA | −0.08 | −0.08 | −0.80 | ||||
| EA vs. Other | 0.19 | 0.19 | 2.02* | ||||
| Step 3 | Personality | 0.13 | 0.04 | 1.00 | |||
| Absorption | −0.04 | −0.05 | −0.46 | ||||
| Constraint | 0.05 | 0.06 | 0.54 | ||||
| Agentic PEM | 0.12 | 0.12 | 1.27 | ||||
| Communal PEM | −0.19 | −0.19 | −2.00* | ||||
| NEM | 0.02 | 0.02 | 0.21 | ||||
|
| |||||||
| AUC Heart Rate | |||||||
| Step 1 | Time of day | 0.20 | 0.21 | 1.85 | 0.04 | 0.04 | 4.25* |
| Step 2 | Race | 0.06 | 0.01 | 0.52 | |||
| EA vs. AA | 0.00 | 0.00 | 0.01 | ||||
| EA vs. Other | 0.15 | 0.15 | 1.40 | ||||
| Step 3 | Personality | 0.11 | 0.04 | 0.82 | |||
| Absorption | −0.05 | −0.05 | −0.44 | ||||
| Constraint | −0.13 | −0.15 | −1.20 | ||||
| Agentic PEM | 0.21 | 0.23 | 1.99* | ||||
| Communal PEM | −0.14 | −0.15 | −1.26 | ||||
| NEM | −0.02 | −0.02 | −0.21 | ||||
|
| |||||||
| Peak Change Mean Arterial Pressure | |||||||
| Step 1 | Sex | 0.06 | 0.06 | 3.80* | |||
| Men vs. Foll | −0.17 | −0.17 | −0.18 | ||||
| Men vs. Lut | −0.14 | −0.16 | −0.15 | ||||
| Step 2 | Time of day | 0.28 | 0.31 | 3.10*** | 0.11 | 0.05 | 5.96* |
| Step 3 | Personality | 0.18 | 0.08 | 2.10 | |||
| Absorption | 0.12 | 0.13 | 1.33 | ||||
| Constraint | 0.12 | 0.12 | 1.27 | ||||
| Agentic PEM | −0.03 | −0.03 | −0.32 | ||||
| Communal PEM | −0.20 | −0.19 | −2.13* | ||||
| NEM | −0.19 | −0.20 | −2.10* | ||||
|
| |||||||
| Peak Change Positive Mood | |||||||
| Step 1 | Personality | 0.11 | 0.11 | 2.82* | |||
| Absorption | −0.05 | −0.05 | −0.50 | ||||
| Constraint | 0.14 | 0.14 | 1.48 | ||||
| Agentic PEM | −0.08 | −0.08 | −0.87 | ||||
| Communal PEM | 0.14 | 0.14 | 1.54 | ||||
| NEM | −0.19 | −0.20 | −2.16* | ||||
Asterisks indicate levels of significance
p<0.05,
0.01
0.001.
r=partial correlation of predictor with dependent variable; β=standardized coefficient, t=statistic of each predictor variable, Foll=women tested in the follicular phase, Lut=women tested in the luteal phase, EA=European American, AA=African American.
Cortisol Response
Together, demographic and personality variables accounted for 13% of the variance in peak cortisol responses [F(9,122)=1.82 p=0.072, Cohen’s f2=0.15] and 12% of the variance in total cortisol responses [F(9,122)=1.77 p=0.083, Cohen’s f2=0.14] to stress. After controlling for demographic characteristics, personality variables explained 4% of the variance in the peak magnitude of cortisol responses to stress [p=0.40, Cohen’s f2=0.04, Table 2] and 6% of the variance in total cortisol responses [p=0.24, Cohen’s f2=0.06, data not shown]. ComPEM was a significant negative predictor of peak cortisol increases (Table 2, Figure 2A) and total cortisol responses to stress [β=−0.22 t(124)=−2.27 p=0.025]. That is individuals with high ComPEM scores exhibited smaller cortisol responses to stress; for each unit increase in trait ComPEM, peak and total cortisol responses to stress decrease by 0.19 and 0.22 units respectively. AgPEM positively predicted total cortisol responses after stress [β=0.19 t(124)=2.0 p=0.052].
Figure 2.
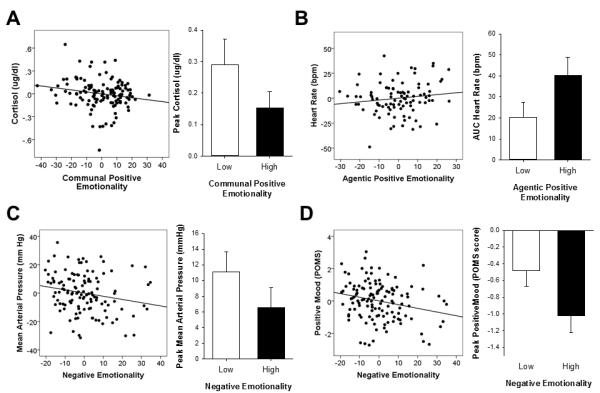
Significant relationships between personality factors and net peak change (TSST minus control) in stress outcome measures. Scatterplots show partial correlations controlling for other demographic, experimental and personality factors. Bars represent mean±SEM responses for individuals with scores in the top and bottom quartiles for the given trait.
Cardiovascular response
Together, demographic and personality variables accounted for 11% of the variance in peak HR responses [F(6,93)=1.71 p=0.13, Cohen’s f2=0.12] and 12% of the variance in total HR responses [F(8,93)=1.39 p=0.21, Cohen’s f2=0.14] to the TSST. After controlling for demographic and experimental variables, personality variables explained 4% of the observed variation in peak HR responses [p=0.54, Cohen’s f2=0.06, data not shown] and 6% of the variance in total heart rate responses [p=0.33, Cohen’s f2=0.07, Table 2] to stress. AgPEM significantly positively predicted total HR responses after the TSST (Table 2, Figure 2B).
Together, demographic and personality variables accounted for 18% of the observed variance in peak MAP responses [F(8,121)=3.12 p<0.005, Cohen’s f2=0.22] and 23% of the variance in total MAP responses [F(10,121)=3.22 p<0.001, Cohen’s f2=0.30] to the TSST. After controlling for demographic and experimental variables, personality explained 8% of the variance in peak MAP responses to stress [p=0.072, Cohen’s f2=0.08, Table 2] and 4% of the variation in total MAP responses [p=0.15, Cohen’s f2=0.04, data not shown]. ComPEM and NEM were significant negative predictors of peak changes in MAP (Table 2, Figure 2C). That is, individuals with high trait ComPEM or NEM exhibited smaller stress-induced peak increases in MAP; for each unit increase in trait ComPEM and NEM, peak changes in MAP decrease by 0.19 and 0.20 units respectively. Simple correlations showed that these relationships were driven by the traits Well Being (r=−0.15 p=0.10) and Stress Reaction (r=−.19 p<0.05).
Mood response
Personality factors explained a significant proportion of the observed variance in both the peak increase in emotional distress [11%, F(5,124)=2.82 p<0.02, Cohen’s f2=0.12, Table 2], and the total mood response after the TSST [11%, F(5,124)=3.02 p<0.02, Cohen’s f2=0.12]. NEM was a significant predictor of changes in mood ratings (Table 2, Figure 2D). That is, individuals with high NEM scores exhibited greater emotional distress after the TSST [Peak response p=0.033 Table 2; Total response β=−0.19 t(124)=−2.02 p<0.05]; for each unit increase in trait NEM, the peak magnitude and total emotional distress are increased by 0.20 and 0.19 units respectively. The greatest predictors of this relationship were trait Stress Reaction (Peak: r=−.28 p<0.002; AUC: r=−.28 p<0.001) and Alienation (Peak: r=−0.19 p<0.05; AUC: r=−.21 p<0.05).
Experimental and demographic factors
Time of day explained a significant proportion of the variance in peak MAP responses [5%, p<0.02, Cohen’s f2=0.05] and total MAP responses to stress [8%, p<0.001, Cohen’s f2=0.09]; participants tested in the afternoon exhibited greater peak and total MAP responses when tested in the afternoon, than participants tested in the morning. Time of day also explained a significant proportion of the observed variance in peak HR changes [6%, p<0.02 Cohen’s f2=0.07] after stress; peak HR responses were greater when participants performed the TSST in the afternoon.
Sex and menstrual cycle phase explained a significant proportion of the variance in peak cortisol responses to stress [5%, p<0.05, Cohen’s f2=0.05]. Overall, men exhibited greater peak cortisol responses to stress than women tested in the follicular and luteal phases of the menstrual cycle. Sex also explained a significant proportion of the variance in peak MAP responses [6%, p<0.025, Cohen’s f2=0.06] and total MAP responses to the TSST [8%, p<0.005, Cohen’s f2=0.09]; men exhibited greater peak and total MAP responses to stress than women tested in both phases of the menstrual cycle. Luteal phase testing did not remain a significant predictor in the final model after addition of the Time of day variable, as these two variables were confounded i.e., no women were tested in the luteal phase of the cycle in the afternoon study.
Finally, race explained 4% of the variance in peak cortisol responses to stress [R2 Change F(2,118)=2.38 p<0.01, Cohen’s f2=0.04] and 3% of the variation in total MAP responses to stress [R2 Change F(2,117)=1.78 p<0.02, Cohen’s f2=0.03,]; participants of Asian and other descent exhibited greater peak increases in cortisol than participants of European descent [β=0.19 t(123)=2.02 p<0.05] and participants of African descent exhibited greater total MAP responses than participants of European descent [β=0.20 t(122)=2.03 p<0.05].
Discussion
In this study, we investigated relationships between personality factors and psychobiological responses to acute psychosocial stress, controlling for the influences of demographic characteristics. We found that personality explained a significant proportion (11%) of the variance in emotional responses to stress. Personality also explained 8% of the variance in blood pressure responses to stress, and 4% of the variance in heart rate and cortisol responses to stress, and while this did not denote a significant proportion of the variance in these outcome measures, certain super-factors independently predicted stress-induced changes in cardiovascular and hormonal measures. Overall, personality contributed approximately the same amount of variance to stress response outcomes as the demographic and experimental variables, sex, race and time of day, and together all of the variables accounted for 11-23% of the variance. This highlights that other variables not considered here, such as trauma history (Gutman and Nemeroff, 2003), genetic variation (Wust et al., 2004), and physical fitness (Rimmele et al., 2009) among others, contribute up to 90% of the variance in stress response measures. Thus, our findings emphasise the importance of considering all of these factors when designing and interpreting stress reactivity research.
In line with our hypothesis and others’ findings (Bolger 1990; Bolger and Zuckerman 1995; Chida and Hamer 2008), high trait NEM was associated with greater aversive mood after stress. A tendency to emotional distress after negative events is a documented risk-factor for depression (Siegrist 2008; Forbes 2009; Mezulis et al. 2010; Morris et al. 2010), and our findings suggest that high trait NEM may identify individuals at elevated risk for chronic stress-related depression and anxiety disorders. NEM has also been linked to poor health and cardiovascular disease (Christensen et al. 2002; Suls and Bunde 2005; Smith and MacKenzie 2006), and in line with others’ findings (Habra et al. 2003; Jonassaint et al. 2009; Williams et al. 2009), we also found that NEM inversely predicted peak MAP responses to stress. Thus, high trait NEM may also be a risk factor for blunted or inefficient cardiovascular responses to stress, which may increase the risk of cardiovascular disease.
The separate measures of extraversion, i.e., reward sensitivity (AgPEM) and affiliation (ComPEM), differentially predicted patterns of cortisol and cardiovascular responses to stress in our sample. First, in line with our original hypothesis ComPEM (a measure of affiliative nature and social warmth) predicted smaller cortisol and also blood pressure responses to stress, suggesting that individuals with high trait ComPEM may be more resilient to negative environmental stimuli and stress-linked disease. Close interpersonal and social relationships may promote stress resilience by playing a role in the development and organization of neurobiological systems (Greenough et al. 1987; Hofer 1987; Silk et al. 2007), and nurturing early attachment relationships appear to be central to the development of the stress response system (Hofer 1987; Hertsgaard et al. 1995; Meaney 2001). Thus, a tendency to close social relationships may protect against excessive stress responses because it reflects processes that encourage the development of less reactive HPA and noradrenaline systems. In contrast, high AgPEM (a measure of social dominance and assertiveness) predicted larger cortisol and HR responses to stress. At first, this appears counterintuitive and we hypothesised that socially dominant and confident individuals might exhibit lower physiological responses to a public speech task. However, such individuals also tend to view themselves as achievers, and a specific feature of the TSST is that participants receive no positive feedback. Thus, individuals with high trait AgPEM may lack a sense of achievement during the TSST causing a more prolonged activation of the sympathetic nervous system and the HPA axis (Papousek et al. 2011). This is consistent with the finding that trait AgPEM contributed to total cortisol and HR responses indicating a sustained response and delayed recovery. On the other hand, prolonged responses and delayed recovery may also reflect engagement of active coping strategies by these individuals, such as cognitive reappraisal, which would increase the duration of engagement in the stress event (Compas et al. 2001; Derryberry et al. 2003; Rueda and Rothbart 2009).
Contrary to our hypotheses, we found no significant relationships between trait CON and emotional or cortisol responses to stress. Thus, we can infer that trait cognitive impulsivity does not significantly influence responses to stress, at least among healthy young adults in response to a relatively brief psychosocial stress task.
Consistent with previous reports, participants’ responses to stress were related to demographic and experimental factors included in the analyses. First, men exhibited larger peak and overall cortisol and blood pressure responses than women tested in two phases of the menstrual cycle. These differences have been observed elsewhere (Kirschbaum et al. 1992, 1999; Steptoe et al. 1996; Kelly et al. 2008; Childs et al. 2010) and could relate to sex differences in the incidence of stress-related disorders such as cardiovascular disease and chronic inflammatory disease (Carter-Snell and Hegadoren 2003). Although, luteal phase testing and time of day were confounded in the analyses of blood pressure responses, we consider it unlikely that there would be a menstrual cycle phase x time of day interaction. Race also influenced stress responses in our sample. First, African Americans exhibited greater MAP responses to stress than European Americans, which is consistent with the results of several previous studies (Murphy et al. 1992; Saab et al. 1992; Kelsey et al. 2000; Wilcox et al. 2005), and is thought to contribute to the greater prevalence of hypertension among this group compared to other ethnicities in U.S. samples (Anderson 1989; Ong et al. 2007). Second, Asian and other Americans (i.e., American Indians, Hawaiian Islanders) exhibited greater cortisol responses than European or African Americans. There has been one other report of greater cortisol responses to speech tasks among Asian Americans compared to European Americans which the author suggested may be related to cultural differences in responses to speech-based tasks (Kim, 2008). Finally, time of day also influenced participants’ cardiovascular responses to stress; HR and MAP responses to stress were greater when tasks were performed in the afternoon. This finding replicates prior reports (Lovallo et al. 2010) and reflects diurnal rhythms in cardiovascular measures which are elevated in the morning.
The findings of this study are important for several reasons. First, the approach used in the current study may be useful to identify individuals at risk for stress-linked disorders such as depression, addiction and cardiovascular disease (Everson et al. 1992; Lee et al. 2002). Indeed, cardiovascular and hormonal reactivity to stress has been shown to predict elevations in blood pressure and the development of hypertension later in life (Treiber et al. 2003; Matthews et al. 2004; Flaa et al. 2008; Chida and Steptoe 2010). Thus, since physiological responses to stress tests have been found to be relatively stable over time (Hassellund et al. 2010), the TSST could be used clinically to identify individuals at greater risk for stress-related disorders e.g. among individuals who are already at an elevated risk due to family history of disease. Together with personality assessments, individuals with high scores for traits conferring heightened stress reactivity could be identified for stress resilience training or intervention. Personalized interventions that increase individuals’ subjective well-being and positive affect, both of which have been linked to stress resilience and multiple quality of life measures including better immunity, mental health and self-reported marriage quality (Lyubomirsky et al. 2005), are likely to impact both health outcomes as well as personal well-being.
Second, the findings may help us to understand the underlying biological links between stress and health. Personality traits such as PEM and NEM, have been linked to momentary positive and negative affect and also to the biologically relevant behavioral activation and inhibition systems (Gray 1970). The relationships between biologically derived personality constructs and reactivity of biological stress systems may provide a behavioral phenotype, through which the mechanisms that mediate the development of adverse health consequences may be investigated.
The current study has several limitations. First, the findings may not readily generalise to other studies because the sample was limited to healthy young adults. In addition to age-related differences in the basal activity and responsivity of stress systems (Seals and Dinenno, 2004; Scott et al., 2013; Hidalgo et al., 2014), personality characteristics may also change across the lifespan (Allemand et al., 2008). Thus, it is feasible that relationships between personality and stress responses may also differ in older adults. Second, we utilised a particular type of laboratory stress (psychosocial) that may not generalise to other non-psychosocial stressors. Third, we did not assess individuals’ coping strategies which may well vary with personality and could also influence stress outcomes in real-world settings. Finally, the sex and race of the TSST panel was not controlled between participants and this may have contributed to the sex and race differences in stress responses reported here.
In conclusion, the current study reports that psychobiological responses to acute psychosocial stress varied with relation to specific personality traits. First, participants with an overall tendency toward situational anxiety and alienation exhibited greater emotional distress and blunted blood pressure responses to the TSST; such individuals may be at greater risk for chronic stress-related mood disorders and cardiovascular disease. Second, participants with a tendency toward social dominance and assertiveness exhibited delayed heart rate recovery to the psychosocial stressor possibly due to a lack of positive feedback or prolonged rumination. Finally, highly sociable participants exhibited smaller cortisol and blood pressure responses to this stressor, which is consistent with increased stress resilience in these individuals. These results indicate that high trait ComPEM is protective while high trait NEM and high trait AgPEM are risk factors for greater psychobiological stress responses to psychosocial stress.
Acknowledgements
We thank Ben Cunningham, Stephen Sittler and Lesley Sidney, who conducted the TSST interviews, Lisa Vicini, and Heather Phillips, who assisted with collection of the data, and Matthew Kirkpatrick for valuable comments on the final versions of the manuscript.
This research was supported by NIDA (DA02812) and the University of Chicago Hospital’s GCRC (USPHS MO1RR000555).
Footnotes
Conflicts of Interest and Source of Funding The authors declare no conflicts of interest.
References
- Allemand M, Zimprich D, Hendriks AA. Age differences in five personality domains across the life span. Dev Psychol. 2008;44:758–70. doi: 10.1037/0012-1649.44.3.758. [DOI] [PubMed] [Google Scholar]
- Altman DG. Practical Statistics for Medical Research. 1st ed Chapman and Hall; New York: 1991. [Google Scholar]
- Anderson NB. Racial differences in stress-induced cardiovascular reactivity and hypertension: current status and substantive issues. Psychol Bull. 1989;105:89–105. doi: 10.1037/0033-2909.105.1.89. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed Author; Washington, DC: 1994. [Google Scholar]
- Arnetz BB, Fjellner B. Psychological predictors of neuroendocrine responses to mental stress. J Psychosom Res. 1986;30:297–305. doi: 10.1016/0022-3999(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50:529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Bohnen N, Nicolson N, Sulon J, Jolles J. Coping style, trait anxiety and cortisol reactivity during mental stress. J Psychosom Res. 1991;35:141–147. doi: 10.1016/0022-3999(91)90068-y. [DOI] [PubMed] [Google Scholar]
- Bolger N. Coping as a personality process: a prospective study. J Pers Soc Psychol. 1990;59:525–537. doi: 10.1037//0022-3514.59.3.525. [DOI] [PubMed] [Google Scholar]
- Bolger N, Zuckerman A. A framework for studying personality in the stress process. J Pers Soc Psychol. 1995;69:890–902. doi: 10.1037//0022-3514.69.5.890. [DOI] [PubMed] [Google Scholar]
- Bossert S, Berger M, Krieg JC, Schreiber W, Junker M, von Zerssen D. Cortisol response to various stressful situations: relationship to personality variables and coping styles. Neuropsychobiology. 1988;20:36–42. doi: 10.1159/000118470. [DOI] [PubMed] [Google Scholar]
- Britton KT, Segal DS, Kuczenski R, Hauger R. Dissociation between in vivo hippocampal norepinephrine response and behavioral/neuroendocrine responses to noise stress in rats. Brain Res. 1992;574:125–130. doi: 10.1016/0006-8993(92)90808-m. [DOI] [PubMed] [Google Scholar]
- Carter-Snell C, Hegadoren K. Stress disorders and gender: implications for theory and research. Can J Nurs Res. 2003;35:34–55. [PubMed] [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol Bull. 2008;134:829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology (Berl) 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Dlugos A, De Wit H. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology. 2010;47:550–559. doi: 10.1111/j.1469-8986.2009.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AJ, Ehlers SL, Wiebe JS, Moran PJ, Raichle K, Ferneyhough K, et al. Patient personality and mortality: a 4-year prospective examination of chronic renal insufficiency. Health Psychol. 2002;21:315–320. doi: 10.1037//0278-6133.21.4.315. [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Compas BE, Connor-Smith JK, Saltzman H, Thomsen AH, Wadsworth ME. Coping with stress during childhood and adolescence: problems, progress, and potential in theory and research. Psychol Bull. 2001;127:87–127. [PubMed] [Google Scholar]
- Derryberry D, Reed MA, Pilkenton-Taylor C. Temperament and coping: advantages of an individual differences perspective. Dev Psychopathol. 2003;15:1049–1066. [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dlugos A, Childs E, Stuhr KL, Hillard CJ, de Wit H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology. 2012;37:2416–2427. doi: 10.1038/npp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson SA, Lovallo WR, Sausen KP, Wilson MF. Hemodynamic characteristics of young men at risk for hypertension at rest and during laboratory stressors. Health Psychol. 1992;11:24–31. doi: 10.1037//0278-6133.11.1.24. [DOI] [PubMed] [Google Scholar]
- Fichera LV, Andreassi JL. Cardiovascular reactivity during public speaking as a function of personality variables. Int J Psychophysiol. 2000;37:267–273. doi: 10.1016/s0167-8760(00)00106-9. [DOI] [PubMed] [Google Scholar]
- Flaa A, Eide IK, Kjeldsen SE, Rostrup M. Sympathoadrenal stress reactivity is a predictor of future blood pressure: an 18-year follow-up study. Hypertension. 2008;52:336–341. doi: 10.1161/HYPERTENSIONAHA.108.111625. [DOI] [PubMed] [Google Scholar]
- Forbes EE. Where’s the fun in that? Broadening the focus on reward function in depression. Biol Psychiatry. 2009;66:199–200. doi: 10.1016/j.biopsych.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Mascetti GG, Gardini S, Zambelli U, Timpano M, et al. Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuroendocrinology. 2001;26:91–107. doi: 10.1016/s0306-4530(00)00046-9. [DOI] [PubMed] [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion. Behav Res Ther. 1970;8:249–266. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987;58:539–559. [PubMed] [Google Scholar]
- Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav. 2003;79:471–8. doi: 10.1016/s0031-9384(03)00166-5. [DOI] [PubMed] [Google Scholar]
- Habra ME, Linden W, Anderson JC, Weinberg J. Type D personality is related to cardiovascular and neuroendocrine reactivity to acute stress. J Psychosom Res. 2003;55:235–245. doi: 10.1016/s0022-3999(02)00553-6. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Childs E, Conrad M, King A, de Wit H. Stress-induced changes in mood and cortisol release predict mood effects of amphetamine. Drug Alcohol Depend. 2010;109:175–180. doi: 10.1016/j.drugalcdep.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassellund SS, Flaa A, Sandvik L, Kjeldsen SE, Rostrup M. Long-term stability of cardiovascular and catecholamine responses to stress tests: an 18-year follow-up study. Hypertension. 2010;55:131–136. doi: 10.1161/HYPERTENSIONAHA.109.143164. [DOI] [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar M, Erickson MF, Nachmias M. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Dev. 1995;66:1100–1106. [PubMed] [Google Scholar]
- Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT. Neuroendocrine and psychometric evaluation of a placebo version of the ‘Trier Social Stress Test’. Psychoneuroendocrinology. 2009;34:1075–1086. doi: 10.1016/j.psyneuen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Hidalgo V, Almela M, Villada C, Salvador A. Acute stress impairs recall after interference in older people, but not in young people. Horm Behav. 2014;65:264–72. doi: 10.1016/j.yhbeh.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early social relationships: a psychobiologist’s view. Child Dev. 1987;58:633–647. [PubMed] [Google Scholar]
- Jonassaint CR, Why YP, Bishop GD, Tong EM, Diong SM, Enkelmann HC, et al. The effects of neuroticism and extraversion on cardiovascular reactivity during a mental and an emotional stress task. Int J Psychophysiol. 2009;74:274–279. doi: 10.1016/j.ijpsycho.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Kaplan HB. Psychosocial stress from the perspective of self-theory. In: Kaplan HB, editor. Psychosocial stress: Perspective on structure, theory, life-course, and methods. Academic Press; San Diego, CA: 1996. pp. 175–244. [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and physiological responses to the Trier Social Stress Test. J Behav Ther Exp Psychiatry. 2008;39:87–98. doi: 10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey RM, Alpert BS, Patterson SM, Barnard M. Racial differences in hemodynamic responses to environmental thermal stress among adolescents. Circulation. 2000;101:2284–2289. doi: 10.1161/01.cir.101.19.2284. [DOI] [PubMed] [Google Scholar]
- Kim HS. Culture and the cognitive and neuroendocrine responses to speech. J Pers Soc Psychol. 2008;94:32–47. doi: 10.1037/0022-3514.94.1.32. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Bartussek D, Strasburger CJ. Cortisol responses to psychological stress and correlations with personality traits. Person individ Diff. 1992a;13:1353–1357. [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992b;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Ann N Y Acad Sci. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4:117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Vincent AS. Use of a resting control day in measuring the cortisol response to mental stress: diurnal patterns, time of day, and gender effects. Psychoneuroendocrinology. 2010;35:1253–1258. doi: 10.1016/j.psyneuen.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: does happiness lead to success? Psychol Bull. 2005;131:803–855. doi: 10.1037/0033-2909.131.6.803. [DOI] [PubMed] [Google Scholar]
- Magnus K, Diener E, Fujita F, Pavot W. Extraversion and neuroticism as predictors of objective life events: a longitudinal analysis. J Pers Soc Psychol. 1993;65:1046–1053. doi: 10.1037//0022-3514.65.5.1046. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, et al. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- McCann BS, Carter J, Vaughan M, Raskind M, Wilkinson CW, Veith RC. Cardiovascular and neuroendocrine responses to extended laboratory challenge. Psychosom Med. 1993;55:497–504. doi: 10.1097/00006842-199311000-00005. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of Mood States. Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Mezulis AH, Funasaki KS, Charbonneau AM, Hyde JS. Gender Differences in the Cognitive Vulnerability-Stress Model of Depression in the Transition to Adolescence. Cogn Ther Res. 2010;34:501–513. [Google Scholar]
- Mikolajczak M, Roy E, Luminet O, Fillee C, de Timary P. The moderating impact of emotional intelligence on free cortisol responses to stress. Psychoneuroendocrinology. 2007;32:1000–1012. doi: 10.1016/j.psyneuen.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Morris MC, Ciesla JA, Garber J. A prospective study of stress autonomy versus stress sensitization in adolescents at varied risk for depression. J Abnorm Psychol. 2010;119:341–354. doi: 10.1037/a0019036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JK, Alpert BS, Walker SS. Ethnicity, pressor reactivity, and children’s blood pressure. Five years of observations. Hypertension. 1992;20:327–332. doi: 10.1161/01.hyp.20.3.327. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The role of corticotropin-releasing factor in the pathogenesis of major depression. Pharmacopsychiatry. 1988;21:76–82. doi: 10.1055/s-2007-1014652. [DOI] [PubMed] [Google Scholar]
- Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Zandi P, Nestadt G, Potash JB, Kalaydjian AE, Wand GS. Relationship between cortisol responses to stress and personality. Neuropsychopharmacology. 2006;31:1583–1591. doi: 10.1038/sj.npp.1301012. [DOI] [PubMed] [Google Scholar]
- Papousek I, Paechter M, Lackner HK. Delayed psychophysiological recovery after self-concept-inconsistent negative performance feedback. Int J Psychophysiol. 2011;82:275–282. doi: 10.1016/j.ijpsycho.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assess. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Gaab J, Hellhammer DH, Lintz D, Schommer N, Kirschbaum C. Increasing correlations between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology. 1997;22:615–625. doi: 10.1016/s0306-4530(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Seiler R, Marti B, Wirtz PH, Ehlert U, Heinrichs M. The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology. 2009;34:190–8. doi: 10.1016/j.psyneuen.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK. The Influence of Temperament on the Development of Coping: The role of maturation and experience. In: Skinner EA, Zimmer-Gembeck MJ, editors. Coping and the development of regulation. New Directions for Child and Adolescent Development. Vol. 124. Jossey-Bass; San Francisco: 2009. pp. 19–31. [DOI] [PubMed] [Google Scholar]
- Saab PG, Llabre MM, Hurwitz BE, Frame CA, Reineke LJ, Fins AI, et al. Myocardial and peripheral vascular responses to behavioral challenges and their stability in black and white Americans. Psychophysiology. 1992;29:384–397. doi: 10.1111/j.1469-8986.1992.tb01712.x. [DOI] [PubMed] [Google Scholar]
- Schneider TR, Rench TA, Lyons JB, Riffle RR. The influence of neuroticism, extraversion and openness on stress responses. Stress Health. 2011;28:102–110. doi: 10.1002/smi.1409. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Kudielka BM, Hellhammer DH, Kirschbaum C. No evidence for a close relationship between personality traits and circadian cortisol rhythm or a single cortisol stress response. Psychol Rep. 1999;84:840–842. doi: 10.2466/pr0.1999.84.3.840. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom Med. 2003;65:450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Scott SB, Sliwinski MJ, Blanchard-Fields F. Age differences in emotional responses to daily stress: the role of timing, severity, and global perceived stress. Psychol Aging. 2013;28:1076–87. doi: 10.1037/a0034000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Dinenno FA. Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol. 2004;287:H1895–905. doi: 10.1152/ajpheart.00486.2004. [DOI] [PubMed] [Google Scholar]
- Siegrist J. Chronic psychosocial stress at work and risk of depression: evidence from prospective studies. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 5):115–119. doi: 10.1007/s00406-008-5024-0. [DOI] [PubMed] [Google Scholar]
- Silk JS, Vanderbilt-Adriance E, Shaw DS, Forbes EE, Whalen DJ, Ryan ND, et al. Resilience among children and adolescents at risk for depression: Mediation and moderation across social and neurobiological contexts. Dev Psychopathol. 2007;19:841–865. doi: 10.1017/S0954579407000417. [DOI] [PubMed] [Google Scholar]
- Smith TW, MacKenzie J. Personality and risk of physical illness. Annu Rev Clin Psychol. 2006;2:435–467. doi: 10.1146/annurev.clinpsy.2.022305.095257. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Fieldman G, Evans O, Perry L. Cardiovascular risk and responsivity to mental stress: the influence of age, gender and risk factors. J Cardiovasc Risk. 1996;3:83–93. [PubMed] [Google Scholar]
- Strutton D, Pelton LE, Lumpkin JR. Personality charactristics and salespeople’s choice of coping strategies. Journal of Academy of Marketing Science. 1995;23:132–140. [Google Scholar]
- Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Ikeda K, Ishikawa M, Kitamura N, Tsukasaki T, Nakama D, Kameda T. Anxiety, reactivity, and social stress-induced cortisol elevation in humans. Neuro Endocrinol Lett. 2005;26:351–354. [PubMed] [Google Scholar]
- Thiagarajan AB, Gleiter CH, Mefford IN, Eskay RL, Nutt DJ. Effect of single and repeated electroconvulsive shock on the hypothalamic-pituitary-adrenal axis and plasma catecholamines in rats. Psychopharmacology (Berl) 1989;97:548–552. doi: 10.1007/BF00439562. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Wier LM, Anderson GM, Wilkinson CW, Price LH, Carpenter LL. Temperament and response to the Trier Social Stress Test. Acta Psychiatr Scand. 2007;115:395–402. doi: 10.1111/j.1600-0447.2006.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eck MM, Nicolson NA, Berkhof H, Sulon J. Individual differences in cortisol responses to a laboratory speech task and their relationship to responses to stressful daily events. Biol Psychol. 1996;43:69–84. doi: 10.1016/0301-0511(95)05159-7. [DOI] [PubMed] [Google Scholar]
- Wilcox S, Bopp M, Wilson DK, Fulk LJ, Hand GA. Race differences in cardiovascular and cortisol responses to an interpersonal challenge in women who are family caregivers. Ethn Dis. 2005;15:17–24. [PubMed] [Google Scholar]
- Williams L, O’Carroll RE, O’Connor RC. Type D personality and cardiac output in response to stress. Psychol Health. 2009;24:489–500. doi: 10.1080/08870440701885616. [DOI] [PubMed] [Google Scholar]
- Wirtz PH, Elsenbruch S, Emini L, Rudisuli K, Groessbauer S, Ehlert U. Perfectionism and the cortisol response to psychosocial stress in men. Psychosom Med. 2007;69:249–255. doi: 10.1097/PSY.0b013e318042589e. [DOI] [PubMed] [Google Scholar]
- Wüst S, Federenko IS, van Rossum EF, Koper JW, Kumsta R, Entringer S, Hellhammer DH. A psychobiological perspective on genetic determinants of hypothalamus-pituitary-adrenal axis activity. Ann N Y Acad Sci. 2004;1032:52–62. doi: 10.1196/annals.1314.005. [DOI] [PubMed] [Google Scholar]